Measuring Bacterial Load and Immune Responses in Mice Infected with Listeria monocytogenes
Summary
Listeria monocytogenes is a model organism for studying immune responses and genetic susceptibility to intracellular bacteria in mice. This method enables one to measure bacterial load and generate single-cell suspensions of the liver and spleen from mice for FACS analysis to determine changes in immune cells due to Listeria infection.
Abstract
Listeria monocytogenes (Listeria) is a Gram-positive facultative intracellular pathogen1. Mouse studies typically employ intravenous injection of Listeria, which results in systemic infection2. After injection, Listeria quickly disseminates to the spleen and liver due to uptake by CD8α+ dendritic cells and Kupffer cells3,4. Once phagocytosed, various bacterial proteins enable Listeria to escape the phagosome, survive within the cytosol, and infect neighboring cells5. During the first three days of infection, different innate immune cells (e.g. monocytes, neutrophils, NK cells, dendritic cells) mediate bactericidal mechanisms that minimize Listeria proliferation. CD8+ T cells are subsequently recruited and responsible for the eventual clearance of Listeria from the host, typically within 10 days of infection6.
Successful clearance of Listeria from infected mice depends on the appropriate onset of host immune responses6 . There is a broad range of sensitivities amongst inbred mouse strains7,8. Generally, mice with increased susceptibility to Listeria infection are less able to control bacterial proliferation, demonstrating increased bacterial load and/or delayed clearance compared to resistant mice. Genetic studies, including linkage analyses and knockout mouse strains, have identified various genes for which sequence variation affects host responses to Listeria infection6,8-14. Determination and comparison of infection kinetics between different mouse strains is therefore an important method for identifying host genetic factors that contribute to immune responses against Listeria. Comparison of host responses to different Listeria strains is also an effective way to identify bacterial virulence factors that may serve as potential targets for antibiotic therapy or vaccine design.
We describe here a straightforward method for measuring bacterial load (colony forming units [CFU] per tissue) and preparing single-cell suspensions of the liver and spleen for FACS analysis of immune responses in Listeria-infected mice. This method is particularly useful for initial characterization of Listeria infection in novel mouse strains, as well as comparison of immune responses between different mouse strains infected with Listeria. We use the Listeria monocytogenes EGD strain15 that, when cultured on blood agar, exhibits a characteristic halo zone around each colony due to β-hemolysis1 (Figure 1). Bacterial load and immune responses can be determined at any time-point after infection by culturing tissue homogenate on blood agar plates and preparing tissue cell suspensions for FACS analysis using the protocols described below. We would note that individuals who are immunocompromised or pregnant should not handle Listeria, and the relevant institutional biosafety committee and animal facility management should be consulted before work commences.
Protocol
1. Culturing and long-term storage of Listeria monocytogenes (Listeria)
- Make or purchase pre-made horse blood agar (HBA) plates. Dry plates prior to use by pre-incubating at 37°C overnight or placing uncovered in a laminar flow cabinet for 1 hour.
- Obtain viable Listeria from one of the following: an infected mouse tissue homogenate, a lyophilized stock, a frozen glycerol stock, or a colony from a recent Listeria culture (i.e. less than one-week old and not sub-cultured more than 10 generations). All Listeria should be obtained using a sterile inoculating loop and employing aseptic techniques for all methods described in this protocol.
- Streak Listeria on a HBA plate as shown in Figure 1 using a sterile inoculating loop: streak Listeria over ¼ of the plate surface as the primary inoculum spread. Make a series of unidirectional streaks from the primary spread. Repeat streaking 2-3 times using a fresh or re-sterilized loop before each set of streaks. Incubate plates at 37°C overnight.
- Store Listeria HBA plate at 4°C for a maximum period of 1 month or go to step 1.5 to prepare Listeria culture for long-term storage.
- Pick a single colony from a fresh Listeria culture on a HBA plate using a sterilized inoculating loop and inoculate 10 mL of brain heart infusion (BHI) broth (prepare according to manufacturer’s instructions) in a 30 mL McCartney bottle.
- Incubate the Listeria culture in an orbital shaker at 180rpm at 37°C overnight.
- Add sterile 80% glycerol (v/v) to liquid Listeria culture at 1:1 ratio to obtain a final glycerol concentration of 40% (v/v). Transfer 0.5-1 mL aliquots into cryovials and store Listeria/glycerol stocks at -70°C.
Tips & notes:
- Growth of Listeria can be supported on different non-selective media, such as a range of blood agar (supplemented with horse, sheep, guinea pig or human blood), brain heart infusion (BHI) agar or broth, or tryptic soy broth with yeast extract (TSB-YE). Contaminating growth can be minimized in culture by adding antibiotics to the media for Listeria strains with defined antibiotic resistance (e.g. L. monocytogenes 10403S), or by using Oxford media, which selectively support the growth of Listeria.
