Cryopreservation of Cortical Tissue Blocks for the Generation of Highly Enriched Neuronal Cultures
Summary
Here, we describe a method for efficient cryopreservation and thawing of cortical brain tissue blocks to generate highly enriched neuronal cultures. This simple protocol provides flexibility for later generation of neuronal, astrocyte, and neuronal precursor cell cultures.
Abstract
In this study, we outline a standardized protocol for the successful cryopreservation and thawing of cortical brain tissue blocks to generate highly enriched neuronal cultures. For this protocol the freezing medium used is 10% dimethyl sulfoxide (DMSO) diluted in Hank’s Buffered Salt Solution (HBSS). Blocks of cortical tissue are transferred to cryovials containing the freezing medium and slowly frozen at -1°C/min in a rate-controlled freezing container. Post-thaw processing and dissociation of frozen tissue blocks consistently produced neuronal-enriched cultures which exhibited rapid neuritic growth during the first 5 days in culture and significant expansion of the neuronal network within 10 days. Immunocytochemical staining with the astrocytic marker glial fibrillary acidic protein (GFAP) and the neuronal marker beta-tubulin class III, revealed high numbers of neurons and astrocytes in the cultures. Generation of neural precursor cell cultures after tissue block dissociation resulted in rapidly expanding neurospheres, which produced large numbers of neurons and astrocytes under differentiating conditions. This simple cryopreservation protocol allows for the rapid, efficient, and inexpensive preservation of cortical brain tissue blocks, which grants increased flexibility for later generation of neuronal, astrocyte, and neuronal precursor cell cultures.
Protocol
1. Cryopreservation of Cortical Tissue Blocks
Material Preparation
- Prepare 1X Hank’s Buffered Salt Solution (HBSS) by diluting at 10X stock solution in sterile water (1:9). Store HBSS at 4oC.
- Prepare 10% DMSO freezing medium by diluting DMSO in HBSS (1:9). The freezing medium should be made fresh before cryopreservation and stored at 4oC until chilled.
- A steel razor blade is used for the systematic chopping of tissue. Before use, the razor blade is sterilized by submersion in 70% ethanol for 2 hr. Immediately before tissue processing, rinse the razor blade with sterile water 3 times. Avoid leaving the razor in sterile water for an excessive amount of time, as it is prone to oxidation.
- A Nalgene freezing container is loaded with cryovials (2.5 mL) and then brought into a sterile biological cabinet. 1 mL of chilled freezing medium is added to each vial. The freezing container is then placed at 4oC for at least 2 hrs.
Cleaning, Chopping, and Freezing
- The brain tissue to be frozen is cleaned of meningeal membrane and blood vessels. Use sterile needle tips to carefully remove debris from the tissue while working on top of an ice pack. Cleaned tissue should be rinsed lightly with cold HBSS and transferred to a new Petri dish for chopping.
- Working on top of an ice pack, use a sterilized razor blade to rapidly chop the tissue into approximately 1 mm3 blocks. Working with small portions of cleaned tissue at a time results in better control over the chopping procedure, resulting in more uniform block sizes.
- Slowly add chopped tissue into 50 mL of HBSS in a 50 mL conical tube. Gently rinse the Petri dish with HBSS in order to collect any remaining tissue blocks. Allow the tissue blocks to descend to the bottom of the tube, creating a loosely packed tissue block pellet.
- While the tissue is settling, bring the previously chilled freezing container and cryovials into the biological safety cabinet. Uncap all cryovials to hasten the process of adding tissue.
- After all the tissue blocks have settled, gently aspirate the excess HBSS, leaving a very thin layer of media above the pellet. Centrifugation is discouraged as it will cause the tissue blocks to adhere to each other, significantly diminishing freezing efficiency.
