Assessing Endothelial Vasodilator Function with the Endo-PAT 2000
Summary
A noninvasive procedure to assess endothelial function is demonstrated using the Endo-PAT 2000.
Abstract
The endothelium is a delicate monolayer of cells that lines all blood vessels, and which comprises the systemic and lymphatic capillaries. By virtue of the panoply of paracrine factors that it secretes, the endothelium regulates the contractile and proliferative state of the underlying vascular smooth muscle, as well as the interaction of the vessel wall with circulating blood elements. Because of its central role in mediating vessel tone and growth, its position as gateway to circulating immune cells, and its local regulation of hemostasis and coagulation, the the properly functioning endothelium is the key to cardiovascular health. Conversely, the earliest disorder in most vascular diseases is endothelial dysfunction.
In the arterial circulation, the healthy endothelium generally exerts a vasodilator influence on the vascular smooth muscle. There are a number of methods to assess endothelial vasodilator function. The Endo-PAT 2000 is a new device that is used to assess endothelial vasodilator function in a rapid and non-invasive fashion. Unlike the commonly used technique of duplex ultra-sonography to assess flow-mediated vasodilation, it is totally non-operator-dependent, and the equipment is an order of magnitude less expensive. The device records endothelium-mediated changes in the digital pulse waveform known as the PAT ( peripheral Arterial Tone) signal, measured with a pair of novel modified plethysmographic probes situated on the finger index of each hand. Endothelium-mediated changes in the PAT signal are elicited by creating a downstream hyperemic response. Hyperemia is induced by occluding blood flow through the brachial artery for 5 minutes using an inflatable cuff on one hand. The response to reactive hyperemia is calculated automatically by the system. A PAT ratio is created using the post and pre occlusion values. These values are normalized to measurements from the contra-lateral arm, which serves as control for non-endothelial dependent systemic effects. Most notably, this normalization controls for fluctuations in sympathetic nerve outflow that may induce changes in peripheral arterial tone that are superimposed on the hyperemic response.
In this video we demonstrate how to use the Endo-PAT 2000 to perform a clinically relevant assessment of endothelial vasodilator function.
Protocol
I.Prepare the Patient for an Endo-PAT Study
- Prior to the study, ensure the patient has fasted for at least 4 hours, and has refrained for at least 8 hours from caffeine, tobacco, vitamins or medications that might affect vascular tone. The patient may wish to use the restroom prior to the study.
- The Endo-PAT study should be conducted in a quiet, dimly lit, temperature-controlled exam room to reduce fluctuations in vascular tone.
- Cell phones or paging devices should be silenced, and restrictive clothing that could interfere with blood flow to the arms should be removed. The patient should also remove watches, rings, or other jewelry on the hands or fingers.
- Inspect the patient’s fingers for any deformities or injuries that could affect the study. Do not place the probes on a finger that is cut or injured. Fingernails should not extend more than 5mm or 1/5 of an inch beyond the tip of the finger tissue. Trim or file fingernails if necessary to avoid damaging the internal membranes of the PAT probes and displacing the finger from the sensing region of the probe.
- The index finger is recommended for the study; however, if this finger is unsuitable, a different digit (except the thumb) may be used, as long as the same finger is used on both hands.
- The patient should be supine and comfortable for 15 minutes so as to attain a cardiovascular steady-state. Place the two arm supporters along each of the patient’s sides.
- Measure the blood pressure using the control arm (the arm that is not occluded during the Endo-PAT study.
- Place a blood pressure cuff on the arm to be occluded during the Endo-PAT study. Apply the cuff snugly, but without excess pressure. Do not inflate the cuff at this time.
II.Prepare the Endo-PAT System for Study
- Launch the Endo-PAT 2000 software and click the “Patient Information” icon on the tool bar to create a new patient file.
- Complete the Patient Information dialog box, including patient ID, name (optional), age, gender, height, weight, systolic and diastolic blood pressures. Optional fields allow for free text comments. Select your name from the pre-defined list in the Patographer name field. If your name has not been inserted add it the list and select.
- Select two new PAT probes and connect to the pneumo-electrical tubing. To connect the probes, insert the connector tab into the probe slit and gently press the connector onto the probe until it clicks into place.
