RNA Isolation of Pseudomonas aeruginosa Colonizing the Murine Gastrointestinal Tract
Summary
A reliable method for the RNA isolation of Pseudomonas aeruginosa recovered from murine cecums is described. The RNA recovered is of sufficient quantity and quality for subsequent qPCR, transcription profiling, and RNA Seq experiments. This technique can be adapted for RNA isolation of other intestinal microbes.
Abstract
Pseudomonas aeruginosa (PA) infections result in significant morbidity and mortality in hosts with compromised immune systems, such as patients with leukemia, severe burn wounds, or organ transplants1. In patients at high-risk for developing PA bloodstream infections, the gastrointestinal (GI) tract is the main reservoir for colonization2, but the mechanisms by which PA transitions from an asymptomatic colonizing microbe to an invasive, and often deadly, pathogen are unclear. Previously, we performed in vivo transcription profiling experiments by recovering PA mRNA from bacterial cells residing in the cecums of colonized mice 3 in order to identify changes in bacterial gene expression during alterations to the host’s immune status.
As with any transcription profiling experiment, the rate-limiting step is in the isolation of sufficient amounts of high quality mRNA. Given the abundance of enzymes, debris, food residues, and particulate matter in the GI tract, the challenge of RNA isolation is daunting. Here, we present a method for reliable and reproducible isolation of bacterial RNA recovered from the murine GI tract. This method utilizes a well-established murine model of PA GI colonization and neutropenia-induced dissemination4. Once GI colonization with PA is confirmed, mice are euthanized and cecal contents are recovered and flash frozen. RNA is then extracted using a combination of mechanical disruption, boiling, phenol/chloroform extractions, DNase treatment, and affinity chromatography. Quantity and purity are confirmed by spectrophotometry (Nanodrop Technologies) and bioanalyzer (Agilent Technologies) (Fig 1). This method of GI microbial RNA isolation can easily be adapted to other bacteria and fungi as well.
Protocol
1. Murine Model of P. aeruginosa GI Colonization and Dissemination
- C3H/HeN mice (6-8 wks old, female, Harlan) are treated with oral antibiotics to deplete commensal flora and then mono-colonized with PA as previously described4.
2. Harvesting Murine Cecal Luminal Contents
- Immerse a cleaned stainless steel mortar into a liquid nitrogen bath.
- Euthanize mice by carbon dioxide asphyxiation.
- Secure mouse carcass on a styrofoam board and shower with 95% ethanol. Make a midline longitudinal incision through the skin from the sternum to the perineum. Reflect the skin and peritoneum to expose the abdominal cavity.
- Resect the entire cecum. Hold the cecum with forceps over the stainless steel mortar. Snip both ends of the cecum with dissection scissors.
- Insert a P1000 pipette tip filled with 1 ml of cecal flushate buffer (10 mM TrisHCl, 1 mM EDTA and 200 mM NaCl)5 into the proximal end of the cecum. Flush the cecal flushate buffer and cecal luminal contents into the stainless steel mortar. 1 ml of cecal flushate buffer will be sufficient for flushing all of the cecal contents. Cecal flushate contents will freeze immediately upon coming into contact with the mortar.
- Grind the cecal flushate contents with a sterile pestle.
- Place ground frozen cecal luminal contents into a 50 ml polypropylene conical tube submerged in a dry ice/ethanol bath.
- Repeat Steps 2.2 through 2.7 for each additional mouse.
- Store at -80°C.
3. Bacterial RNA Isolation
- Warm 1 volume (based on final volume of two cecal luminal contents, approximately 3 ml) of acid phenol/chloroform (5:1, v/v) contained in a 50 ml Oak Ridge centrifuge tube with the cap secured in parafilm in a 65°C water bath.
- Boil 0.5 volume of lysis buffer (2% SDS, 16 mM EDTA and 200 mM NaCl)6 contained in a 50 ml polypropylene conical tube for 5 minutes.
- Add boiling lysis buffer to cecal luminal contents. Homogenize. Boil for 5 minutes with periodic vortexing.
- Add the 100°C cecal contents/lysis sample to the 65°C acid phenol/chloroform in the Oak Ridge centrifuge tube. Seal cap with parafilm.
- Incubate at 65°C for 10 minutes with periodic vortexing (every 2 minutes).
- Centrifuge sample at 2,500 g at 4° for 15 minutes.
- Carefully transfer aqueous phase (avoiding any of the white interface) to a fresh 50 ml Oak Ridge centrifuge. The volume of the aqueous phase will be approximately 50% of the total volume of cecal contents, lysis buffer, and acid phenol/chloroform. Add an equal volume of acid phenol/chloroform.
- Seal cap with parafilm and mix well by vortexing at high speed.
- Centrifuge sample at 2,500 g at 4° for 15 minutes.
- Repeat Steps 3.7 to 3.9 until there is no visible white interface between the aqueous and organic phases.
