Patch-clamp Capacitance Measurements and Ca2+ Imaging at Single Nerve Terminals in Retinal Slices
Summary
Here we describe a protocol for the preparation of agar-embedded retinal slices that are suitable for electrophysiology and Ca2+ imaging. This method allows one to study ribbon-type synapses in retinal microcircuits using direct patch-clamp recordings of single presynaptic nerve terminals.
Abstract
Visual stimuli are detected and conveyed over a wide dynamic range of light intensities and frequency changes by specialized neurons in the vertebrate retina. Two classes of retinal neurons, photoreceptors and bipolar cells, accomplish this by using ribbon-type active zones, which enable sustained and high-throughput neurotransmitter release over long time periods. ON-type mixed bipolar cell (Mb) terminals in the goldfish retina, which depolarize to light stimuli and receive mixed rod and cone photoreceptor input, are suitable for the study of ribbon-type synapses both due to their large size (~10-12 μm diameter) and to their numerous lateral and reciprocal synaptic connections with amacrine cell dendrites. Direct access to Mb bipolar cell terminals in goldfish retinal slices with the patch-clamp technique allows the measurement of presynaptic Ca2+ currents, membrane capacitance changes, and reciprocal synaptic feedback inhibition mediated by GABAA and GABAC receptors expressed on the terminals. Presynaptic membrane capacitance measurements of exocytosis allow one to study the short-term plasticity of excitatory neurotransmitter release 14,15. In addition, short-term and long-term plasticity of inhibitory neurotransmitter release from amacrine cells can also be investigated by recordings of reciprocal feedback inhibition arriving at the Mb terminal 21. Over short periods of time (e.g. ~10 s), GABAergic reciprocal feedback inhibition from amacrine cells undergoes paired-pulse depression via GABA vesicle pool depletion 11. The synaptic dynamics of retinal microcircuits in the inner plexiform layer of the retina can thus be directly studied.
The brain-slice technique was introduced more than 40 years ago but is still very useful for the investigation of the electrical properties of neurons, both at the single cell soma, single dendrite or axon, and microcircuit synaptic level 19. Tissues that are too small to be glued directly onto the slicing chamber are often first embedded in agar (or placed onto a filter paper) and then sliced 20, 23, 18, 9. In this video, we employ the pre-embedding agar technique using goldfish retina. Some of the giant bipolar cell terminals in our slices of goldfish retina are axotomized (axon-cut) during the slicing procedure. This allows us to isolate single presynaptic nerve terminal inputs, because recording from axotomized terminals excludes the signals from the soma-dendritic compartment. Alternatively, one can also record from intact Mb bipolar cells, by recording from terminals attached to axons that have not been cut during the slicing procedure. Overall, use of this experimental protocol will aid in studies of retinal synaptic physiology, microcircuit functional analysis, and synaptic transmission at ribbon synapses.
Protocol
1. External and internal Solutions
- Prepare slicing solution (low calcium) from 10x stock solution and add MgCl2, CaCl2, and D-glucose daily. The final 1x solution consists of (in mM): 119 NaCl, 2.5 KCl, 3.2 MgCl2, 0.25 CaCl2, 12 D-glucose, 0.2 L-ascorbic acid, 12 HEPES. Set the pH to 7.4 (with NaOH), and adjust osmolarity to 260 mOsm (using H2O and 10x stock solution).
- Weigh out 3% low gelling-temperature agar (Agarose type VII-A, A0701, Sigma; Rieke, 2001) and mix with slicing solution. Heat the agar containing solution in a microwave for 1-2 min, or until it dissolves completely, and incubate the agar in a water-bath (30-33°C) to prevent the rapid solidification (gel transition temperature: 26 ± 2°C).
- Prepare recording solution (normal calcium) from 10x stock solution and add MgCl2, CaCl2, and D-glucose daily. The final 1x solution consists of (in mM): 100 NaCl, 2.5 KCl, 1 MgCl2, 25 NaHCO3, 0.2 L-ascorbic acid, 2.5 CaCl2, 12 D-glucose. After bubbling the solution in 95% O2 / 5% CO2 (carbogen) for 5-10 minutes, set the pH to 7.4 (with NaOH), and adjust osmolarity to 260 mOsm (using H2O and 10x stock solution).
