State-Dependency Effects on TMS: A Look at Motive Phosphene Behavior
Summary
In this article, we examine the effects of visually relevant state dependency on TMS induced motive phosphenic presentations.
Abstract
Transcranial magnetic stimulation (TMS) is a non-invasive neurostimulatory and neuromodulatory technique that can transiently or lastingly modulate cortical excitability (either increasing or decreasing it) via the application of localized magnetic field pulses.1,2 Within the field of TMS, the term state dependency refers to the initial, baseline condition of the particular neural region targeted for stimulation. As can be inferred, the effects of TMS can (and do) vary according to this primary susceptibility and responsiveness of the targeted cortical area.3,4,5
In this experiment, we will examine this concept of state dependency through the elicitation and subjective experience of motive phosphenes. Phosphenes are visually perceived flashes of small lights triggered by electromagnetic pulses to the visual cortex. These small lights can assume varied characteristics depending upon which type of visual cortex is being stimulated. In this particular study, we will be targeting motive phosphenes as elicited through the stimulation of V1/V2 and the V5/MT+ complex visual regions.6
Protocol
1) Preparation
- To begin, seat the subject in a comfortable chair in front of a computer screen.
- Use a tape measure to ensure the distance between the screen and the subject’s nasion is 60 cm.
- Finally, place a light-blocking mask over the subject’s eyes.
2) Determining Phosphene Threshold over V1/V2
- Knowing the subject’s phosphene threshold will be important when exploring phosphenic behavior later in this protocol. To determine this threshold, first set the TMS machine to 70% power.
- Hold the coil so the top of the figure-8 faces cranial midline.
- Begin single-pulse stimulation at a point about 3 cm above the inion.
- After each pulse, ask the subject to report any phosphenic experience.
- Begin moving the coil in a small search grid around this region (V1/V2).
- Determine the location where stimulation elicits consistent and unambiguous phosphene reports from the subject. It has been theorized that TMS will be unable to elicit phosphenes in around 40% of the population, so there is a chance the subject will not report anything. If this is the case, unfortunately, this experiment will not work. The subject should be allowed to leave.
- Once the phosphenic “hotspot” has been located, adjust the TMS power output up and down until the subject reports phosphenes for exactly 3 of 6 consecutive pulses. This power level is the subject’s V1/V2 threshold. If available, neuronavigation can be used to achieve greater spatial precision when locating and targeting the V1/V2 hotspot.
3) Determining Phosphenic Threshold over the V5/MT+ Complex
- Using the same aforementioned procedure and parameters, we will now determine phosphene threshold over the V5/MT+ complex. To locate this region, begin at a point 3 cm dorsal and 5 cm lateral to the subject’s inion.
- After each pulse, ask the subject to report any phosphenic experience.
- Again, using single pulses, begin a small search grid pattern until unambiguous and consistent phosphenes are elicited.
- Finally, adjust the TMS power output up and down until the subject reports phosphenes for exactly 3 of 6 consecutive pulses. This power level is the subject’s V5/MT+ complex threshold. Again, if available, neuronavigation can be used to achieve greater spatial precision when locating and targeting the V5/MT+ hotspot.
4) Determining Baseline Phosphene Behavior
- Once phosphene threshold has been determined for both visual areas, we need to measure baseline phosphenic behavior. To do this, first remove the subject’s light-blocking mask.
- Next, instruct the subject to stare at a stable fixation cross presented in the center of the computer screen for 60 seconds.
- Replace the mask and, over the V1/V2 hot spot, generate a single pulse train for 3-seconds at 120% V1/V2 threshold.
- Wait five-seconds and conduct another train.
- Again, wait five-seconds and conduct a third train.
- After the third single pulse train, ask the subject to describe the location and motive characteristics of any elicited phosphenes. This will be the baseline.
- Repeat this same procedure over the V5/MT+ hot spot (remember to reset the TMS power to 120% of the V5/MT+ threshold).
5) Condition Number One
- Once the phophenic baseline has been determined, remove the subject’s light-blocking mask once again.
- Instruct the subject to stare at a stable fixation cross presented in the center of the computer screen for 60 seconds. This time, rather than a blank screen, we will embed a series of moving dots at several locations around the cross.
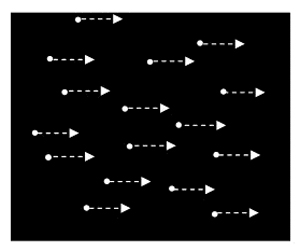
Figure 1. Adapting stimuli for condition one: simple translational motion. All dots move coherently either to the left or right.
Make sure all the dots are moving in the same direction. This stimulus should serve to engender visual adaptation: a phenomenon in which changes in neural excitability as induced by prolonged exposure to a stimuli serves to bias the perception of subsequently presented stimuli. - After 60 seconds, replace the mask and, over the V1/V2 hotspot, generate a single pulse train for 3 seconds at 120% V1/V2 threshold.
- Wait five-seconds and repeat two more times.
- After three trains, ask the subject to report the locations and motive characteristics of any elicited phosphenes. If available, the use of an eye movement tracking system in a dark room can help achieve greater precision and quantitative monitoring of the eye movements made by subjects during phosphene elicitations.
- Continue this three-train/report sequence until phosphene behavior returns to baseline activity.
- Repeat this procedure for the V5/MT+ complex hotspot.
6) Condition Number Two
- In this second condition, repeat the same procedure utilized for condition one. This time, however, rather than presenting the subject with a series of dots moving in a singular direction, present the subject with a series of dots each moving in its relative cardinal direction away from a central point – similar to a “star burst” pattern.