- If Listeria is obtained from an untested source, it is recommended that additional tests be performed to confirm the identity of Listeria (L. monocytogenes is Gram positive, non-spore forming, motile at <30°C, facultatively anaerobic, catalase positive, and oxidase negative). The obtained culture can also be grown on Listeria selective media to ensure that a pure culture is obtained.
- Drying the HBA plates ensures that there is optimal separation of bacterial colonies on the plate.
- When making frozen glycerol stocks of Listeria, use fresh HBA Listeria cultures that are less than 1 week old and sub-cultured for as few generations as possible (<5 is best).
- When deriving fresh Listeria cultures from a frozen glycerol stock, the first passage should be made on solid medium (i.e. HBA plates) because Listeria obtained from frozen glycerol stocks grow poorly when directly subcultured in liquid medium.
2. Preparation and storage of Listeria infectious stock for in vivo infection studies
- Streak Listeria from a frozen glycerol stock (Step 1.7) onto a HBA plate as described in step 1.3. Incubate plates at 37°C overnight for use on the next day.
- Pick a single Listeria colony from a streak culture and inoculate a 10 mL BHI broth. Avoid transferring agar into the broth.
- Incubate the Listeria BHI culture in an orbital shaker at 180 rpm and 37°C to mid-logarithmic phase (OD600 = ~0.4). This typically takes 3-4 hours.
- Take a 0.9 mL aliquot aseptically and obtain an OD reading at 600 nm at 3-4 hours of shaking. If OD reading is <0.3, then continue to culture in an orbital shaker at 180 rpm and 37°C until OD reading is ~0.4.
- Add 1 mL sterile 80% glycerol (v/v) to 10 mL Listeria BHI broth culture (OD600 ~0.4). Mix gently and transfer into 1.5 mL microfuge tubes in 0.5-1 mL aliquots.
- Place the aliquots on ice for 10 min and store at -70°C. Leave one aliquot unfrozen to determine the concentration of Listeria in the infectious stock, which is typically in the order of 109 CFU/mL.
- Perform 1:10 serial dilutions of the unfrozen Listeria infectious stock in PBS to 10-8 dilution. Spread 100 μL of undiluted and each subsequent dilution onto pre-dried HBA plates (step 1.1) in duplicate using a glass spreader. Incubate plates at 37°C overnight.
- Count the number of colonies on each plate and determine the Listeria concentration of the frozen infectious stock culture using the following calculation: concentration (CFU/mL) = number of colonies x dilution factor / 0.1 mL. The plates chosen for counting should ideally have 30-300 colonies to ensure a reliable estimate of the CFU concentration of viable bacteria.
- A few days after storing Listeria infectious stock at -70°C, thaw out one tube. Determine the concentration of viable Listeria as described in steps 2.7 and 2.8. This concentration should be similar to that determined for the freshly prepared infectious stock in step 2.8.
Tips & notes:
- Listeria infectious stock is frozen at a lower percentage glycerol (8%) than for Listeria frozen for general long-term storage (40%).
- Frozen Listeria infectious stocks are typically stable at -70°C for up to 3 months. If using beyond 3 months, it is recommended that a new infectious stock be prepared from a fresh Listeria culture on a HBA plate.
- If the concentration of the thawed Listeria infectious stock (step 2.9) is different from the freshly prepared stock as described in steps 2.7 and 2.8 (i.e. >20% decline in CFU), then a new frozen infectious stock should be prepared.
- Although initially more time consuming than preparing a fresh bacterial inoculum for a single experiment, preparing frozen Listeria infectious stocks enables accurate determination of the CFU concentration prior to infection and better consistency between experiments when mice are infected from the same frozen stock.
3. Preparation of Listeria inoculum and intravenous injection of mice
- Determine the concentration of Listeria to be injected per mouse. For intravenous injection, CFU in 200 μL inoculum per mouse is used.
- Thaw out a frozen aliquot of Listeria infectious stock (from Step 2.6). Dilute the infectious stock in PBS to the desired Listeria CFU concentration. Double the volume needed for injecting all mice to ensure that there is enough inoculum for injection and determination of the Listeria CFU pre- and post-injection (step 3.3 and 3.7).
- Remove 2x 0.1 mL aliquots from the prepared Listeria inoculum, and prepare a 1:10 dilution in PBS for each aliquot before injection of mice. Plate 0.1 mL of each dilution on HBA plates, and incubate the plates overnight at 37°C. Count the number of colonies and determine the pre-injection concentration of the Listeria inoculum: concentration (CFU/mL) = (number of colonies x dilution factor) / mL plated for that dilution factor.
- Transfer the mouse to be injected into a clean cage. Warm the mouse by placing the cage ~50 cm under a 250 watt infrared lamp for 5 min (other suitable thermal devices that will not burn the mouse or increase the temperature of the cage above 40°C can also be used). Place the mouse in a restraining device for tail vein injections.