- Place the connected probes into the sockets of the arm-supports and press the “Deflate” button on the top of the Endo-PAT 2000 device.
III.Conduct an Endo-PAT Study
- Place the patient’s index fingers completely into the probes, confirm with the patient that he or she can feel the very end of the probes, and press the “Inflate” button on the top of the Endo-PAT 2000 device.
- Place a foam anchor ring at the base of the adjacent middle finger. Ensure that the foam ring and the PAT sensor do not touch. Otherwise the ring may mechanically interfere with the sensor.
- Create an approximately 7-10cm loop with the pneumo-electrical tubing. The loop should extend from the PAT sensor and return to the foam ring on the adjacent finger while the rest of the tubing that connects to the EndoPAT device is pointing out tubing to the tip of the finger.
- Position the patient’s arms so the forearms are supported on the arm supports and the fingers dangle freely off the edge of the support. Make sure the probes are not in contact with any object, including the arm support, foam ring, tubing, the mattress or another finger.
- Ask the patient to refrain from moving the fingers, as this will create mechanical artifacts. It is important for the patient to be relaxed throughout the study. Explain to the patient that during the test you will inflate the arm cuff, and during that time they may feel some discomfort, numbness, or tingling.
- Click the “Standby” icon on the Endo-PAT’s computer interface. Adjust the time base to 1 minute and adjust the signal gain on the screen to maximize signal clarity. Inspect the tracings of the PAT signals from the two probes to confirm that they are free of artifactual signals. If artifactual signals are present, veriify that the probes are not touching anything and that the patient is not moving the fingers.
- To begin the study, click the “Go” icon on the computer interface. Start the stopwatch, by clicking the “Start/Stop Timer” icon. This will initiate a five minute count down for the baseline recording period. After five minutes, stop the stopwatch by clicking the “Start/Stop Timer” icon.
- Tell the patient that you are going to inflate the cuff for the occlusion phase and that he or she should stay relaxed and not move the fingers.
- Rapidly inflate the blood pressure cuff to a supra-systolic pressure of 60mmHg above the patient’s systolic pressure or 200mmHg, whichever is higher and start the stopwatch again. Complete cessation of blood flow to the hand is verified by the absence of a PAT signal from the occluded arm. To confirm occlusion increase the gain on the screen of the channel of the occluded side to 20,000 while keeping the gain of the contra-lateral side constant. Decrease the time base of both channels to 30 seconds. Verify that you do not observe any signals at a periodicity that matches the signal from the control arm as this indicates an incomplete occlusion. If this is the case then further inflate the cuff until no signals are seen. The cuff may be inflated to a maximum of 300mmHg.
- This will initiate a five minute count down for the arterial occlusion recording period. Toward the end of the occlusion period tell the patient you are going to release the cuff and that they should continue to refrain from moving their fingers. After exactly five minutes, deflate the cuff abruptly as quickly as possible and stop the stopwatch by clicking the “Start/Stop Timer” icon.
- Click the “Start/Stop Timer” icon again to initiate a five-minute post occlusion recording period. Stop the timer after five minutes and click the “Test Stop” icon to complete the study. The probes will automatically deflate.
- Remove the probes, tape, and foam rings from the patient’s fingers and disconnect the PAT probes from the pneumo-electrical tubing. Discard the used probes.
IV.Review and Analysis
- Load the study file to the screen using the load icon. To run the automatic analysis, click the “magician stick” icon. The occlusion period will be highlighted in blue and the test result will be displayed, including the Reactive Hyperemia Index (RHI) and Heart Rate (HR), in the right hand column of the screen.
- To review additional data, including study parameters, calculated variables, patient information, and measures of signal quality, click the “Open Results of Last Calculation” icon. This will open a spread sheet with study parameters and results for all analyses performed to date, with the last line in the table containing data from the most recent analysis.
V.Representative Results
A representative Endo-PAT screen of a study performed on an individual with normal endothelial vasodilator function is shown in Figure 1. A representative screen of an Endo-PAT study performed on an individual with endothelial vasodilator dysfunction is shown in Figure 2.