- Aliquots (1 ml each) of internal pipette solutions are prepared in advance and stored in a freezer (-20°C). Two internal pipette solutions are typically used to isolate bipolar cell calcium currents (in mM):
- 40 CsCl, 60 Cs-gluconate, 10 tetraethylammonium (TEA)-Cl, 28 HEPES, 3 Mg-ATP, 1 Na-GTP, and 2 EGTA. Adjust pH to 7.2 (with CsOH) and osmolarity set to 250 mOsm. This high chloride internal solution typically provides lower series resistance recordings (better voltage clamp conditions) and larger inhibitory postsynaptic currents (IPSCs) at a bipolar cell resting membrane potential of -60 mV.
- 95 Cs-gluconate, 10 TEA-Cl, 25 HEPES, 3 Mg-ATP, 0.5 Na-GTP, and 0.5 EGTA. Adjust pH to 7.2 (with CsOH), and set osmolarity to 250 mOsm 22. This low EGTA internal solution typically leads to higher membrane capacitance changes elicited by depolarizing step pulses and thus allows studies of vesicle pool depletion and short-term depression.
2. Patch-clamp pipette electrodes
- Prepare thick-walled (1.5 mm outer diameter) borosilicate glass (1B150F-4; World Precision Instruments, Sarasota, FL) and pull the patch pipettes using a vertical puller (Narishige, PP830; Tokyo, Japan). Open-tip patch pipette resistances in recording solutions are 7-8 MΩ when the pipette is filled with internal pipette solution #1.
- Coat the patch-pipette evenly, from the tip to the level of the shaft that reaches the pipette holder, with dental wax (Cavex, West Chester, PA). This will minimize the pipette capacitance and electrical noise and enable more accurate and low-noise capacitance measurements. A low pipette capacitance also helps the electronic C-fast (fast capacitance) compensation of the EPC-9 (or EPC-10) patch clamp amplifier.
3. Preparation of agar-embedded retinal slices
- Select a goldfish (Carassius auratus; 8-16 cm) and place in a covered bucket in a dark room for 30 min of dark adaptation. After anesthesia, euthanize the sedated goldfish by quick decapitation and double-pithing of the spinal cord and brain stem. Then remove the eyes with curved-tip scissors and curved-tip tweezers. These procedures have been approved by the Institutional Animal Care and Use Committee (IACUC) at the Oregon Health & Science University.
- Hemisect each eye by cutting evenly around the front of the eye with spring scissors (15003-08, Fine Science Tools; initiate cut by puncturing with scissor tips), and place the eyecups in chilled slice solution (low calcium). If necessary, remove the lens with tweezers (the lens will usually detach with the front of the eye). Remove the retina with pigment epithelium attached by using a pair of 45° angled tip fine forceps (11251-35, Fine Science Tools) to peel the retina away from the eyecup, progressing slowly around the full 360°. Sever or cut the optic nerve with either fine forceps or spring scissors. Remove the retina gently with a combination of angled fine tip forceps and suction applied from a modified Pasteur pipette or transfer pipette (large tip). Use angled fine tip forceps to remove the remainder of the pigment epithelium attached to the retina. The surface of the cleaned and healthy retina should appear dark, smooth and red colored. For complete removal of all dark pigment epithelium cells dark-adapt the retina for 1 hour (8, 1992).
- Treat isolated retina with ~0.03% (wt/vol; ~0.36 mg/mL in 1x slice solution) hyaluronidase (3; H6254, Sigma) for 15-30 min at room temperature (20-23°C) to remove vitreous humor. During this incubation, you can prepare ice-cold slice solution.