- Gently mix the Listeria suspension to maintain a consistent suspension each time a syringe is loaded.
- Locate the lateral tail vein of the mouse, gently wipe with a 70% ethanol wipe and inject 200 μL of the Listeria inoculum (at desired concentration) using a syringe with a 27 gauge needle. Briefly apply pressure to entry wound with a sterile gauze. Return the mouse to its cage.
- When injection of all mice is completed, repeat step 3.3 using the remaining amount of inoculum to determine the post-injection concentration of the Listeria inoculum.
- The amount of Listeria injected into mice is reported as the average CFU determined from the pre- and post-injection Listeria inoculum samples.
Tips & notes:
- We typically inject 500 – 3,000 CFU (200 μL of 2,500 CFU/mL – 15,000 CFU/mL inoculum) when comparing bacterial load and immune responses between a resistant mouse strain (e.g. C57BL/6) and a susceptible mouse strain (e.g. BALB/c).
- If the infectious stock will be diluted in PBS by at least 105, then a washing step is not generally required to remove residual media/glycerol. If the infectious stock will not be diluted in PBS by at least 105, then a washing step should be added in step 3.2 to remove residual media/glycerol as follows: Add 9 mL PBS to thawed infectious stock and bacteria collected by centrifugation at 2100 x g for 10 min at 4°C. Remove supernatant and resuspend the pelleted bacterial cells in 10 mL sterile PBS, repeat centrifugation, and resuspend the bacterial cell pellet in the desired volume. Determine the average CFU from the pre- and post-injection Listeria inoculum samples as described in Step 3.3 and 3.7. It is noted that this washing step may result in some loss of bacteria, and it is recommended that such loss be determined beforehand and accounted for in making the inoculum of required concentration.
- Safely discard syringe and needle after injecting mouse with Listeria.
4. Removal of liver and spleen from Listeria-infected mice for analysis
- Euthanase the infected mouse at the desired time point post-infection using a method approved by the local Animal Ethics Committee, such as CO2 asphyxiation.
- Perform cardiac bleed immediately after euthanasia using a 1 mL syringe and 25 gauge needle. Collect as much blood as possible. Remove gall bladder and sever the inferior vena cava.
- Expose the hepatic portal vein by carefully pushing intestines to the right, flip the liver lobes toward the head and to the left so the liver is sitting where the diaphragm is located. The hepatic portal vein feeds into the bottom of the liver from the lower right hand side.
- Perfuse the liver by injecting 10 mL PBS into the hepatic portal vein using a 26 gauge needle. PBS injected through the portal vein will exit from the severed inferior vena cava. Liver perfusion ensures that harvested cells are from the liver and not the circulating blood.
- Remove the perfused liver from the mouse’s body cavity, place in FACS buffer, and store on ice. Go to step 5.1 for the preparation of the liver for FACS analysis.
- Remove the spleen from the mouse, place in FACS buffer, and store on ice.
- Weigh the whole spleen, cut in half, and weigh one of the halves. Place one half in FACS buffer and go to step 6.1. Place the other half in a Stomacher bag on ice and go to step 7.1.
Tips & notes:
- In steps 4.5 and 4.6, use sufficient FACS buffer to completely submerge the tissue to minimize exposure of any part of the tissue to air.
- A ½ inch length 26 gauge needle that is bent by ~30 degrees can better facilitate injection of PBS into the hepatic portal vein.
- When perfused properly, the liver turns from a dark red color to light brown after injecting 1-2 mL PBS.
- If the liver is not required for FACS analysis, perfusion is not necessary. The liver can be isolated and collected directly into a Stomacher bag and processed as described in step 7.
- For euthanasia of mice, CO2 asphyxiation is the preferred method because it has minimal impact on bacterial CFU and immune cell viability in the spleen and liver. If blood collection is not required, cervical dislocation may be used as alternative euthanasia method. It is recommended that euthanasia methods that may affect organ weight or immune cell viability not be used (eg thiobarbiturates).
5. Preparation of hepatic cell suspension for FACS analysis
- Generate single-cell suspensions by placing liver with FACS buffer (step 4.5) in a 70 μm cell strainer that is inside a 60 mm Petri dish. Push the tissue through the cell strainer using the plunger of a 5 mL syringe until a single-cell suspension is formed.
- Transfer the hepatic single-cell suspension from the Petri dish to a 50 mL conical screw-cap tube and bring the total volume to 40 mL with cold FACS buffer.
- Vortex cell suspension and take a 1 mL aliquot to determine bacterial load for the liver. Go to step 7.3 and use this 1 mL aliquot in place of tissue homogenate in Stomacher bag.
