A Turbidimetric Analysis to Monitor Collagen-Induced Aggregation of Pretreated Human Platelets
Abstract
Source: Molica, F. et al., Turbidimetry on Human Washed Platelets: The Effect of the Pannexin1-inhibitor Brilliant Blue FCF on Collagen-induced Aggregation. J. Vis. Exp. (2017)
This video demonstrates turbidimetric analysis to assess collagen-induced platelet aggregation in pre-treated, washed human platelets. Control wells show collagen-induced aggregation, whereas a high concentration of inhibitors blocks Pannexin-1 channels, thereby inhibiting collagen-induced platelet aggregation, evident from unchanged turbidity.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Buffer Preparation for Human Blood Collection and Washed Platelet Isolation
- Prepare a 100 mL aqueous solution of acid-citrate-dextrose (ACD) by dissolving 1.4 g citric acid monohydrate (C6H8O7•H2O, 66.6 mM), 2.5 g trisodium citrate dihydrate (Na3C6H5O7•2 H2O, 85 mM) and 2 g of anhydrous D(+)-glucose. The pH of the solution is about 4.5.
- Prepare stock solutions for Tyrode's buffer as follows
- Prepare stock solution 1 by dissolving 80 g sodium chloride (NaCl), 2 g potassium chloride (KCl), 10 g sodium bicarbonate (NaHCO3), and 0.58 g sodium dihydrogen phosphate monohydrate (NaH2PO4*H2O) in 500 mL of distilled H2O. The respective final concentrations are 2.73 M, 53.6 mM, 238 mM, and 8.4 mM. Keep the solution at 4 °C.
- Prepare stock solution 2 by dissolving 10.15 g magnesium dichloride hexahydrate (MgCl2*6 H2O) (100 mM) in 500 mL distilled water (H2O). Keep the solution at 4 °C.
- Prepare stock solution 3 by dissolving 10.95 g (100 mM) calcium chloride hexahydrate CaCl2*6 H2O in 500 mL distilled H2O. Keep the solution at 4 °C.
- Prepare Tyrode's buffer by diluting 2.5 mL of stock solution 1 in a final volume of 50 mL with distilled H2O. This corresponds to final concentrations of 136.5 mM NaCl, 2.68 mM KCl, 11.9 mM NaHCO3, and 0.42 mM NaH2PO4*H2O. Adjust the pH to 7.35 and sterilize by filtering with 0.22-μm filters.
- Prepare Tyrode's albumin 0.35% buffer (TA buffer) by diluting 5 mL of stock solution 1, 1 mL of stock solution 2, 2 mL of stock solution 3, 0.5 mL 1M HEPES, 1.8 mL of 200 g/L human serum albumin and 0.1 g of anhydrous D(+)-glucose in a final volume of 100 mL distilled H2O.
- Adjust the pH to 7.35 with 1N hydrochloric acid (HCl) and set the osmolarity to 295 mOsm/L by adding distilled H2O (10% of total volume). The final concentrations in this solution are 124 mM NaCl, 2.44 mM KCl, 10.82 mM NaHCO3, 0.38 mM NaH2PO4*H2O, 0.91 mM MgCl2*6 H2O, 1.82 mM CaCl2*6 H2O. Keep TA buffer at 37 °C during the whole experiment.
2. Blood Collection
- Collect 45-50 mL of venous blood, from the antecubital vein using a 19 G needle and no or low tourniquet, into 50 mL tubes containing ACD anticoagulant (1 volume ACD for 6 volumes of blood). Discard the first 1-2 mL of blood to avoid the presence of thrombin and tissue factor.
- After collection, mix the blood with the ACD by gently inverting the tube. Incubate the sample for 10 min at 37 °C.
3. Preparation of Human Washed Platelets
- Pre-heat the centrifuge to 37 °C. All centrifugation steps below are performed at this temperature.
- Dispatch the collected blood into 15 mL tubes (5 mL per tube) and centrifuge at 250 x g for 13 min to obtain platelet-rich plasma, PRP.
NOTE: This centrifugation step results in the production of three layers in the sample: 1) The upper layer, composed of plasma, platelets, and a small fraction of white blood cells. 2) The intermediate layer, a portion rich in white blood cells. 3) The bottom layer, which is essentially composed of red blood cells. - Collect the PRP by pipetting the upper layer carefully into a new 15 mL tube to maximally prevent contamination with red and white blood cells, and incubate for 10 min at 37 °C.
- Centrifuge the PRP at 2,200 x g for 12 min (for 5 mL PRP).
NOTE: This centrifugation step should be performed with low brake or without brake. - Remove the supernatant (platelet-poor plasma), and carefully resuspend the pellet with 10 mL of TA buffer containing 2 µL/mL of heparin (5,000 U/mL) and 2.5 µL/mL of 25 μM prostacyclin (PGI2) using a plastic Pasteur pipet. Incubate for 10 min at 37 °C.