- Cut a rectangular piece of retina (~2×2 mm, containing the full thickness of the retina) by removing the curved edges with a small segment of razor blade (Personna, double-edged, cleaned with 70% ethanol and H2O) attached to the body of a 1 mL plastic syringe, and transfer the retinal piece to a small container filled with agar solution (prepared in (1.2)). Try to minimize the amount of solution around the retina as it is placed into the liquid agar. Immerse directly in ice-cold slice solution (prepared in (3.3)) to solidify the agar 20, 18, 9. We use a small, handmade container. Briefly, a small, cylindrical tube (Fisherbrand, Polyethylene sample vials 2.5 ml, 03-338-1B) was cut at both ends and sealed with Parafilm (PM-996, Pechiney Plastic Packaging) patches.
- Cut the solid agar block into a 1x1x1 cm cube containing the rectangular piece of retina.
- Transfer the block to the slice chamber and glue it to the surface of the slicing plate. The slice chamber is pre-cooled in a freezer (-20°C). Cut the block into 200-250 μm thick slices using a Vibratome slicer (VT1000S or VT 1200S, Leica) in ice-cold slice solution. A total of 5 to10 slices can be obtained, which are viable for about 5 to 6 hours.
- Transfer one of the slices to the recording chamber. Place a grid of nylon threads glued to a U-shaped platinum frame 19 on top of the slice, and perfuse continuously (rate of 1-2 mL per minute) with recording solution bubbled with 95% O2 / 5% CO2 (carbogen).
Trouble Shooting:
If the retinal piece embedded in the agar block is detached or comes out of the agar during slicing, one can increase the slice thickness from 200 μm up to 300 μm in increments of 20 μm. Also try to minimize the amount of solution around the retina as it is transferred and placed into the liquid agar by sucking up extra solution with a rolled “Kimwipe” paper tip. Another way to avoid this problem is to reduce the size of retinal piece initially placed in the agar. Because vitreous humor prohibits agar from fully attaching to the retinal piece, it may be necessary to make fresh hyaluronidase or adjust the incubation time (step (3.3)).
4. Identification of the bipolar cell synaptic terminal in a retinal slice
- Position the retinal slice under the upright microscope (e.g. BX51WI, Olympus). We view the retinal slice with infrared differential interference contrast (IR-DIC) optics through a 60x water-immersion objective (NA 0.90, Olympus) and CCD camera (XC-75, Sony). The output of the CCD camera is sent to a camera controller (C2400, Hamamatsu) for contrast enhancement before visualization on an analog Sony 13″ black and white monitor. The microscope is mounted on an X-Y translation stage, and the recording chamber is placed on a fixed stage 14.
- Find either intact and axotomized (axon-cut) bipolar cell terminals, which can be identified by their size, shape, and position in the slice (Figure 1). Axotomized and intact terminals can also be distinguished by examination of their capacitative transient current decay times (single exponential versus double-exponential decays, respectively) and Ca2+ currents (Figure 2; 12, 14, 15). Mb terminals are located in the most proximal layer of sublamina b (ON-type layer; adjacent to ganglion cell layer) in the inner plexiform layer of the goldfish retina. Mb terminals, with their dull surface and flat appearance, can be easily distinguished from ganglion and displaced amacrine somas, which appear phase-bright and spherical. Furthermore, the edge of Mb terminals is covered with a high density of synaptic contacts, and appears rough and irregular when compared to the smooth, clean surfaces of ganglion and amacrine cells (Figure 1a-c). Axotomized Mb terminals appear round and near circular (Figure 1c), while intact terminals appear elliptical and stretched, often with a pinched end pointing toward the inner nuclear layer of the retina (Figure 1a,b).
- Damaged or unhealthy terminals often look swollen and display several small granular structures on the surface. It may be possible to obtain a GΩ seal on these terminals, but they are likely to rupture shortly after break-in. Other signs of unhealthy terminals include a hollow appearance, contrast and/or brightness identical to the surrounding slice, and sometimes location at the surface of the slice. It is possible to record both from healthy terminals deep in the slice (advantage: more intact synaptic circuitry) and also near the surface of the slice (advantage: quicker access to perfused drugs in the external solution; easier to seal).