- Pellet cells by centrifugation at 500 x g for 5 min at 4°C, discard supernatant, resuspend pellet in 40 mL cold FACS buffer, pellet the cells by centrifugation at 500 x g for 5min at 4°C, and discard supernatant.
- Resuspend the cell pellet in 20 mL isotonic Percoll at room temperature. Centrifuge cell suspension at 700 x g for 12 min at room temperature. Discard supernatant and disc-like sheet of cells floating on top (i.e. hepatocytes). Resuspend the leukocyte-containing pellet in 4 mL TAC buffer to lyse erythrocytes and incubate cells at room temperature for up to 10 min with frequent inversion.
- Filter cell suspension through a 100 μm nylon membrane into a new 10 mL centrifuge tube.
- Underlay filtered cell suspension with 1 mL FCS/EDTA buffer. Centrifuge at 350 x g for 5 min at 4°C, discard supernatant, and resuspend in 5 mL FACS/EDTA buffer.
- Centrifuge cell suspension at 350 x g for 5 min at 4°C, discard supernatant, and resuspend cell pellet in 0.5-3 mL FACS/EDTA buffer depending on the size of the pellet.
- Dilute cell suspension 1:5-1:20 in trypan blue depending on suspension volume in step 5.8. Count viable hepatic leukocytes using hemacytometer (Figure 4A) and determine total viable cells as follows: viable cells/organ = number of cells in the counting area x dilution factor x 104 x volume (mL). Liver single-cell suspensions can then be labeled with antibodies specific for desired cell-surface markers and analyzed by FACS.
Tips & notes:
- Keep cells on ice unless otherwise specified.
6. Preparation of splenic single-cell suspension for FACS analysis
- Generate single-cell suspensions by placing one half of the spleen (step 4.7) with FACS buffer in a 70 μm cell strainer that is inside a 60 mm Petri dish. Gently push the tissue through the cell strainer using the plunger of a 5 mL syringe until a single-cell suspension is formed.
- Transfer the spleen single-cell suspension from the Petri dish to a 10 mL centrifuge tube and bring the total volume to 10 mL using cold FACS buffer.
- Pellet cells by centrifugation at 350 x g for 5 min at 4°C, discard supernatant, resuspend cell pellet in 4 mL TAC buffer to lyse erythrocytes, and incubate cells at room temperature for up to 10 min with frequent inversion.
- Filter cell suspension through a 100 μm nylon membrane into a new 10 mL centrifuge tube.
- Underlay filtered cell suspension with 1 mL FCS/EDTA buffer. Centrifuge at 350 x g for 5 min at 4 °C, discard supernatant, and resuspend cell pellet in 5 mL FACS/EDTA buffer.
- Centrifuge cell suspension at 350 x g for 5 min at 4°C, discard supernatant, and resuspend cell pellet in 3-8 mL FACS/EDTA buffer depending on the size of the pellet.
- Dilute cell suspension 1:10 – 1:20 in trypan blue (0.4% in distilled water) depending on suspension volume in step 7.6. Count viable splenocytes using hemacytometer – see Step 5.9 for calculating total viable cells per organ Spleen single-cell suspensions can then be labeled with antibodies specific for desired cell-surface markers and analyzed by FACS.
Tips & notes:
- Keep cells on ice unless otherwise specified.
7. Measurement of bacterial load in tissues from Listeria-infected mice
- Fold the top of the Stomacher bag containing the spleen half. While holding the Stomacher bag shut to prevent leakage, lay it flat on a clean bench or in a laminar flow cabinet. Roll a thick round pen over the tissue until the tissue is mashed within the bag.
- Add 5 ml of PBS to each Stomacher bag containing mashed tissue. Place the Stomacher bag into the Stomacher, and run the Stomacher on high speed for 10 minutes.
- Mix the homogenate in the Stomacher bag (or the 1 mL aliquot of hepatic cell suspension) with a pipette. Perform the following in duplicates. Transfer 300 μL to a 96-well flat bottom plate. Within the wells of the 96-well plate perform 1:10 serial dilutions (30 μL into 270 μL PBS) to a 10-5 dilution for each tissue homogenate sample.
- Carefully spread 0.1 mL of each dilution onto a pre-dried HBA plate using a glass spreader. Incubate plates at 37°C overnight.
- Count the number of CFU for each dilution and calculate the CFU per tissue taking into account the portion of the tissue (e.g. half a spleen), dilution factor, and the total volume of tissue homogenate (5 mL or 40 mL): CFU/tissue = CFU/0.1 mL x dilution factor x mL/tissue; for spleen divide this number by the percentage weight of the spleen sample used for tissue homogenization (see step 4.7).