- Add 2.5 µL/mL of 25 μM prostacyclin-I2 (PGI2) and centrifuge for 8 min at 1,900 x g (with low brake or without brake).
- Remove the supernatant and resuspend the pellet with 5 mL TA buffer containing 2.5 µL/mL of 25 μM PGI2 using a plastic Pasteur pipet. Incubate for 10 min at 37 °C.
- During the incubation period, pipet 150 µL of the platelet suspension into a 1.5 mL tube and count platelets, using an automatized cell counter (that detects the size of blood cells by measuring the changes in direct-current resistance).
- After the 10 min incubation, add 2.5 µL/mL of 25 μM PGI2 to the platelet suspension and immediately centrifuge at 1,900 x g for 8 min.
- Remove the supernatant and resuspend the pellet to a concentration of 250,000 platelets/µL with an adequate volume of TA buffer (i.e. if the cell count is 500,000 per µL, resuspend in 10 mL TA buffer) containing 32 µL/mL of apyrase at 0.01 U/mL (final concentration 0.32 U/mL).
NOTE: A high concentration of apyrase is used to avoid the desensitization of P2X purinoceptor 1, P2X1 receptors induced by spontaneous secretion of adenosine triphosphate (ATP) in the absence of agonists. This is important because collagen-induced responses are induced by fast paracrine/autocrine activation of P2X1 by ATP released from activated platelets. If the platelet signaling pathway does not critically require the preservation of P2X1 function, use 0.02 U/mL apyrase. Several studies demonstrated that 0.02 U/mL apyrase avoids adenosine diphosphate ADP receptor P2Y purinoceptor 1, and P2Y1 desensitization with negligible P2X1 responses. - Incubate the cell suspension for at least 30 min at 37 °C before performing the aggregometric measurements. The preparation is stable for 5 to 8 hours.
4. Aggregometry
- Prepare fibrinogen (56 mg/mL) in Tyrode's buffer.
- Pipet 260 µL of platelet suspension into glass cuvettes (Figure 1A; left cuvette) containing 10 µL of fibrinogen (56 mg/mL) and a magnetic stirring rod, then incubate the suspension for 2 – 3 min at 37 °C in incubation wells present in the aggregometer (Figure 1B and 1C).
- Pre-incubate with the Panx1 inhibitor Brilliant Blue FCF by adding 10 µL of a 2.8 mM or 28 mM stock solution (final concentration 100 μM and 1 mM, respectively) for 7 min at 37 °C.
- Calibrate the aggregometer to an assumptive 100% aggregation value by measuring the optical density (OD) of a cuvette containing 10 µL fibrinogen (56 mg/mL), 10 µL Brilliant Blue FCF (2.8 mM or 28 mM), and TA buffer without platelets.
- Place the cuvette in an aggregation well under automatic stirring and press the corresponding button on the keyboard of the computer linked to the aggregometer (i.e. press F1 if aggregation well 1 is used).
NOTE: This experiment described below includes Brilliant Blue FCF. The compound used for calibration has to be adjusted to the experimental condition.
- Place the cuvette in an aggregation well under automatic stirring and press the corresponding button on the keyboard of the computer linked to the aggregometer (i.e. press F1 if aggregation well 1 is used).
- Calibrate the aggregometer to an assumptive 0% aggregation value by using the same platelet sample that will be used for the experiment under automatic stirring.
- Place the cuvette in the aggregation well and press the corresponding button on the keyboard of the computer linked to the aggregometer. Wait for about 20-30 s before proceeding. This delay serves to assure that no aggregation happens before adding the agonists.
NOTE: As any difference in platelet number may have an effect on the measured OD, the 0% calibration step needs to be repeated for each individual measurement.
- Place the cuvette in the aggregation well and press the corresponding button on the keyboard of the computer linked to the aggregometer. Wait for about 20-30 s before proceeding. This delay serves to assure that no aggregation happens before adding the agonists.
- Add 20 µL of desired agonist, such as 15 µg/mL collagen (1 µg/mL final) or 1.125 mM arachidonic acid (75 µM final), into the cuvette. Immediately start the recording under continuous automatic stirring by pressing the corresponding button on the keyboard of the computer linked to the aggregometer.
NOTE: The addition of the agonist induces platelet activation. Platelet aggregates can clearly be distinguished in the glass cuvette at the end of the experiment (Figure 1A; right cuvette). - The recording automatically stops after 6 min. At this point, save the data by clicking on the save icon on the computer.
NOTE: The calculation of the rate of aggregation is performed by the computer, which expresses the end results of the aggregation process as a percentage.