- Carefully transfer aqueous phase to a fresh 50 ml Oak Ridge centrifuge. Add an equal volume of chloroform/isoamyl alcohol (24:1, v/v).
- Seal cap and mix well by vortexing.
- Centrifuge sample at 2,500 g at 4° for 15 minutes. Remove aqueous phase (volume will be approximately 50% of the total reaction volume) to 15 ml polypropylene conical tube and add an equal volume of isopropanol.
- Incubate at -20° for at least 2 hours or overnight.
- Centrifuge sample at 2,500 g at 4° for 45 minutes. A gel-like pellet will form at the bottom of the tube. Remove supernatant.
- Wash the pellet with 1 ml of ice-cold 70% ethanol. Vortex. Centrifuge sample at at 10,000 g at 4° for 5 minutes.
- Remove the supernatant and invert the tube to let the pellet dry at room temperature for 10 minutes.
- Resuspend the RNA pellet in 200 μl of RNase-free water.
4. DNase Treatment (Turbo DNA-Free, Applied Biosystems)
- Add 20 μl of 10X Turbo DNase buffer and 2 μl Turbo DNase and mix gently.
- Incubate at 37° for 20 minutes.
- Add 20 μl of resuspended DNase inactivation reagent and mix well.
- Incubate 5 minutes at room temperature, mixing occasionally.
- Centrifuge at 10,000 g at room temperature for 1.5 minutes and transfer the supernatant to a new microfuge tube.
- Add one volume of cold (4°) acid phenol/chloroform and mix thoroughly by vortexing for 1 minute.
- Centrifuge at 12,500 g at room temperature for 2 minutes.
- Transfer the aqueous phase to a new tube and add 1 volume of chloroform/isoamyl alcohol (49:1 v/v), vortex.
- Centrifuge at 12,500 g at room temperature for 2 minutes.
- Transfer the aqueous phase (approximately 50% of the total reaction volume) to a new tube.
- Add 0.1 volume of 3M sodium acetate, pH 5.5 and 2.5 volumes of ice-cold 100% ethanol. Vortex.
- Incubate at -20° for at least 2 hours or at -80° for 30 minutes.
- Centrifuge at 12,000 g for 30 minutes at 4°
- Remove supernatant.
- Wash the pellet with 1 ml of ice-cold 70% ethanol by centrifuging for 5 minutes at 10,000 g at 4°.
- Remove the supernatant and air dry for 10 minutes.
- Resuspend the pellet in 100 μl of RNase-free water. May need to place in 65° water bath to resuspend and dissolve the pellet.
- To test the efficacy of the DNase treatment, a standard RT-PCR reaction for the amplification of a ribosomal protein (or other reference gene) could be performed for treated and non-treated samples, using no RT controls.
5. RNA Cleanup Step (Qiagen, RNeasy Kit)
- Refer to pages 56-57 of Qiagen RNeasy Mini Handbook (4th Edition, April 2006). Follow as indicated.
6. Representative Results
The amount of bacterial total RNA recovered by using this protocol is approximately 2-3 μg from two cecums. The RNA recovered is of sufficient quantity and quality for subsequent qPCR, transcription profiling, and RNA Seq experiments. RNA purity is routinely assessed by measuring the 260nm/280nm ratio7 of the sample, yet this method provides no information about RNA integrity. The Agilent Bioanalyzer is a microfluidics-based platform for sizing, quantification and quality control of DNA, RNA, proteins and cells; and utilizes an RNA integrity metric known as RNA Integrity Number (RIN)8. RNA extracted with this protocol produces 260/280 ratios ranging from 1.7 to 2.0 and RIN values ≥ 7.0. An example of an Agilent Bioanalyzer analysis of the bacterial RNA recovered by this protocol is shown in Figure 1.
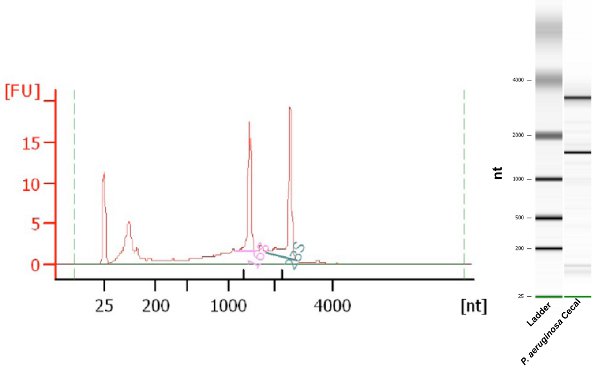
Figure 1. Agilent Bioanalyzer electropherogram and gel-like image of Pseudomonas aeruginosa total RNA sample isolated and recovered from murine cecal contents. RIN 8.0.
