Summary
Human pluripotent stem cells (hPSCs) have the potential to treat a myriad of different diseases. The utility of these cells lies in the fact that they can differentiate into any cell type in the body. Here we describe the teratoma assay, which is used to demonstrate the pluripotence of hPSCs.
Abstract
Pluripotent stem cells (PSCs) have the unique characteristic that they can differentiate into cells from all three germ layers. This makes them a potentially valuable tool for the treatment of many different diseases. With the advent of induced pluripotent stem cells (iPSCs) and continuing research with human embryonic stem cells (hESCs) there is a need for assays that can demonstrate that a particular cell line is pluripotent. Germline transmission has been the gold standard for demonstrating the pluripotence of mouse embryonic stem cell (mESC) lines1,2,3. Using this assay, researchers can show that a mESC line can make all cell types in the embryo including germ cells4. With the generation of human ESC lines5,6, the appropriate assay to prove pluripotence of these cells was unclear since human ESCs cannot be tested for germline transmission. As a surrogate, the teratoma assay is currently used to demonstrate the pluripotency of human pluripotent stem cells (hPSCs)7,8,9. Though this assay has recently come under scrutiny and new technologies are being actively explored, the teratoma assay is the current gold standard7. In this assay, the cells in question are injected into an immune compromised mouse. If the cells are pluripotent, a teratoma will eventually develop and sections of the tumor will show tissues from all 3 germ layers10. In the teratoma assay, hPSCs can be injected into different areas of the mouse. The most common injection sites include the testis capsule, the kidney capsule, the liver; or into the leg either subcutaneously or intramuscularly11. Here we describe a robust protocol for the generation of teratomas from hPSCs using the testis capsule as the site for tumor growth.
Protocol
Note: All animal procedures must be approved by IACUC or equivalent.
All surgical equipment must be sterilized prior to surgery. Sterile gloves, drapes and gauze must be used.
1. Preparation prior to surgery
- Obtain 6 week old Mus Musculus CbySmn.CB17-Prkdc SCID/J male mice or other immunocompromised strain of mouse.
- Sterilize all surgical instruments, gloves, and gauze.
- Dissociate hPSCs to be injected with accutase.
- Count cells and resuspend 1,000,000 cells per 20-30 μL in matrigel diluted 1:1 in DMEM/F-12. Keep cells on ice until finished.
- Note that often it is useful to do a run through of the experiment where Trypan Blue is injected. This will help to identify any potential problems before any cells are wasted.
- In the vivarium, anesthetize your mouse according to accepted procedures at your institution. In our experiments, we used an anesthesia machine with isoflurane. Mice were initially put in an induction chamber with 1L/min oxygen and 3-4% isoflurane. Once anesthetized, a nose cone with 1L/min oxygen and 2-3% isoflurane was used.
- Shave the abdomen with clippers, and clean the anterior wall of the mouse abdomen, starting from the center of the abdomen and working clockwise outward. First use Povidone-Iodine solution then wash with 70% ethanol. Repeat 3 times, changing swabs each time. Transfer the animal to a heating pad to keep the animal warm inside a tissue culture or dissecting hood.
- Make a 1 cm longitudinal incision through the skin and peritoneum with sterile surgical scissors just below the level of the hip joint.
- While holding the peritoneum with forceps, reach down toward the right hip with another sterile forcept and pull out the white fatty tissue along with attached testes.
- Place the testes on sterile gauze.
- Fill a tuberculin or Hamilton (1cc) syringe with the hPSCs to be injected. Note that it is a good idea to include a control hPSC line that you know is pluripotent such as WA09 (also known as H9). This way you have a positive control which you can use to determine whether your injection or surgery was flawed.
- Slowly inject the hPSCs (20-30 μL) into the center of the testis capsule away from any major blood vessels, stopping if the testicular capsule begins to swell.
- Remove the needle slowly to avoid reflux of the cells.
- Using forceps, transfer the testes and fatty tissue back to its original position in the abdomen.
- Close up the peritoneum with 2 or 3 reabsorbable sutures and close the skin with autoclips.
- The mouse should be kept warm until it recovers and given some form of analgesic (see what is accepted in your vivarium) after surgery twice a day for 1-2 days.
Note that neither the anesthetic nor the analgesic with interfere with tumor development. - Monitor the animal for tumor growth for 6-12 weeks. Very rarely, tumors may grow to 5 mm in size prior to 6 weeks after injection, thus it is important to monitor the animals. If this happens, animals should be euthanized and the tumors processed and analyzed as usual.