5. Electrophysiological recordings and Ca2+ imaging
- Using a syringe with a 0.2 μm filter tip (Nalgene), fill the glass patch pipette with internal solution to a level 1-2 centimeters from the back of the pipette. After removing air bubbles from the tip, secure the pipette in the holder, apply positive pressure (1.2-1.6 psi), and move it toward the targeted terminal using a micromanipulator (MPC-200, Sutter Instrument). While moving the tip downward through the slice, move the pipette in a lateral sweeping direction to ensure that the slice is not pulled along with the tip.
- Move the pipette tip around the terminal to clean the membrane surface. Push the tip downward on the edge of the terminal to create a dimple (indent), and release the positive pressure. If a GΩ seal does not form immediately, apply slight negative pressure.
- After a GΩ seal, switch to the on-cell recording mode of the EPC-9 patch clamp amplifier and change the holding potential to -60 or -70 mV. Apply the C-fast (fast-capacitance) correction, wait for the seal to stabilize between 5-10 GΩ, and then rupture the membrane with sharp, gentle suction applied by mouth to establish the whole-cell recording configuration. If the whole-cell configuration has been correctly established, series resistance will be between 14-30 MΩ. Input resistance will be 200-500 MΩ for intact terminals and 1-3 GΩ for axotomized terminals 14.
- Observe the capacitive current transient (Figure 2a and 2c), to distinguish between intact and axotomized bipolar cell terminals. Intact and axotomized Mb terminals have a baseline membrane capacitance of 9-16 pF and 3-8 pF, respectively.
- Apply a 200 ms duration voltage-clamp step from -70 to 0 mV to an axotomized Mb terminal. This will allow the direct measurement of large (~200-600 pA), isolated inward Ca2+ currents activated by the opening of voltage-dependent L-type Ca2+ channels (Figure 2b). In parallel, one is able to monitor the capacitance jump caused by the exocytosis of synaptic vesicles, and the rapid, pH- mediated inhibition of Ca2+ current that follows exocytosis 15, and GABAergic reciprocal feedback inhibition (Figure 3c; 22). If one patches an intact Mb bipolar cell terminal the result will be as shown in Figure 2c and 2d. No discernible Ca2+ currents are observed because of the large “leak” currents due to gap-junction coupling in the dendrites of the Mb bipolar cells 1.
- Membrane capacitance changes can be evoked by a step depolarization from the holding potential of -70 mV to 0 mV using the “sine + DC” method of time-resolved membrane capacitance measurements 6. Membrane capacitance jumps indicate the fusion of synaptic vesicles with the plasma membrane. A 2 kHz sinusoidal voltage-clamp command (30 mV peak-to-peak) was added to the holding potential of -70 mV. The recording current was analyzed at two orthogonal phase angles by the EPC-9 patch-clamp amplifier software emulation of a lock-in amplifier (HEKA, Lambrecht, Germany). In the intact terminal of Figure 2d, a high frequency (2 kHz) sinusoidal stimulus confines the membrane capacitance that is analyzed to the terminal endings and axon, which have a baseline capacitance Cm ~6.8 pF. The membrane of the cell body and dendrites of the bipolar cell are thus filtered out by the high frequency voltage-clamp sinewave 13.
- For Ca2+-imaging experiments, thoroughly mix the internal patch pipette solution with a Ca2+-sensitive fluorescent dye (e.g. Oregon Green 488 BAPTA-1 (100-200 μM; O-6806, Invitrogen)) and repeat the protocol from 5.1 to 5.5. This will allow one to combine fluorescence imaging and electrophysiological recording. For example, it is possible to image the Ca2+ transient associated with a 200 ms step from -70 to 0 mV in the small telodendria appendages of the Mb terminal (Figure 3a,b). We have integrated our upright Olympus microscope with a spinning disk laser confocal microscope system (CSU-X1, Yokogawa). The confocal microscope uses 488 nm and 561 nm laser lines that are modulated by an acousto-optic tunable filter. Data are acquired using Slidebook software (3i; Intelligent Imaging Instruments). For further details on calcium imaging techniques using goldfish retinal neurons see 24, 17, 3.