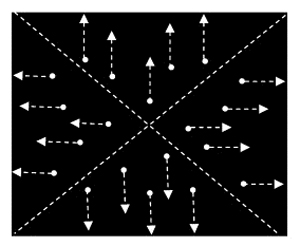
Figure 2. Adapting stimuli for condition two: radial motion. Dots move in respective cardinal directions either towards or away from a central point. - As before, after 60 seconds, replace the mask, conduct a series of three, 120% threshold trains over V1/V2, ask for a subject report, and continue until baseline returns.
- Repeat for V5/MT+.
7) Diagram
- As an option, after each condition (or after all conditions are completed), ask the subject to draw the regions and motive behavior of each series of phopsphenes onto a graph. This is not an integral step but will supply you with another set of interpretable data.
8) Representative Results
Visual adaptation to the uni-directional motive stimuli should elicit an identical phosphenic movement over V1/V2.
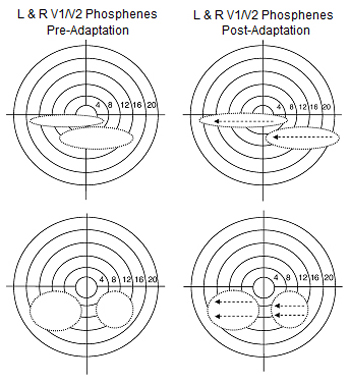
Figure 3. Examples of phosphenes induced from V1/V2 in the right and left hemisphere during condition one (based on the drawings of subjects).
The V5/MT+ complex phosphenes should also be affected, such that the phosphene now will appear as a sum of the motion direction in the adapting stimulus and the baseline phosphene.
However, visual adaptation to the starburst pattern should elicit an identical starburst phosphenic movement from the V5/MT+ complex, but should not change the V1/V2 baseline.
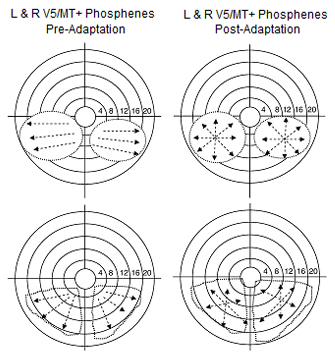
Figure 4. Examples of phosphenes induced from the V5/MT+ complex in the right and left hemisphere during condition two (based on the drawings of subjects).
Discussion
This experiment gets at the heart of state dependency. Neurons in V1/V2 are thought to correspond with simple, directional movement.7,8 Therefore, adaptation to the uni-directional motive stimuli increased the excitability of neurons registering this movement – as such, they should be the first to react to the TMS pulse. The V5/MT+ complex also contains neurons tuned to simple translational motion, therefore the phosphenes induced from this region should also be affected by the adapting stimulus. However, the V5/MT+ complex is also thought to contain distinct neurons which register radial movement.9,10 Because of this, the starburst pattern should change the state of V5 but not V1/V2. By adapting to simple translational or complex radial motion, we can effectively control which neuronal population in the V5/MT+ complex reacts quickest to the TMS pulse.
Knowing that a specific neuronal population can be primed to be more responsive to TMS than surrounding neuronal population holds incredible promise for both the fields of cognitive neuroscience and therapeutic treatment. Exploiting state dependency effects, perhaps an argument could be made for prescribing physical therapy, psychotherapy, or other behavioral “priming” therapies before administering therapeutic TMS trains.
Divulgations
The authors have nothing to disclose.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Light Blocking Eye Mask | ||||
| Ear Plugs | ||||
| Swim Cap | ||||
| Marker | ||||
| Tape Measure | ||||
| Blank Graph Paper | ||||
| Stimuli Developed & Presented on Computer using Adobe Photoshop | ||||
| Any Single Pulse Capable TMS Device | ||||
| Any Figure-of-Eight Coil |
References
- Pascual-Leone, A., Davey, M., Wassermann, E. M., Rothwell, J., Puri, B. . Handbook of Transcranial Magnetic Stimulation. , (2002).
- Walsh, V., Pascual-Leone, A. . Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. , (2005).
- Silvanto, J., Muggleton, N. G., Cowey, A., Walsh, V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. European Journal of Neuroscience. 25, 1874-1881 (2007).
- Silvanto, J., Pascual-Leone, A. State-Dependency of Transcranial Magnetic Stimulation. Brain Topography. 21, 1-10 (2008).
- Silvanto, J., Cattaneo, Z., Battelli, L., Pascual-Leone, A. Baseline cortical excitablility determines whether TMS disrupts or facilitates behavior. Journal of Neurophysiology. 99, 2725-2730 (2008).
- Silvanto, J., Muggleton, N. G. Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations. Neuroimage. 40, 1841-1848 (2008).
- Tootell, R. B., Rappas, J. B., Kwong, K. K., Malach, R., Born, R. T., Brady, T. J., Rosen, B. R., Belliveau, J. W. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience. 15, 3215-3230 (1995).
- Singh, K. D., Smith, A. T., Greenlee, M. W. Spatiotemporal frequency and direction sensitivities of human visual areas measured using fMRI. Neuroimage. 12, 550-564 (2000).
- Rutchmann, R. M., Schrauf, M., Greenlee, M. W. Brain activation during dichoptic presentation of optic flow stimuli. Exp Brain Res. 134, 533-537 (2000).
- Morrone, M. C., Tosetti, M., Montanaro, D., Fiorentini, A., Cioni, G., Burr, D. C. A cortical area that responds specifically to optic flow revealed by fMRI. Nat. Neuroscience. 3, 1322-1328 (2000).