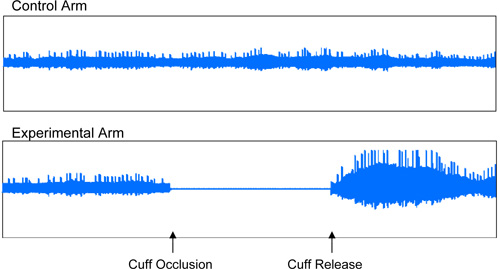
Figure 1: Normal Endothelial Vasodilator Function. Representative recording of an individual with normal endothelial vasodilator function, characterized by an increase in the signal amplitude after cuff release relative to baseline.
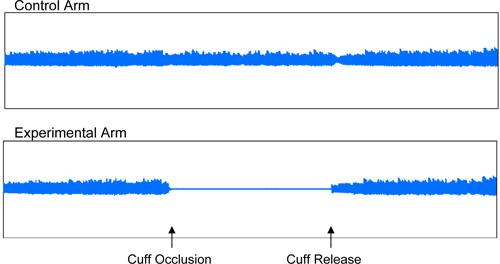
Figure 2: Endothelial Vasodilator Dysfunction. Representative recording of an individual with endothelial vasodilator dysfunction.
Discussion
The major cause of morbidity and mortality worldwide is atherosclerotic vascular disease, leading to stroke, myocardial infarction, heart failure, renal insufficiency, aneurysm rupture or embolism, intermittent claudication and gangrene 1,2. Endothelial dysfunction is one of the earliest events in the pathophysiological process leading to these atherosclerotic disorders 3. Furthermore endothelial dysfunction contributes to the progression of disease, by facilitating inflammation and thrombosis. 4-6. The traditional cardiovascular risk factors are associated with endothelial vasodilator dysfunction 7-11. Endothelial vasodilator dysfunction can be detected in seemingly healthy individuals that are at risk for developing cardiovascular disease 12. Furthermore, the finding of endothelial vasodilator dysfunction is predictive of major adverse cardiovascular events as well as mortality 13-16.
The vasodilators released by the endothelium include vasodilator prostanoids such as prostacyclin, peptides such as adrenomedullin and atrial natriuretic peptide, and small molecules such as endothelium dependent hyperpolarizing factor, carbon monoxide and nitric oxide 17. In addition, the effect of hyperpolarizing currents generated in the endothelium can be transmitted to underlying vascular smooth muscle of smaller vessels, relaxing them 18. An indirect assessment of the generation of these endothelial vasodilator influences can be gained by studying vascular reactivity. The most prevalent method to assess endothelial regulation of vascular reactivity non-invasively has been duplex ultrasonography to detect flow-mediated vasodilation of the brachial artery 19.
The tractive force of fluid flow stimulates the endothelium to release vasodilators, most prominently nitric oxide 20. This phenomenon can be observed by ultrasound in the brachial artery during increases in forearm blood flow induced by reactive hyperemia 19. This technology has been widely utilized to document the association of endothelial vasodilator dysfunction with cardiovascular risk factors; the relationship of endothelial vasodilator dysfunction to various biomarkers, such as C-reactive peptide, or asymmetric dimethylarginine (the endogenous antagonist of nitric oxide synthase); and the correction of endothelial vasodilator function with nutritional and lifestyle modifications as well as with the use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, statins, insulin sensitizing agents or aspirin 21-23.
The assessment of flow-mediated vasodilation by brachial artery ultrasound requires expensive equipment, is highly operator-dependent, the response has a very small dynamic range and the signal-to-noise ratio is low. New approaches to address these problems with assessment of endothelial vasodilator function are needed 24. The Endo-PAT 2000 is a new approach to assess endothelial vasodilator function in a rapid non-invasive manner. The technique provides values for the calculation of a Reactive Hyperemia Index (RHI), which gives an indication of the endothelial vasodilator function. The RHI is the post-to-pre occlusion PAT signal ratio in the occluded arm, relative to the same ratio in the control arm, and corrected for baseline vascular tone. Studies using the EndoPAT have shown that the RHI score reflects NO-bioavailability 25. The RHI correlates with the measurement of endothelial vasodilator function in the coronary arteries 26 and with brachial FMD 27. Patients with a greater degree of cardiovascular disease exhibit a lower score 28 and values are also lower in other conditions associated with impaired endothelial function and risk of cardiovascular disease 29-33. Notably, RHI values appear to be predictive of cardiovascular outcomes 35. A low RHI (indicating endothelial dysfunction) can be reversed with treatment 36.