Trouble Shooting:
If one frequently fails to establish a whole-cell configuration, even after formation of a stable GΩ seal, it may be helpful to reduce the negative pressure used to puncture the terminal membrane. It may also be the case that optimal suction pressure for the establishment of whole-cell configuration varies as a function of membrane curvature (soma > terminal). It is also possible to use a zap protocol (Amplitude: 400 mV, Duration: 100 μs) to enter the whole-cell configuration, either alone or in combination with a sharp pulse of negative pressure. We have found the zap protocol to be particularly useful for break-in when using a pipette tip with a bath resistance in excess of 12 MΩ.
6. Representative Results
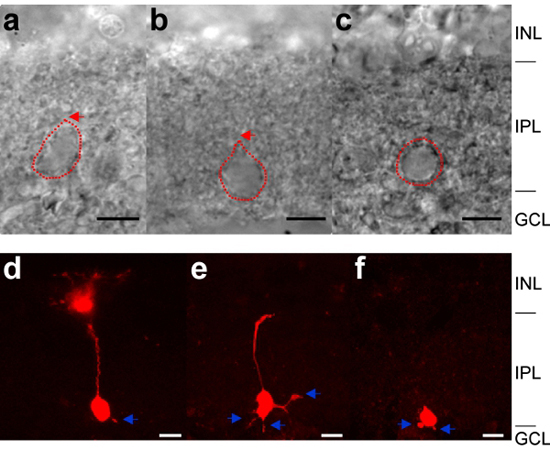
Figure 1. Identification of Mb bipolar cell terminals in goldfish retinal slices. (a-c) IR-DIC images of Mb bipolar cell terminals. We can identify likely axotomized (axon-cut) and intact terminals in the IR-DIC image. An axotomized terminal appears round and circular, as shown in c while an intact terminal is more likely to appear elliptical, as shown in a and b. This classification can be confirmed by transient capacitance measurement (see Figure 2) or by the dye filling method (d–f). Red arrows indicated the location of the intersection between the axon and axon terminal for the intact terminals in a and b. Red dotted line indicated the contour of Mb bipolar cell terminals. (d-f). Fluorescence images of Mb bipolar cell terminals with an intact axon (d), a partially cut axon (e), or a fully removed axon (f). Alexa 555 (A20501MP, Invitrogen) fluorescent dye was filled with internal solution prior to recording. Immediately after patch-clamp recording, the retinal slice was transferred into 4% (wt/vol) paraformaldehyde (P6148, Sigma) in phosphate buffer solution (70013, GIBCO) and incubated for 30 min. Slices were mounted onto Superfrost slides (Fisher Scientific) in aqueous mounting medium with anti-fading agents (Biomeda corp). Alexa 555 containing Mb bipolar cell terminals were viewed with a 555 nm laser line (red) using a 40x water-immersion objective on a confocal laser-scanning microscope (LSM 710, Carl Zeiss). Note the presence of small telodendria appendages protruding from the axon terminals (blue arrows). Scale bar indicate 10 μm (a–f). INL: inner nuclear layer, IPL: inner plexiform layer, GCL: ganglion cell layer.
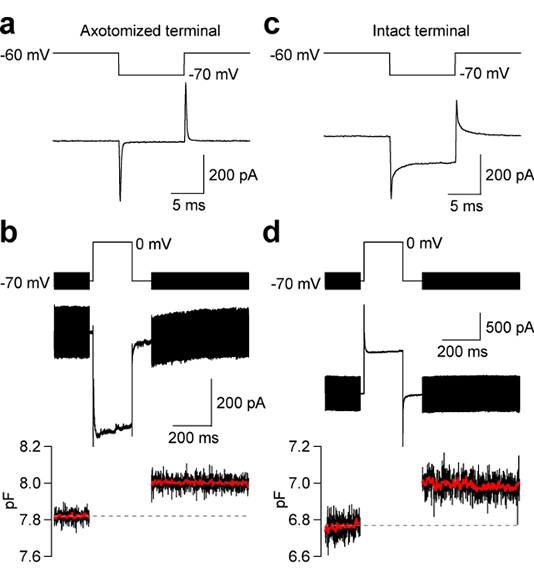
Figure 2. Electrical properties of axotomized and intact Mb bipolar cell terminals. (a,c) Transient current response activated by a voltage-clamp step hyperpolarization from the holding potential of -60 mV to -70 mV. The short current response to a -10 mV voltage-clamp step can result in: 1) a single fast exponential current decay with high input resistance at -70 mV (indicative of an axotomized terminal; panel a), or 2) a double-exponential decay with low input resistance at -70 mV (indicative of an intact terminal; panel c; see also 12, 14, 15). (b,d) Ca2+ currents and membrane capacitance jumps were evoked by a step depolarization from -70 to 0 mV. Time-resolved membrane capacitance measurements use a 2-kHz sinewave stimulus superimposed on the resting holding potential of -70 mV 6. The red trace is the averaged value of the capacitance data points. Note that the capacitance jump in Figure 2b and 2d are both equal to about 200 fF.