- Once the tumor is palpable and reaches approximately 5mm in size, anesthetize the mouse and sacrifice it according to accepted animal protocol procedures.
- Remove tumor and document accordingly – photograph, measure size, weigh.
- Cut tumor into small pieces and fix in 4% paraformaldehyde solution. Store in 4% paraformaldehyde until samples are sent to a pathologist for sectioning, staining and analysis.
2. Representative Results:
When this protocol is done as described and the injected cell line is pluripotent, a palpable, visually obvious, tumor should form within 12 weeks at the most. For established hPSC lines like WA09, we typically see tumors within 6 weeks. For iPSC lines it is not unusual to see tumors within 8-10 weeks. It is very important to inject mice with a line that is known to be pluripotent, as a positive control, in order to be sure that the procedure was performed correctly. Tumors usually look very heterogeneous and have many attached cysts (Figure 1). Analysis of the tumor samples by a pathologist should show differentiated tissues from all three germ layers (Figure 2).
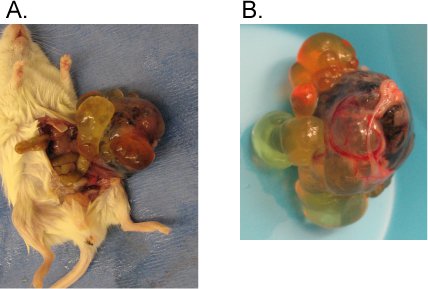
Figure 1. Typical hPSC derived teratoma in the testis capsule.
Following the described protocol, one million WA09 cells were injected into the testis capsule of an immune compromised mouse. Six weeks later a teratoma was observed. A) Teratoma pulled from the mouse. B) Close up picture of the teratoma. Note the heterogeneity and cyst structures.
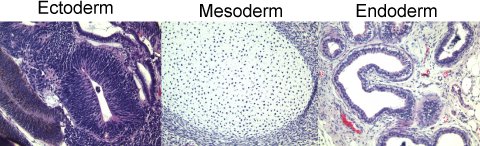
Figure 2. Hematoxylin and Eosin stained sections from the teratoma show tissues from each germ layer.
Following fixation, the teratoma was sectioned and stained with Hematoxylin and Eosin. Analysis by a pathologist revealed the presence of cells from each of the 3 germ layers.
Discussion
The method presented here provides a highly reliable, straightforward means of generating teratomas from hPSCs in the testis capsule. There are several critical parameters in this technique. In particular, it is important to inject hPSC cell lines that are known to be pluripotent as a control. Other important parameters include the time interval between injection and tumor observation. For cell lines like WA09, teratomas should be observed in 6-8 weeks. For new iPSC lines we find that often 10 weeks are required. Another concern is the number of cells injected. We inject one million cells but the assay can easily be done with fewer cells. In addition, the injection medium is important. We find that we get the best results when the cells are injected in a 1:1 mixture of matrigel and DMEM/F-12, as opposed to PBS or DMEM/F-12.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by CIRM grants #TR-1250, RT1-01108, and CL1-00502.
Materials
| Name of the reagent | Company | Catalogue number |
| Accutase | Invitrogen | A1110501 |
| DMEM/F-12 | Invitrogen | 113300-032 |
| Matrigel | BD Biosciences | 354277 |
References
- Bradley, A., Evans, M., Kaufman, M. H., Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 309, 255-255 (1984).
- Evans, M. J., Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 292, 154 (1981).
- Martin, G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U. S. A. 78, 7634-7634 (1981).
- Downing, G. J., Battey, J. F. Technical assessment of the first 20 years of research using mouse embryonic stem cell lines. Stem Cells. 22, 1168-1168 (2004).
- Thomson, J. A. Embryonic stem cell lines derived from human blastocysts. Science. 282, 1145-1145 (1998).
- Reubinoff, B. E. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 18, (2000).
- Muller, F. J., Goldmann, J., Loser, P., Loring, J. F. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 6, 412-412 (2010).
- Brivanlou, A. H. Stem cells. Setting standards for human embryonic stem cells. Science. 300, 913-913 (2003).
- Lensch, M. W., Schlaeger, T. M., Zon, L. I., Daley, G. Q. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 1, 253-25 (2007).
- Gertow, K. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem. Cells. Dev. 13, 421-421 (2004).
- Gertow, K. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol. Chapter 1, Unit1B 4-Unit1B 4 (2007).