- Slowly collect 200 μL from the bottom of the loose tissue block pellet by using pipette tip with wide orifices (cut tip with sterile scissors). Transfer the material to one cryovial and move on to the next, again collecting tissue from the bottom of the pellet. It is essential that the entire tissue allocation procedure does not take more than 3-4 min per freezing container. This ensures that the tissue is exposed to DMSO for only a short period of time before freezing. After transferring the tissue to the vials, place the freezing container in a -80oC freezer for at least 4 hrs. Alternatively, the freezing container can be left overnight at -80 oC. Repeat this process for any remaining freezing containers.
- Transfer the cryovials from the freezing container(s) to a cryobox and place in a liquid nitrogen tank for long-term storage.
2. Thawing and Culturing of Frozen Cortical Tissue Blocks
Material Preparation
- One day prior to thawing tissue samples, poly-L-lysine coated Petri dishes/coverslips are prepared. In general, for cortical neurons we prepare coated dishes with 500 μg/ mL or 1mg/ mL. Add enough poly-L-lysine solution (made in borate buffer) to fully cover the bottom of the dish. If glass coverslips are required, ensure that they are completely submerged under the poly-L-lysine solution. Incubate for at least 12 hr. Before use, thoroughly rinse the dishes with a liberal amount of sterile water 3 times, 5 min each.
- Warm HBSS is used to clean thawed tissue as well as to dissociate the tissue into cell suspensions. The volume of HBSS needed will vary depending on the amount of tissue to be thawed, but typically 1 cryovial will dilute in 50 mL of HBSS and will be dissociated in 10 mL of HBSS.
- Dulbecco’s Modified Eagle Medium with 10% iron-supplemented bovine calf serum (EBS) and 1% antibiotic/antimycotic (AA) mixture (DMEM(++)) should be placed in a 37°C water bath.
- Plating medium (NB(+++)) should be made fresh before use. This medium is prepared by adding 1% antibiotic/antimycotic plus B27 and N2 supplements.
Note: Media names appended with crosses (+) indicate the inclusion of additives to the base composition of the media. In this text, DMEM(++) denotes DMEM plus 10% EBS plus 1% AA, while NB(+++) denotes NB plus 1% AA plus B27 plus N2.
Thawing and Culturing
- Remove the cryovials from the liquid nitrogen tank and rapidly thaw at 37°C in a water bath until only a small ice pellet is observed inside the vial content.
- Gently transfer the vial content to 50mL of warm HBSS in a conical tube. Use a wide orifice tip to gently dislodge any remaining tissue blocks stuck in the cryovial. Invert the tube 3-4 times and allow the tissue to slowly collect at the bottom. Tissue should settle quickly, but if the blocks are too small this may not occur and light centrifugation (~200 x g) is recommended.
- Aspirate excess HBSS.
- Add 10 mL of warm HBSS to the tissue pellet and 300 μL of 0.25% trypsin and 50 μL of DNAase. Incubate in a 37°C water bath for 5 min, then place in an orbital shaker set to 80 rpm and 37°C for an additional 5 min.
- After this period, bring the tissue blocks into a biological safety cabinet and use a 10 mL pipette to carefully agitate the tissue blocks until a cloudy cell suspension is formed. Deactivate the trypsin by adding 10 mL of warm DMEM(++) to the cell suspension.
- Centrifuge the dissociated cells at 1200 x g for 5 min. Aspirate and discard the supernatant, resuspend the cell pellet in 10 mL of warm DMEM(++) and quantify cell number using a hemocytometer.
- Plate cells on poly-L-lysine coated dishes. Typically, a 60-mm dish will be plated with 1×106 cells.
- Allow the cells to attach to the dish/coverslips for approximately 1 hr in the tissue culture incubator. It is recommended to monitor the progress of attachment in one plate every 10 min until effective attachment is observed. Then, gently replace the medium with fresh DMEM(++).