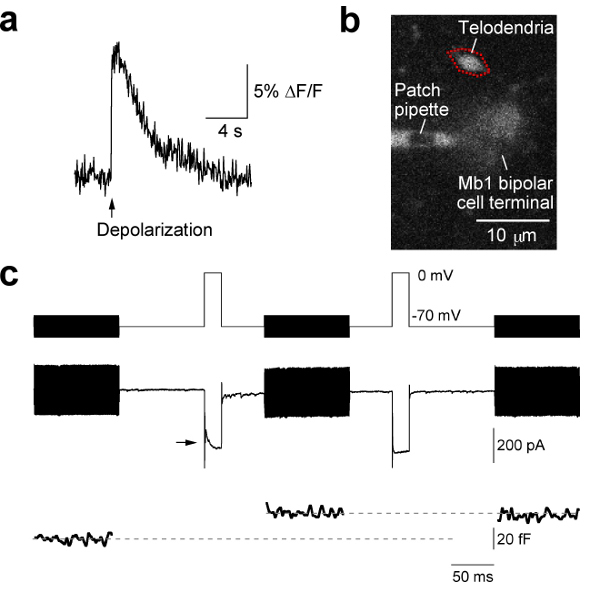
Figure 3. Direct measurements of Ca2+ imaging signals, exocytosis, and inhibitory feedback in an Mb bipolar cell terminal. (a) A transient rise in Ca2+ concentration in the telodendria of an Mb terminal was activated by a voltage-clamp step depolarization from -70 to 0 mV for 200 ms (arrow). Oregon Green 488 BAPTA-1 (100 μM), a Ca2+ indicator dye, was included in the patch pipette. (b) Corresponding fluorescence image of the Mb telodendria (region of interest indicated by red dotted line). (c) Short-term synaptic depression of neurotransmitter release (exocytosis). Ca2+ currents (middle trace) and membrane capacitance (lower trace) evoked by a pair of 20 ms depolarizations to 0 mV (upper trace; 200 ms inter-pulse interval). The arrow indicates the effect of exocytosed protons on the Ca2+ current and also the GABAergic reciprocal feedback inhibition from neighboring amacrine cells 15, 21. Note that the proton-mediated inhibition of the Ca2+ current and also the GABAergic reciprocal feedback inhibition are present only in the first depolarizing response, which also has a capacitance jump due to the exocytosis of synaptic vesicles.
Discussion
A critical and difficult step in our protocol is the transfer of the piece of retina into the agar solution (protocol 3.4). It is necessary to carefully remove the vitreous humor and residual slice solution from the retinal piece and transfer it without distortion or bending. In order to accomplish this, we use a small spatula (21-401-25B, Fisherbrand) with a tip bent to form a 90° angle, along with angled tip forceps (11251-35, Fine Science Tools), to position the retinal piece throughout the transfer process in slice solution. Residual slice solution on the retinal slice and spatula can be removed by careful dabbing of surface with the corner of a folded or rolled Kimwipes (Kimberly-Clark).