Representative Results
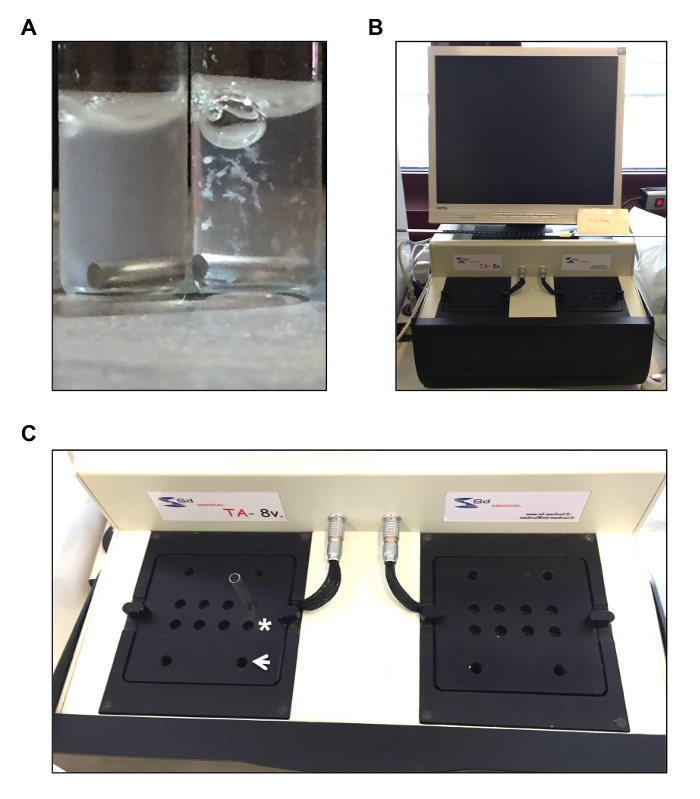
Figure 1: Aggregometry. (A) Representative image of glass cuvettes (containing a stirring magnet) used for aggregometry. The cuvette on the left shows resting platelets while the cuvette on the right illustrates platelet aggregates after collagen-induced activation. (B) Representative image of an 8-well aggregometer for turbidimetric measurements. The wells used (asterisk) to incubate platelet suspensions present in a glass cuvette at 37 °C, and those used for the measurements (white arrow) are indicated in C.
Divulgations
The authors have nothing to disclose.
Materials
| Citric acid monohydrate (C6H8O7*H2O) | Roth | 5949-29-1 | danger of eye damage/irritation |
| Trisodium citrate dihydrate (Na3C6H5O7*2H2O) | Sigma-Aldrich | S1804 | – |
| D(+)-glucose | Sigma-Aldrich | G8270 | – |
| Sodium chloride (NaCl) | Sigma-Aldrich | S9888 | – |
| Potassium chloride (KCl) | Sigma-Aldrich | P9541 | – |
| Sodium bicarbonate (NaHCO3) | Sigma-Aldrich | S6014 | – |
| Sodium dihydrogenophosphate monohydrate (NaH2PO4*H2O) | Sigma-Aldrich | S9638 | – |
| Magnesium chloride hexahydrate (MgCl2*6H2O) | Sigma-Aldrich | M9272 | – |
| Calcium chloride hexahydrate (CaCl2*6H2O) | Sigma-Aldrich | 442909 | danger of eye damage/irritation |
| N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (Hepes) | ThermoFisher Scientific | 15630 | – |
| Human serum albumin | CSL Behring | 00257/374 | – |
| Hydrochloric acid | Sigma-Aldrich | 320331 | Corrosive and irritative for the respiratory system. Can cause severe skin and eye damages. |
| Eppendorf 5810 R | Fisher Scientific | – | – |
| Heparin | Drosspharm AG/SA | 20810 | |
| Prostacyclin I2 (PGI2) | Cayman | 18220 | – |
| Apyrase from potatoes | Sigma-Aldrich | A6535 | – |
| Fibrinogen (Haemocomplettan) | CSL Behring | HS 73466011 | – |
| Thrombo-aggragometer SD-Medical | SD-Innovation | TA8V | – |
| Brilliant blue FCF (Erioglaucine disodium salt) | Sigma-Aldrich | 80717 | Harmful to aquatic life with long lasting effects (Avoid release to the environnement) |
| Collagen | Horm, Nycomed | – | |
| Arachidonic acid | Bio/Data corporation | C/N 101297 | – |
| Cell counter Sysmex KX-21N | Sysmex Digitana | – | – |
| HEPES | Gibco | 15630-056 | – |
| Glass cuvettes | SD-Innovation | THCV1000 | – |
| Magnetic stirrers | SD-Innovation | THA100 | – |