Tips and notes:
- More than one Stomacher bag can be placed in the Stomacher at a time as long as total volume does not exceed maximum volume as specified by the manufacturer
- If a Stomacher is not available, a tissue homogenizer can be used instead. Alternatively, the following method, although not as efficient, can also be used to manually macerate the organ in place of steps 7.1 and 7.2: place organ in a sealed sterilized plastic bag and lay on a clean bench while holding the bag shut to prevent leakage. Roll a 500 mL laboratory glass bottle repetitively over the bag until the tissue is thoroughly mashed. Add 5 mL of PBS to each bag and proceed to step 7.3.
- It is very important in step 7.4 that the HBA plates be dried thoroughly (i.e. no moisture on the agar). Otherwise it may be difficult to obtain distinct Listeria colonies that can be counted properly.
- If there are limited HBA plates, an alternative step 7.4 is: Draw lines on the bottom of a pre-dried HBA plate (Step 1.1) to divide it into five to six sections, one for each dilution. Carefully pipette 25 μL of each dilution into corresponding sections on the HBA plate for each tissue homogenate – do not spread with a spreader. Wait until drops of tissue homogenate are dry before inverting the plate. Incubate plate at 37°C overnight. Up to 80 colonies can be distinguished due to one drop on an agar plate.
- Dilution of tissue homogenates may depend on the Listeria growth in the animal and can be estimated based on the symptoms displayed by the animal at the time of euthanasia. For mice that are noticeably moribund, then additional dilutions may be necessary to more accurately determine the bacterial load. For mice inoculated with low doses of Listeria or mice euthanased at the end of the infection period, undiluted tissue homogenate may be used to determine the bacterial load.
- Plates can be incubated at 37°C overnight or at room temperature for 2-3 days.
8. Representative Results:
For a standard infection experiment, Listeria was obtained from a frozen glycerol stock and streaked on a HBA plate as shown in Figure 1. If few colonies are obtained after streaking, this may indicate poor streaking or a less than optimal frozen stock. A Listeria infectious stock was prepared from a freshly streaked HBA plate and stored at -70°C. For measuring bacterial clearance and immune responses, we routinely dilute the thawed Listeria infectious stock to 2,500 CFU/mL – 15,000 CFU/mL and inject mice with 200 μL to infect them with 500 – 3,000 CFU. We observe that mice infected with sub-lethal doses of Listeria using this protocol may transiently exhibit ruffled fur, hunched posture and weight loss within the first few days. These symptoms provide a visual cue as to how severely an individual mouse is affected by the infection, whether it responds differently to the rest of the group (i.e. an “obvious” outlier), and whether euthanasia is necessary to prevent excessive suffering and impending death due to the infection.
In the results represented by Figures 2-5, mice were infected using a freshly thawed aliquot of Listeria infectious stock. At different time points post-infection, mice were euthanased and liver and spleen were removed. Figure 2 shows a HBA plate on which diluted spleen homogenate from a single mouse was cultured. There should be a clear reduction in the number of colonies as the dilution factor becomes higher, such that counting distinct CFU is possible for at least two dilutions. If the mouse has cleared the Listeria infection (i.e. detection limit = 100 CFU/tissue), then <5 Listeria colonies will be present on the HBA plate that was spread with 200 μL of undiluted tissue homogenate. If the liver and/or spleen become contaminated with intestinal or other external bacteria during isolation, the colonies on the HBA plate are likely to exhibit different morphology (i.e. won’t have characteristic halo and pale color of Listeria colonies) and/or may be different in colony counts to what is expected for Listeria (i.e. too few or too many). Figure 3 shows a typical Listeria clearance curve for C57BL/6 mice – note that Listeria are typically cleared more quickly from the spleen than the liver and that clearance does not happen until five days after infection.
Figure 4 shows cell counts based on single-cell suspensions prepared from spleen and liver of infected mice at different time points after infection. This method can achieve >95% leukocyte purity in single-cell suspensions prepared from liver and >80% from spleen. Leukocyte purity can be determined by FACS analysis using a monoclonal antibody specific for the pan-leukocyte marker CD45 (clone 30-F11). Typically, the number of splenocytes and hepatic leukocytes increase during the period it takes to clear the infection with the liver exhibiting a greater fold increase in hepatic leukocytes, but a substantially smaller total, compared to the increase of splenocytes in the spleen. The type and number of the immune cells can be determined by labeling the single-cell suspensions with antibodies specific for cell-surface markers. Labeled cells can then be detected by FACS analysis. Figure 5 provides representative results for CD8+ T cells. During a standard Listeria infection in C57BL/6 mice, the number of CD8+ T cells transiently decreases due to lymphopenia in the spleen at days 2-3 before increasing noticeably from day 5 post-infection in both the spleen and liver.
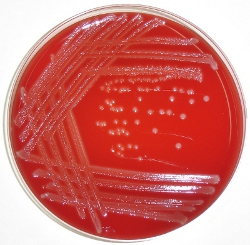
Figure 1. HBA plate streaked with Listeria. A sterile inoculating loop was used to streak Listeria from a frozen glycerol stock. The plate was incubated at 37°C overnight. The characteristic halo surrounding individual colonies is due to β-hemolysis.