Another common way to prepare transverse retinal slices is the filter paper-based protocol 23. Briefly, an inverted piece of whole retina is attached to a rectangle piece of Millipore filter disk (ganglion cell layer against the filter paper, photoreceptor layer facing up) and cut into 250 μm thick slices with a manual vertical “thumb ” slicer (e.g. Narishige ST-20). We find that the advantages of the filter paper-based protocol include healthier photoreceptors and an increase ratio of axotomized to intact Mb terminals. We thus use this filter paper method to elicit light-evoked responses from single Mb terminals embedded in retinal slices.
The advantages of our agar-based protocol over the filter paper-based protocol are that 1) the retinal slice produced is flat, even, and relatively undamaged with clear separation between retinal layers, which enables patching in any inner nuclear layer or plexiform layer retinal cell that is desired, and 2) immunohistochemistry following electrophysiological recording is performed more easily because of the intact and flat geometry of the slice and the factors mentioned in item (1) above. A protocol for recording light responses of retinal neurons in an agar-based preparation was previously published in JoVE 2. A horizontal retinal slice preparation that used a combination of agar and filter paper techniques was also previously published in JoVE 5. We recommend watching these videos in combination with our own to compare the different techniques.
Direct access to ribbon-type presynaptic terminals in vertebrate retinal slices allows us to investigate synaptic transmission at ribbon-type active zones. It also allows us to directly study the synaptic physiology in retinal microcircuits. This technique will be particularly useful for paired recordings of pre- and post-synaptic signals, with postsynaptic partners including inhibitory amacrine cells and spiking ganglion cells 1, 16, 10. Paired recordings at ribbon-type synapses between rat cochlear inner hair cells and afferent dendrites are possible and have been described by 7. Calcium imaging of the Mb bipolar cell terminals has been performed in acutely dissociated cells 8 and in retinal slices 17, as well as in goldfish amacrine cells 3. Recent studies have shown great improvements for in vivo and in vitro optogenetic approaches in transgenic zebrafish retina 4. Future directions will likely involve these transgenic techniques, together with electrophysiological methods, which will allow us to bridge the gap between in vitro slice recordings and in vivo imaging, leading ultimately to behavioral studies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Fred Rieke for his kind explanation of the agar-embedded retinal slice preparation when we started using the protocol in our lab. We also thank Lori Vaskalis for the illustration of schematic overview and Drs. Veeramuthu Balakrishnan and Soyoun Cho for helpful comments on the text and video. This work was supported by an NEI-NIH RO1 grant, and was also partially supported by a Korea Research Foundation Grant funded by the Korean Government [KRF-2008-357-E00032].
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Low gelling-temperature agar | Sigma | A0701 | Agarose type VII-A |
| Patch pipette | World Precision Instruments | 1B150F-4 | Thick-walled (1.5 mm outer diameter) borosilicate glass |
| Vertical puller | Narishige | PP830 | |
| Dental wax | Cavex | ||
| Spring scissors | Fine Science Tools | 15003-08 | |
| 45° angled fine tip forceps | Fine Science Tools | 11251-35 | |
| Razor blade | Personna | Double-edged, cleaned with 70% ethanol and H2O | |
| Cylindrical tube | Fisherbrand | 03-338-1B | Polyethylene sample vials 2.5 ml |
| Hyaluronidase | Sigma | H6254 | |
| Vibratome slicer | Leica | VT1000S or VT1200S | |
| Upright microscope | Olympus | BX51WI | |
| 60x water-immersion objective | Olympus | LUMPlanFl | NA 0.90 |
| CCD camera | Sony | XC-75 | |
| Camera controller | Hamamatsu | C2400 | |
| Monitor | Sony | 13” black and white monitor | |
| Syringe filter | Nalgene | 0.2 μm | |
| Micromanipulator | Sutter Instrument | MPC-200 | |
| Lock-in amplifier | HEKA | EPC-9/10 amplifiers have software emulation | |
| Spinning disk laser confocal microscope | Yokogawa | CSU-X1 | Live cell imaging after patch clamp whole cell recording |
| Slidebook software | Intelligent Imaging Instruments (3i) | Imaging data acquisition and analysis | |
| Paraformaldehyde | Sigma | P6148 | |
| Phosphate buffer solution | GIBCO | 70013 | |
| Superfrost slide | Fisher Scientific | Slide glass | |
| Anti-fading agents | Biomeda corp. | ||
| Confocal laser-scanning microscope | Carl Zeiss | LSM 710 | Imaging of fixed tissue |
| Spatula | Fisherbrand | 21-401-25B | |
| Manuel vertical slicer | Narishige | ST-20 | |
| Oregon Green 488 BAPTA-1 | Invitrogen | O-6806 | Ca2+ sensitive fluorescent dye |
| Alexa Fluor 555 Hydrazide | Invitrogen | A-20501MP | Fluorescent dye |
References
- Obeso, J. A., Olanow, C. W., Nutt, J. G. Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 23, S2-S7 (2000).