- After 24 hr, replace the DMEM(++) with warm NB(+++). Partial medium changes (50%) are carried out every 5 days with fresh NB(+++). Healthy cultures usually show signs of differentiation and growth of processes after 24 hrs. Under these conditions, and performing partial medium changes every 4-5 days, the cultures can be maintained for prolonged periods of time up to 4-6 weeks.
- For the generation of neuronal precursor cells (NPCs), a similar protocol is used with the exception that after centrifugation, DMEM without EBS is used. Cells are plated in T-25 flasks in DMEM/F12 (1:3) supplemented with B27 and EGF (20 ng/ mL) to allow formation of neuropheres. To differentiate NPCs, neurospheres are plated on laminin (10 ug/ mL) coated coverslips in DMEM/F12 (1:3) medium supplemented with N2.
3. Representative Results
Healthy cultures will show significant growth and differentiation after 5 days post-thawing and will usually stabilize in its growth by 10 days (Figure 1). Immuncocytochemical staining of these cultures reveals numerous astrocytes (Figure 2A) and neurons (Figure 2B) for both human and rat primary neuronal cultures. This protocol is also suitable for the generation of NPCs as free-floating neurospheres (Figure 3A), which under differentiating conditions, result in high quality mixed cultures (Figure 3B,C).
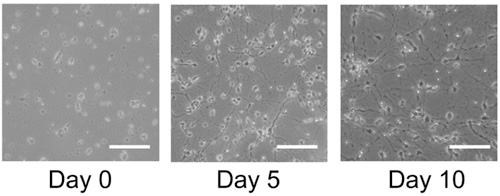
Figure 1. Cell cultures of frozen human cortical tissue. Frozen human cortical tissue blocks are thawed and plated on poly-lysine coated coverslips and grown for 10 days. By day 5 cell expansion is apparent and by day 10 confluency is achieved. Scale bar: 100 μm.
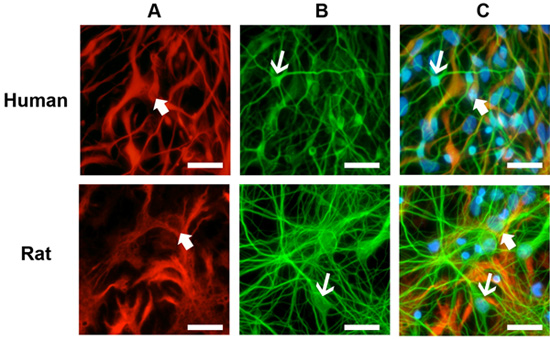
Figure 2. Immunocytochemical staining of cell cultures. (A) Cultures were stained with the glial marker glial fibrillary acidic protein (GFAP, thick arrows). (B) Neurons were detected with the neuronal marker beta-tubulin III (TIII, thin arrows). (C) Both human and rat cultures show abundant glia and neurons after 10 days in culture. Primary antibodies: mouse anti-GFAP (1:1000) and rabbit anti-TIII (1:1000). Secondary antibodies: anti-mouse Alexa 594 (1:500) and anti-rabbit Alexa 488 (1:500). Nuclei staining: Hoestch blue (1:1000). Scale bar: 50 μm.
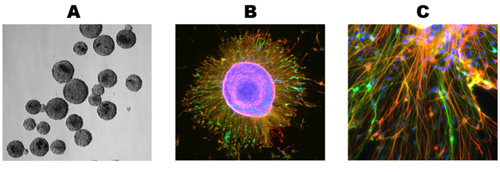
Figure 3. Generation of neurospheres. (A) Frozen tissue was processed as described above and allowed to propagate as neurospheres. (B) Neurospheres were plated on glass coverslips under differentiating conditions and grown for 10 days, resulting in cells migrating away from the neurosphere. (C) Both neurons and astrocytes are present in the expanding fringe of a differentiating neurosphere. Nuclear counterstaining, primary and secondary antibodies used as in Fig 2.