Discussion
The RNA extraction method described here allows for recovery of sufficient quantities of high-quality Pseudomonas aeruginosa total RNA harvested from the murine GI tract. This method is not restricted to P. aeruginosa and can potentially be applied to other bacteria. The recovery of sufficient microbial organisms from the intestine will vary significantly from organism to organism. In our murine model, P. aeruginosa typically colonizes the murine GI tract at levels between 5 x 107 to 5 x 108 cfu/g feces4. Since the recovered cecal contents are roughly 0.5 gram, the estimated number of P. aeruginosa recovered from two cecums is between 5 x 107 to 5 x 108 cfu. If other microorganisms are used, it would be prudent to verify GI colonization levels and then calculate the number of cecums needed to recover the targeted number of cfu. It is also important to note that when using this particular murine model, antibiotic-treated mice not infected with PA have no quantifiable amounts of RNA isolated from their cecal contents.
Our murine model of Pseudomonas aeruginosa gastrointestinal colonization and dissemination attempts to emulate the pathogenesis of P. aeruginosa bacteremia in cancer and stem cell transplant patients. In this patient population, commensal flora is often depleted secondary to antibiotic or chemotherapeutic treatment (e.g the antibiotic depletion of GI commensal flora) resulting in overgrowth of pathogenic microbes (e.g. mono-association with P. aeruginosa) and then subsequent dissemination after immune suppression. The advantages and limitations of this murine model have already been addressed previously4. The purpose of this current study is to provide a methodology for isolating microbial total RNA from the GI tract. This protocol can easily be adapted to other murine models that study other aspects of microbial pathogenesis in the GI tract (i.e. commensal flora interactions, bacterial effects on inflammatory bowel disease, etc.).
One advantage of this method is the incorporation of multiple lysis steps including freeze/thaw, mechanical disruption (pulverizing with mortar/pestle, homogenization), boiling, and chemical lysis (e.g. SDS). Despite the multitude of lysis steps, some microorganisms (notably Gram-positive bacteria and fungi/yeast) may require additional mechanical disruption. After the hot lysis/acid phenol-chloroform incubation (Step 3.5), the addition of beads (0.1 mm for bacteria and/or 0.5-0.7 mm for yeast) and a subsequent bead-beating step should be sufficient to lyse these organisms9, 10.
Given the complex nature of materials recovered from the murine cecum, the repeated cold acid-phenol/chloroform extractions (Step 3.7 to 3.9) are absolutely required in order to achieve acceptable RNA quality and integrity for further downstream reactions. Between 3-5 cold acid-phenol/chloroform extractions may be required before the white interface between the aqueous and organic phases is eliminated. Finally, the combination of both the DNase treatment (Step 4) and RNeasy Cleanup Protocol (Step 5) are essential for removing contaminating DNA and small non-mRNA (5s and tRNA). As stated previously, the RNA recovered by utilizing this protocol is of sufficient quantity and quality for subsequent transcription profiling 3, qPCR (unpublished), and RNA Seq (unpublished) experiments.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by the National Institutes of Health grants AI62983 (AYK), AI22535 (GPB).
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| Mortar and Pestle | Fisher | 12-947-1 |
| Homogenizer | Omni | TM-125 |
| Oak Ridge centrifuge tubes (50 ml) | Nalgene | 3119-0050 |
| Acid Phenol/Chloroform | Ambion | AM9720 |
| Chloroform | Sigma-Aldrich | C2432 |
| Isoamyl Alcohol | Sigma-Aldrich | W205702 |
| Isopropanol | Sigma-Aldrich | 190764 |
| Diethyl pyrocarbonate (DEPC) | Sigma-Aldrich | 40718 |
| DNase | Ambion | AM2238 |
| RNeasy Kit | Qiagen | 74104 |
| 3M Sodium Acetate | Ambion | AM9740 |
| 100% Ethanol | Sigma-Aldrich | E7023 |
References
- Bodey, G. P., Bolivar, R., Fainstein, V., Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 5, 279-279 (1983).
- Bertrand, X. Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 27, 1263-1268 (2001).
- Koh, A. Y. Utility of in vivo transcription profiling for identifying Pseudomonas aeruginosa genes needed for gastrointestinal colonization and dissemination. PLoS One. 5, e15131-e15131 (2010).
- Koh, A. Y., Priebe, G. P., Pier, G. B. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect Immun. 73, 2262-2272 (2005).
- Alexander, R. J., Raicht, R. F. Purification of total RNA from human stool samples. Dig Dis Sci. 43, 2652-2658 (1998).
- Fitzsimons, N. A., Akkermans, A. D., de Vos, W. M., Vaughan, E. E. Bacterial gene expression detected in human faeces by reverse transcription-PCR. J Microbiol Methods. 55, 133-140 (2003).
- Manchester, K. L. Use of UV methods for measurement of protein and nucleic acid concentrations. Biotechniques. 20, 968-970 (1996).
- Schroeder, A. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 7, 3-3 (2006).
- Turnbaugh, P. J. A core gut microbiome in obese and lean twins. Nature. 457, 480-484 (2009).