- Caprioli, R. M., Farmer, T. B., Gile, J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751-4760 (1997).
- Obeso, J. A. The evolution and origin of motor complications in Parkinson’s disease. Neurology. 55, S13-S20 (2000).
- WHO. Noncommunicable Diseases and Mental Health Cluster, Noncommunicable Disease Prevention and Health Promotion Department, Ageing and Life Course . Active ageing: a policy framework. , (2002).
- Schapira, A. H. Movement disorders: advances in cause and treatment. Lancet Neurology. 9, 6-7 (2010).
- Obeso, J. A., Rodriguez-Oroz, M. C., Rodriguez, M., DeLong, M. R., Olanow, C. W. Pathophysiology of levodopa-induced dyskinesias in Parkinson’s disease: problems with the current model. Ann. Neurol. 47, 22-32 (2000).
- Cenci, M. A., Lee, C. S., Bjorklund, A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur. J. Neurosci. 10, 2694-2706 (1998).
- Andersson, M., Hilbertson, A., Cenci, M. A. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 6, 461-474 (1999).
- Hanrieder, J. Alterations of striatal neuropeptides revealed by imaging mass spectrometry. Molecular & Cellular Proteomics. , (2011).
- Cornett, D. S., Reyzer, M. L., Chaurand, P., Caprioli, R. M. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat. Methods. 4, 828-833 (2007).
- Ljungdahl, . Imaging Mass Spectrometry Reveals Elevated Nigral Levels of Dynorphin Neuropeptides in L-DOPA-Induced Dyskinesia in Rat Model of Parkinson’s Disease. PLoS ONE. 6, e25653-e25653 (2011).
- Groseclose, M. R., Andersson, M., Hardesty, W. M., Caprioli, R. M. Identification of proteins directly from tissue: in situ tryptic digestions coupled with imaging mass spectrometry. J. Mass. Spectrom. 42, 254-262 (2007).
- Andersson, M., Groseclose, M. R., Deutch, A. Y., Caprioli, R. M. Imaging mass spectrometry of proteins and peptides: 3D volume reconstruction. Nat. Methods. 5, 101-108 (2008).
- Deininger, S. -. O., Setou, M. . Imaging Mass Spectrometry. , 199-208 (2010).
- Norris, J. L. Processing MALDI Mass Spectra to Improve Mass Spectral Direct Tissue Analysis. Int. J. Mass. Spectrom. 260, 212-221 (2007).
- Ihaka, R., Gentleman, R. R. A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics. 5, 299-314 (1996).
- Li, H., Chen, S., Hong, D., Li, M., Shyr, Y. . Mass Spectrometry Binning Software GAB. , (2012).
- Tusher, V. G., Tibshirani, R., Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 98, 5116-5121 (2001).
- Bergstrom, L., Christensson, I., Folkesson, R., Stenstrom, B., Terenius, L. An ion exchange chromatography and radioimmunoassay procedure for measuring opioid peptides and substance P. Life. Sci. 33, 1613-1619 (1983).
- Falth, M. Neuropeptidomics strategies for specific and sensitive identification of endogenous peptides. Mol. Cell. Proteomics. 6, 1188-1197 (2007).
- Falth, M. a database designed for endogenous peptides and mass spectrometry. Mol. Cell. Proteomics. 5, 998-1005 (2006).