In conclusion, we have demonstrated how to perform a reliable and reproducible test for endothelial vasodilator function with the Endo-PAT 2000. The test is noninvasive, easy to perform, and is a useful research tool. Its utility in clinical monitoring of endothelial function and in tailoring disease management is under investigation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Koby Sheffy, PhD for his insightful review of this work and William Sotka for ongoing support and technical assistance.
This work was supported in part by grants from the National Institutes of Health (K12 HL087746, RC2HL103400, 1U01HL100397), and the California Tobacco Related Disease Research Program of the University of California (18XT-0098).
References
- Pasternak, R. C., Criqui, M. H., Benjamin, E. J., Fowkes, F. G., Isselbacher, E. M., McCullough, P. A., Wolf, P. A., Zheng, Z. J. Atherosclerotic Vascular Disease Conference. Writing Group I: Epidemiology. Circulation. 109, 21-2605 (2004).
- Mozaffarian, D., Wilson, P. W., Kannel, W. B. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 117 (23), 3031-3038 (2008).
- Davies, P. F. Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circ Res. 101 (1), 10-12 (2007).
- Libby, P., Ridker, P. M., Hansson, G. K. Leducq. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 54 (23), 2129-2138 (2009).
- Lamon, B. D., Hajjar, D. P. Inflammation at the molecular interface of atherogenesis: an anthropological journey. Am J Pathol. 173 (5), 1253-1264 (2008).
- Napoli, C., Ignarro, L. J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 32 (8), 1103-1108 (2009).
- Creager, M. A., Cooke, J. P., Mendelsohn, M. E., Gallagher, S. J., Coleman, S. M., Loscalzo, J., Dzau, V. J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 86 (1), 228-234 (1990).
- Celermajer, D. S., Sorensen, K. E., Georgakopoulos, D., Bull, C., Thomas, O., Robinson, J., Deanfield, J. E. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 88 (5 Pt 1), 2149-2155 (1993).
- Celermajer, D. S., Sorensen, K. E., Bull, C., Robinson, J., Deanfield, J. E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 24 (6), 1468-1474 (1994).
- Stuhlinger, M. C., Abbasi, F., Chu, J. W., Lamendola, C., McLaughlin, T. L., Cooke, J. P., Reaven, G. M., Tsao, P. S. Relationship Between Insulin Resistance and an Endogenous Nitric Oxide Synthase Inhibitor. Journal of the American Medical Association. 287 (11), 1420-1426 (2002).
- Stühlinger, M. C., Oka, R. K., Graf, E. E., Schmölzer, I., Upson, B. M., Kapoor, O., Szuba, A., Malinow, M. R., Wascher, T. C., Pachinger, O., Cooke, J. P. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 108 (8), 933-938 (2003).
- Celermajer, D. S., Sorensen, K. E., Gooch, V. M., Spiegelhalter, D. J., Miller, O. I., Sullivan, I. D., Lloyd, J. K., Deanfield, J. E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 340 (8828), 1111-1115 (1992).
- Schächinger, V., Britten, M. B., Zeiher, A. M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 101 (6), 1899-1906 (2000).
- Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T., Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 104 (22), 2673-268 (2001).
- Halcox, J. P. J., Schenke, W. H., Zalos, G., Mincemoyer, R., Prasad, A., Waclawiw, M. A., Nour, K. R. A., Quyyumi, A. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 106 (6), 653-658 (2002).
- Gokce, N., Keaney, J. F., Hunter, L. M., Watkins, M. T., Menzoian, J. O., Vita, J. A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 105 (13), 1567-1572 (2002).
- Cooke, J. P., Aird, W. C. The diversity of vascular disease: a clinician’s perspective. Endothelial Cells in Health and Disease. , (2005).
- Olesen, S. P., Clapham, D. E., Davies, P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 331 (6152), 168-170 (1988).
- Corretti, M. C., Anderson, T. J., Benjamin, E. J., Celermajer, D., Charbonneau, F., Creager, M. A., Deanfield, J., Drexler, H., Gerhard-Herman, M., Herrington, D., Vallance, P., Vita, J., Vogel, R. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 39 (2), 257-2565 (2002).