Discussion
Cryopreservation offers an opportunity to bank precious brain tissue samples for future use. Here we describe a simple but effective protocol to generate both neuron-enriched cultures and neuronal precursor cells from frozen brain tissue blocks. This economical procedure avoids the costs of traditional cryopreservation techniques that utilize more expensive rate-controlled freezers. The protocol allows for the generation of viable neuronal cultures post-thawing by providing a rapid yet effective means to freeze tissue blocks. The entire freezing process can take as little as 20 minutes. In addition to primary neuronal cultures, using this method tissue blocks can also be thawed to generate NPCs grown as free floating neurospheres. In this regard, the lack of serum in our freezing medium ensures that cells are preserved in an undifferentiated state. Under differentiating conditions, neurospheres produced from frozen tissue show expansion and differentiation rates very comparable to neurospheres generated from fresh tissue.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by grants from the Undergraduate Research Opportunities Program at UCI (A.R. and S.P.) and grants from the State of California Alzheimer’s Disease Initiative and the National Institutes of Health grant no. HD38466, and Alzheimer’s Disease Research Center grant no. AG16573 (J.B.)
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Dulbecco’s Modified Eagle Medium (DMEM) | Invitrogen | 11995-073 | High gluclose 1X | |
| Bovine calf serum supplemented | HYCLONE | SH30072.03 | For culture medium | |
| Neurobasal-A Medium (1X), liquid | Invitrogen | 1088-022 | ||
| B27 Supplement | Invitrogen | 17504-044 | Supplement for Neurobasal medium | |
| N2 Supplement | Invitrogen | 17502-048 | Supplement for Neurobasal medium | |
| Trypsin (10X) | Cellgro | 25-054CI | Tissue dissociation | |
| Deoxyribonuclease 1 from bovine pancreas (DNase) | Sigma | D4527-30KU | Tissue dissociation | |
| Hanks Balanced Salt Solution (HBSS) (10X) | GIBCO | 14065 | Cleaning tissue, washes, and freezing medium | |
| Poly-L-Lysine- 500mg | Invitrogen | P2636 | Substrate for adhesion of neuronal cells | |
| antibiotic-antimycotic (100X), liquid | Invitrogen | 15240-062 | To prevent contamination | |
| Large orifice pipette Tips (1-200 ul) | Fischer Scientific | 02-681-141 | To prevent shear stress to cells | |
| Graduated pipette tips (101-1000 ul) | USA Scientific | 1111-2721 | ||
| 21 G1 precision guide needles | Beckton Dickinson | 305165 | To clean tissue | |
| 10 ml pipette | USA Scientific | 1071-0810 | Individually wrapped | |
| 50 ml tubes | USA Scientific | 926-9-04 | ||
| Single edge razor blade | Smith Brand | 67-0238 | To chop tissue | |
| 60 x 15 mm polystyrene petri dish | USA Scientific | 8609-0160 | For general culture | |
| 100 x 15 mm polystyrene petri dish | USA Scientific | 8609-0010 | For cleaning tissue | |
| Cryogenic box | NALGENE Labware | 5026-1010 | ||
| Freezing container | NALGENE Labware | 5100-0001 | “Mr. Frosty” | |
| 2.0 ml cryogenic vials | NALGENE Labware | 5012-0020 | ||
| DMSO | Fischer Scientific | D128-500 | For freezing medium | |
| Ethanol 200 proof | Sigma | E7023 | For sterilizing razor blade |
References
- Robbins, R. J. Cryopreservation of human brain tissue. Exp Neurol. 107, 208-213 (1990).
- Ware, C. B., Nelson, A. M., Blau, C. A. Controlled-rate freezing of human ES cells. Biotechniques. 38, 879-880 (2005).
- Paynter, S. J. Principles and practical issues for cryopreservation of nerve cells. Brain Res Bull. 75, 1-14 (2008).
- Thirumala, S., Gimble, J. M., Devireddy, R. V. Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med. 4, 224-232 (2010).

