Protocol
Collagenase Preparation
- Weigh out the collagenase into a 50 ml conical and add isolation buffer to make the final concentration, as indicated in the table below. Dissolve the collagenase by vortexing.
(12 mice + 2 extra mice = 14 mice x 5 ml/mouse = 70 ml total collagenase solution)
(70 ml x 0.5 mg/ml or 0.3 mg/ml = 35 mg or 21 mg of collagenase P total)
Strain Age Collagenase P (mg/ml) Time (min) C3H, Balb/C, B6 > 12 weeks 0.8 17 C3H, Balb/C, B6 <= 12 weeks 0.5 13 NOD, NOR, NON > 12 weeks 0.8 17 NOD, NOR, NON <= 12 weeks 0.5 17 - Dispense 2 ml of collagenase solution into each glass vial and place on ice. Load each 5 ml syringe with 3 ml of collagenase add 30g needle and place in ice (prepare in hood).
Distension
- Just before dissection, sacrifice animal by cervical dislocation. Spray the whole body with 70% of ETOH, making it completely wet. Make a V-insision starting at the genital area. Rotate the mouse so the tail is facing away from you.
- Remove the bowel to the left side of the open mouse. This will expose the pancreas and common bile duct.
- Place a hemostat clamp on either side of the small-intestinal, where the bile duct drains, leaving a small pocket for collagenase solution to enter the intestine.
- Inflate the pancreas through the bile duct with a 30g needle and 5 ml syringe containing 3 ml of cold collagenase solution, starting at the gall bladder.
- Remove the pancreas from the body and place it in a siliconized vial containing 2 ml of collagenase solution. This step should be done QUICKLY and as cleanly as possible, minimizing collection of fat and connective tissue.
- Place the vial on ice and repeat step 3 with the next mouse.
Digestion
- Seal each vial and place it into a 37°C water-bath. Incubate for 13-17 minutes (time varies with strain and age of the animal).
- After time has elapsed, shake the vial vigorously. The pancreas should fall apart.
- Pour each digest through a large sterile sink strainer into a sterile 1000 ml beaker and forcefully pipette off the screen with washing buffer. Dispense the digests into 50 ml conicals tubes (3 pancreata/50 ml tube: if n=12, use enough washing buffer to make the final volume 200 ml and dispense it to 4×50 ml tubes). The dilution should be done rapidly and on ice to avoid unwanted islet digestion.
- Spin down: Start the centrifuge. When it reaches 1300 rpm, turn it off.
- Aspirate the supernatant, leaving ~5 mL. Be very cautious not to aspirate the pellet.
- Resuspend pellet by tapping vigorously with your hand, then add 50 ml of wash buffer.
- Spin down: Start the centrifuge. When it reaches 1300 rpm, turn it off.
- Resuspend the pellet and wash with 5 ml of washing buffer.
- Transfer the 5 ml to 15 ml conical and spin down, as above.
- Aspirate the supernatant as completely as possible. (Remaining buffer might cause the change of the density of the Ficoll.)
Ficoll
Perform quickly. Long-term Ficoll exposure is TOXIC to islets.
- Make sure pellet is completely broken up prior to adding ficoll (unbroken tissue is difficult to resuspend in ficoll).
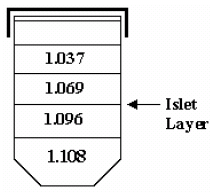
- Resuspend the pellet in 7 ml of ficoll, density 1.108, by vortexing vigorously.
- Layer on top of each density of ficoll 2ml of each of the remaining densities in this order: 1.096, 1.069, then 1.037.
- Spin for 15 minutes at 1800 rpm at 4 degrees, with break OFF!
- Pick islets from the second layer using spoid (sterile plastic eyedropper). Transfer all collections to a 50 ml conical containing ~25 mL of cold buffer.
- Wash, as above, 3 times with washing buffer (repeating step 12-15).
- Resuspend Islets in 20 ml RPMI-1640 (containing 10% FCS and penicillin and streptomycin, HEPES, MEM-NEAA) and mix gently. Remove 100 ul of sample for counting.
- Transfer 100 ul to 35 mm Grid-plate containing 1 ml of media and 1 ml dithizone.
- Incubate remaining islets in RPMI 1640, in a 37°C, 7% CO2 incubator, in 160 mm plates with a total of 30 ml of media/plate.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Dithizone | reagent | Dissolve 5mg Dithizone in 5 ml of DMSO in a 50 ml conical tube. Add 45 ml of normal saline and mix gently. Filter with a 60 cc syringe and a 0.22 “m syringe filter into a new 50 ml tube. Store at 4 OC, until use. Add 1 ml to each 35 mm counting Grid-plate, when needed. | ||
| PBS | Buffer | |||
| HBSS | Buffer | |||
| Isolation soln. | Buffer | HBSS containing 10 mM of HEPES, 1 mM of MgCl2, 5 mM of Glucose, pH 7.4 | ||
| Wash soln. | Buffer | Isolation buffer containing 1mM CaCl2 | ||
| Collagenase P | Boehringer Mannheim-Roche | 1213873 | ||
| Dissection tools | Hemostat clamp (n=2), Forceps (n=2), Scissors (for skin incision an for taking out pancreas from adjacent tissue) | |||
| Syringe | sterile, 5ml, 1/mouse. | |||
| 30 G needles | sterile, 1/syringe | |||
| siliconized glass vials w/Teflon cap | Fisher | 213-018-54 | sterile, 1/mouse | |
| large sink strainer | sterile | |||
| 1000 ml Beaker | sterile | |||
| Gauze pads | 4×4, 2/mouse | |||
| plastic eye dropper | sterile | |||
| Mice | Animal |
Citer Cet Article
Szot, G. L., Koudria, P., Bluestone, J. A. Murine Pancreatic Islet Isolation. J. Vis. Exp. (7), e255, doi:10.3791/255 (2007).