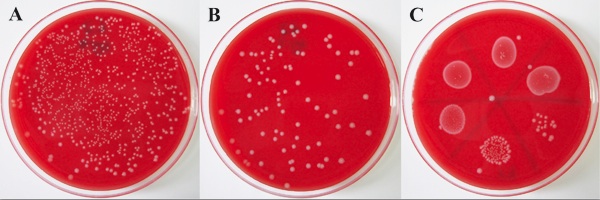
Figure 2. Tissue homogenate from a Listeria-infected mouse cultured on HBA plates. A liver was harvested from a Listeria-infected C57BL/6 mouse at 3 days post-infection. Tissue homogenate was prepared and dilutions placed as 100 μl full plate spread for 10-4 dilution (A) and 10-5 dilution (B), as well as 25 μl drops on a HBA plate for each 1:10 dilution from undiluted to 10-5 (C). The plates were incubated at 37°C overnight. The dilutions that enable counting of individual colonies are used to determine the bacterial load (i.e. CFU/tissue) at that time point post-infection.
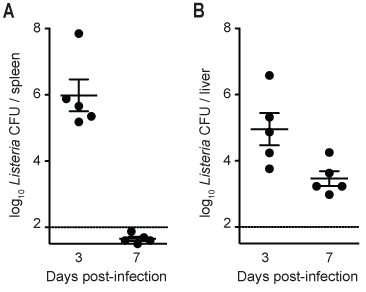
Figure 3. Listeria load in spleen and liver of infected C57BL/6 mice at 3 and 7 days post-infection. C57BL/6 mice were infected with ~2,000 CFU of Listeria. At each time point, mice were euthanased, the liver was perfused and harvested with the spleen, and dilutions of splenic homogenate (A) or hepatic single-cell suspension (B) were cultured on HBA plates to determine the bacterial load. Solid lines indicate geometric mean, and vertical bars indicate SEM. The dotted line indicates that the detection limit for accurate measurement of bacterial load is 100 CFU/organ for this experiment.
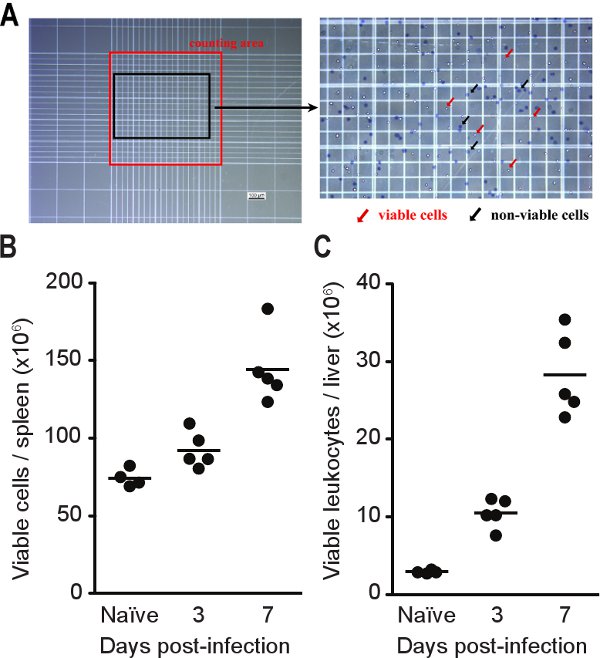
Figure 4. Viable cell counts for tissues from infected mice. C57BL/6 mice were infected with ~2,000 CFU of Listeria. At each time point, mice were euthanased, the liver perfused and harvested with the spleen. Cells were stained with trypan blue and counted using a hemacytometer as described in Step 5.9 (A). Splenocyte (B) and hepatic leukocyte (C) counts obtained from single-cell suspensions prepared from Listeria-infected mice. Lines indicate geometric mean.