- Cooke, J. P., Rossitch, E., Andon, N. A., Loscalzo, J., Dzau, V. J. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 88 (5), 1663-1671 (1991).
- Deanfield, J. E., Halcox, J. P., Rabelink, T. J. Endothelial Function and Dysfunction Testing and Clinical Relevance. Circulation. 115, 1285-1295 (2007).
- Boger, R., Bode-Boger, S., Szuba, A., Tsao, P. S., Chan, J., Tangphao, O., Blaschke, T., Cooke, J. P. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 98 (18), 1842-1847 (1998).
- Charakida, M., Masi, S., Loukogeorgakis, S. P., Deanfield, J. E. The role of flow-mediated dilatation in the evaluation and development of antiatherosclerotic drugs. Curr Opin Lipidol. 20 (6), 460-466 (2009).
- Celermajer, D. S. Reliable Endothelial Function Testing At Our Fingertips?. Circulation. 117 (19), 2428-2430 (2008).
- Nohira, A., Gerhard-Herman, M., Creager, M. A., Hurley, S., Mitra, D., Ganz, P. Role of Nitric Oxide in Regulation of Digital pulse Volume Amplitude in Humans. J Appl Physiology. 101 (2), 545-548 (2006).
- Bonetti, P. O., Pumper, G. M., Higano, S. T., Holmes, D. R., Kuvin, J. T., Lerman, A. Noninvasive Identification of Patients with Early Coronary Atherosclerosis by Assessment of Digital Reactive Hyperemia. J Am Coll Cardiol. 44 (11), 2137-2141 (2004).
- Kuvin, J. T., Patel, R. P., Sliney, K. A., Pandian, N. G., Sheffy, J., Schnall, R. P., Karas, R. H., Udelson, J. E. Assessment of Peripheral Vascular Endothelial Function with Finger Arterial Pulse Wave Amplitude. Am Heart J. 146 (1), 168-174 (2003).
- Bonetti, P. O. Attenuation of Digital Reactive Hyperemia in Patients with Early and Advanced Coronary Artery Disease. J Am Coll Cardiol. 45 (3), 407A-407A (2005).
- Mahmud, F. H., Earing, M. G., Lee, R. A., Lteif, A. N., Driscoll, D. J., Lerman, A. Altered Endothelial Function in Asymptomatic Male Adolescents with Type I Diabetes. Congenit Heart Dis. 1 (3), 98-103 (2006).
- Shachor-Meyouhas, Y., Pillar, G., Shehadeh, N. Uncontrolled Type 1 Diabetes Mellitus and Endothelial Dysfunction in Adolescents. Isr Med Assoc J. 9 (9), 637-640 (2007).
- Mahmud, F. H., Hill, D. J., Cuerden, M. S., Clarson, C. L. Impaired Vascular Function in Obese Adolescents with Insulin Resistance. J Pediatr. 155 (5), 678-682 (2009).
- Hirata, Y., Nagata, D., Suzuki, E., Nishimatsu, H., Suzuki, J., Nagai, R. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 51 (1), 1-6 (2010).
- Hamburg, N. M., Keyes, M. J., Larson, M. G., Vasan, R. S., Schnabel, R., Pryde, M. M., Mitchell, G. F., Sheffy, J., Vita, J. A., Benjamin, E. J. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circulation. 117 (19), 2467-2474 (2008).
- Truschel, E., Jarczok, M. N., Fischer, J. E., Terris, D. D. High-throughput ambulatory assessment of digital reactive hyperemia: Concurrent validity with known cardiovascular risk factors and potential confounding. Prev Med. 49 (6), 468-4672 (2009).
- Rubinshtein, R., Kuvin, J. T., Soffler, M., Lennon, R. J., Lavi, S., Nelson, R. E., Pumper, G. M., Lerman, L. O., Lerman, A. Assessment of Endothelial Function by Non-invasive Peripheral Arterial Tonometry Predicts Late Cardiovascular Adverse Events. Eur Heart. , (2010).
- Yamaoka-Tojo, M., Tojo, T., Kosugi, R., Hatakeyama, Y., Yoshida, Y., Machida, Y., Aoyama, N., Masuda, T., Izumi, T. Effects of ezetimibe add-on therapy for high-risk patients with dyslipidemia. Lipids Health Dis. 8 (1), 41-41 (2009).