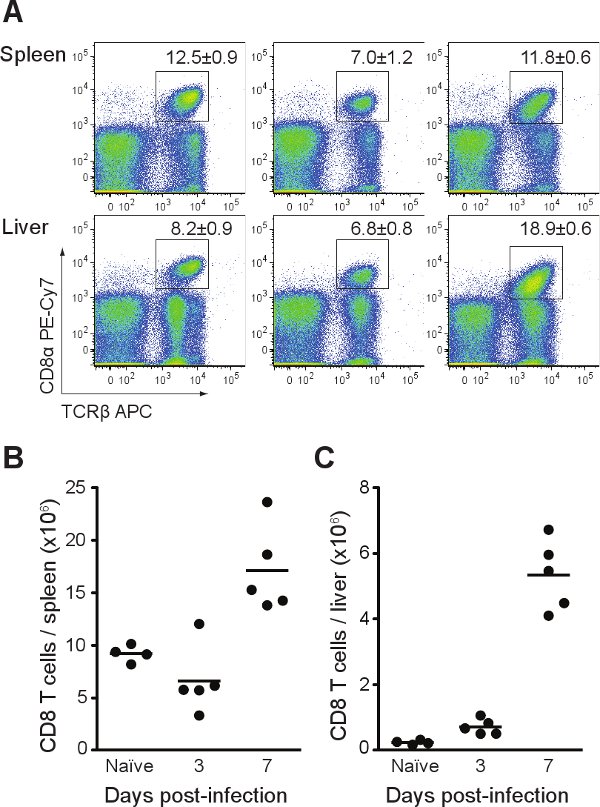
Figure 5. FACS analysis of CD8+ T cells in Listeria-infected mice. C57BL/6 mice were infected with ~2,000 CFU of Listeria. At each time point, mice were euthanased, the liver perfused and harvested with the spleen. Single-cell suspensions were stained with antibodies specific for T cells (CD3, TCRβ, CD4, CD8). (A) Representative FACS profiles with % CD8+ T cells +/- SEM. (B) Total CD8+ T cells in spleen. (C) Total CD8+ T cells in liver.
Discussion
Listeria is one of the most widely used organisms to characterize host immune responses to intracellular bacteria6. The protocol presented here enables one to measure bacterial load and immune cell responses within the same tissue of a given mouse. This dual measurement of a particular tissue for each infected mouse provides for more robust comparisons within and between mouse cohorts (either representing different mouse strains or time points post-infection). While Listeria infection by oral administration could also be used to study immune responses in mice, infection by intravenous injection is often used because: 1) it ensures rapid and effective delivery to the bloodstream; 2) it results in a synchronized and consistent systemic infection; and 3) the Mus species harbors a mutation in the gene encoding the E-cadherin receptor, which limits Listeria infection by oral administration (this mutation affects Listeria‘s ability to bind the mouse E-cadherin receptor and to efficiently cross the epithelial lining of the gastrointestinal tract)16-18.
There are a few critical steps in this protocol. First, the Listeria inoculum stock should be generated from a fresh overnight HBA culture to ensure viability and virulence. Second, it is important to accurately determine the CFU concentration of the inoculum before and after injecting all mice to ensure that the CFU concentration does not differ greatly between the first and last mouse injected. Third, injection of Listeria into the tail vein must be consistent for all mice. Lastly, it is necessary to perfuse the liver to deplete non-resident leukocytes to ensure accurate measurement of immune cells within the liver and not leukocytes passing through in the peripheral blood. All of these steps, if not performed successfully, can result in unwanted variability for bacterial load and/or immune responses between individual mice infected with Listeria.
Two limitations of this protocol are the investigator’s skill for infecting mice by intravenous injection and the detection of bacterial load in tissues. If one person is injecting a large number of mice, Listeria viability (i.e. CFU concentration) may reduce over time once the frozen inoculum is thawed (e.g. >2 hours between injecting first and last mouse). It is up to the investigator to determine how many mice she/he can inject before the inoculum is compromised. Another limitation of this method is that the Listeria load cannot be accurately measured below 100CFU/organ due to the relatively small amounts of cultured tissue homogenate (e.g. Figure 3 indicates that 100CFU/organ is the detection limit). To more accurately measure lower values for CFU/organ, a larger amount of the tissue homogenate can be cultured (up to 0.5mL per plate, multiple plates can be used) so a greater proportion of the tissue is sampled for detection of Listeria. If a Stomacher is not available, then alternative methods to homogenate the tissue, such as using a tissue homogenizer, can be used instead for steps 7.1-7.2. On a practical note, if space in the 37°C incubator is limited for culturing tissue samples from infected mice, then HBA culture plates can be incubated at room temperature for 2-3 days (the colonies will grow slower at room temperature). However, room temperature should not be used when growing cultures for preparation of frozen Listeria stocks.
This protocol provides a basic approach for characterizing Listeria infection in mice and can be used with other Listeria strains besides EGD. In addition to the liver and spleen, single-cell suspensions from lymph nodes can also be generated. In either instance, these single-cell suspensions can be used for a variety of analyses, including measuring immune cell subsets, and in vitro stimulation of sorted immune cells. Once the basic techniques are mastered this protocol can also be modified to isolate Listeria-specific T cells19, characterize dendritic cells20, or perform immune cell depletion at certain time-points after infection21,22 to more thoroughly characterize the immune response in mice infected with Listeria.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Anna Walduck, Christina Cheers, Stuart Berzins, Dale Godfrey, Yifan Zhan and Jonathan Wilksch for advice and reagents. This work was funded by the Juvenile Diabetes Research Foundation (1-2008-602) and the Australian National Health and Medical Research Council (575552). NW is supported by an Australian Postgraduate Award. OW is supported by a R.D. Wright Fellowship from the Australian National Health Medical Research Council.
Materials
| Name of the reagent | Company | Catalogue number | Comments |
|---|---|---|---|
| Horse blood agar base No.2 | Oxoid | CM0271 | Preparation of agar base for HBA plates |
| Horse blood (defibrinated) | Oxoid | HB1000 | Add 5-10% to agar base |
| Brain heart infusion (BHI) broth (10 mL) | Oxoid | TM456 | |
| Brain heart infusion dehydrated media | Oxoid | CM1135 | |
| 96-well flat bottom plate | BD Biosciences | 353072 | |
| 70 μm cell strainer | BD Biosciences | 352350 | |
| Small petri dish | BD Biosciences | 351007 | |
| Stomacher 80 biomaster lab system | Seward | ||
| Plastic Stomacher bags | Sarstedt | 86 9924 530 | |
| Bovine serum albumin | Sigma | A3912 | |
| FACS buffer | 0.1% (w/v) bovine serum albumin in PBS | ||
| Isotonic Percoll (33.75% Percoll in PBS) | GE Healthcare | 17-0891-01 | 33.75mL Percoll, 3.75mL 10x PBS, 62.5mL 1x PBS makes 100mL isotonic Percoll |
| TAC Buffer | 17mM Tris, 140mM ammonium chloride in distilled water | ||
| FCS/EDTA buffer | Fetal calf serum with 10mM EDTA | ||
| FACS/EDTA buffer | FACS buffer + 5mM EDTA | ||
| Trypan blue | Sigma | T6146 | 0.4% (w/v) in PBS, filter sterilized |
| 2mL Cryovial | Greiner Bio One | 121263 | |
| 27 gauge/1mL insulin syringe | Terumo Medical Products | SS10M2713 | |
| Needle | Terumo Medical Products | NN-2516R (25G 5/8in) NN-2613R (26G 1/2in) |
|
| Syringe | Terumo Medical Products | SS-01T (1mL), SS-053 (5mL), SS-10S (10mL) |
References
- Wing, E. J., Gregory, S. H. Listeria monocytogenes: clinical and experimental update. J Infect Dis. 185, S18-S24 (2002).
- Conlan, J. W. Early host-pathogen interactions in the liver and spleen during systemic murine listeriosis: an overview. Immunobiology. 201, 178-187 (1999).
- Neuenhahn, M., Busch, D. H. Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens. Immunol Lett. 114, 66-72 (2007).
- Cousens, L. P., Wing, E. J. Innate defenses in the liver during Listeria infection. Immunol Rev. 174, 150-159 (2000).
- Hamon, M., Bierne, H., Cossart, P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 4, 423-434 (2006).
- Pamer, E. G. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 4, 812-823 (2004).
- Cheers, C., McKenzie, I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 19, 755-762 (1978).
- Garifulin, O., Boyartchuk, V. Listeria monocytogenes as a probe of immune function. Brief Funct Genomic Proteomic. 4, 258-269 (2005).
- Gervais, F., Stevenson, M., Skamene, E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 132, 2078-2083 (1984).
- Gervais, F., Desforges, C., Skamene, E. The C5-sufficient A/J congenic mouse strain. Inflammatory response and resistance to Listeria monocytogenes. J Immunol. 142, 2057-2060 (1989).
- Boyartchuk, V. L. Multigenic control of Listeria monocytogenes susceptibility in mice. Nat Genet. 27, 259-260 (2001).
- Boyartchuk, V. The host resistance locus sst1 controls innate immunity to Listeria monocytogenes infection in immunodeficient mice. J Immunol. 173, 5112-5120 (2004).
- Pan, H. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 434, 767-772 (2005).
- Garifulin, O. Irf3 polymorphism alters induction of interferon beta in response to Listeria monocytogenes infection. PLoS Genet. 3, 1587-1597 (2007).
- Hof, H. Virulence of different strains of Listeria monocytogenes serovar 1/2a. Med Microbiol Immunol (Berl. 173, 207-218 (1984).
- Wollert, T. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 129, 891-902 (2007).
- Lecuit, M. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science. 292, 1722-1725 (2001).
- Marco, A. J. Penetration of Listeria monocytogenes in mice infected by the oral route. Microb Pathog. 23, 255-263 (1997).
- Busch, D. H., Vijh, S., Pamer, E. G. Animal model for infection with Listeria monocytogenes. Curr Protoc Immunol. Chapter. 19, 19-19 (2001).
- Neuenhahn, M., Schiemann, M., Busch, D. H. DCs in mouse models of intracellular bacterial infection. Methods Mol Biol. 595, 319-329 (2010).
- Conlan, J. W., North, R. J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 179, 259-268 (1994).
- Czuprynski, C. J., Brown, J. F., Wagner, R. D., Steinberg, H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 62, 5161-5163 (1994).

