F1FO ATPase Vesicle Preparation and Technique for Performing Patch Clamp Recordings of Submitochondrial Vesicle Membranes
Summary
A method to isolate submitochondrial vesicles enriched in F1FO ATP synthase complexes from rat brain is described. These vesicles allow the study of the activity of F1FO ATPase complex and its modulation using the technique of patch clamp recording.
Abstract
Mitochondria are involved in many important cellular functions including metabolism, survival1, development and, calcium signaling2. Two of the most important mitochondrial functions are related to the efficient production of ATP, the energy currency of the cell, by oxidative phosphorylation, and the mediation of signals for programmed cell death3.
The enzyme primarily responsible for the production of ATP is the F1FO-ATP synthase, also called ATP synthase4-5. In recent years, the role of mitochondria in apoptotic and necrotic cell death has received considerable attention. In apoptotic cell death, BCL-2 family proteins such as Bax enter the mitochondrial outer membrane, oligomerize and permeabilize the outer membrane, releasing pro-apoptotic factors into the cytosol6. In classic necrotic cell death, such as that produced by ischemia or excitotoxicity in neurons, a large, poorly regulated increase in matrix calcium contributes to the opening of an inner membrane pore, the mitochondrial permeability transition pore or mPTP. This depolarizes the inner membrane and causes osmotic shifts, contributing to outer membrane rupture, release of pro-apoptotic factors, and metabolic dysfunction. Many proteins including Bcl-xL7 interact with F1FO ATP synthase, modulating its function. Bcl-xL interacts directly with the beta subunit of F1FO ATP synthase, and this interaction decreases a leak conductance within the F1FOATPasecomplex, increasing the net transport of H+ by F1FO during F1FO ATPase activity8 and thereby increasing mitochondrial efficiency. To study the activity and modulation of the ATP synthase, we isolated from rodent brain submitochondrial vesicles (SMVs) containing F1FO ATPase. The SMVs retain the structural and functional integrity of the F1FO ATPase as shown in Alavian et al. Here, we describe a method that we have used successfully for the isolation of SMVs from rat brain and we delineate the patch clamp technique to analyze channel activity (ion leak conductance) of the SMVs.
Protocol
1. Brain Mitochondrial Isolation (Adapted from Brown M.R. et al.9)
- Sacrifice the rat using methods approved by the Institutional Animal Care and Use Committee (IACUC).
- Cut the head of the animal by decapitation, cut the skin and expose the skull.
- Open the skull gently by cutting with a scissor or rongeur. Remove the brain.
- Mince finely the brain without cerebellum in Isolation Buffer (see Table 1) and transfer it to a 5 ml glass/teflon homogenizer (see equipment list).
- Homogenize tissue gently 10 times (no bubbles), approximately 5 min.
- Centrifuge sample at 1,500 × g for 10 min at 4 °C in a bench-top centrifuge.
- Save the supernatant (mitochondrial and synaptosomes) and discard the pellet (nuclear material and cell debris). Centrifuge at 16,000 × g for 10 min at 4 °C in a bench-top centrifuge.
- Discard supernatant and re-suspend pellet in 500 μl of Isolation Buffer. Disrupt synaptosomes with a cell disruption vessel (see equipment list). Apply a pressure of 1,200 psi for 10 min, followed by rapid decompression.
- Layer the mixture onto Ficoll gradients (see Table 2), place in SW-50.1 rotor and centrifuge at 126,500 × g for 20 min at 4 °C in an ultracentrifuge (see equipment list). The pellet is the purified mitochondria, the layer between the different densities of Ficoll is undisrupted synaptosomes.
- Wash pellet by centrifuging in Isolation Buffer at 16,000 × g for 10 min at 4 °C in a bench-top centrifuge.
2. Submitochondrial Vesicles (SMV) Isolation (Adapted from Chan et al.10)
- Re-suspend mitochondria in 200 μl Isolation Buffer combined with an equal volume of 1% digitonin and allow to sit on ice for 15 min.
- Add more Isolation Buffer and centrifuge at 16,000 × g for 10 min at 4 °C in a bench-top centrifuge. Do this twice.
- Re-suspend pellet in 200 μl Isolation Buffer and add 2 μl of 10% Lubrol PX (C12E9). Mix and allow to sit on ice for 15 min.
- Layer mixture onto Isolation Buffer, place in SW-50.1 rotor and centrifuge at 182,000 × g at 4 °C for 1 hr.
- Wash final pellet by centrifuging in a desk-top centrifuge in Isolation Buffer at 16,000 × g for 10 min at 4 °C.
3. Electrophysiological Recording
- A typical electrophysiology rig includes an amplifier, a PC computer equipped with a Digidata 1440A analog-to-digital converter interface in conjunction with pClamp10.0 software, manipulators, a microscope, a vibration isolation table, Faraday Cage.
- Borosilicate glass capillary tubes are inserted into a Flaming/Brown Micropipette Puller Model P-87. A pipette-puller program is optimized to generate pipettes with resistances between 80 to 100 MΩ.
- SMVs are placed in a physiological intracellular solution (ATP is added at the appropriate time during the recording) (Table 3). Patch clamp pipettes are filled with the same solution (no ATP). Recordings are made by forming a giga-ohm seal onto SMVs at room temperature. Vesicles are visualized by phase-contrast microscopy with a Nikon or Zeiss inverted microscope.
- The membrane potential is maintained at voltages ranging from -100 mV to + 100 mV for periods of 10 seconds. Recordings are filtered at 5 kHz using the amplifier circuitry. Level of stray electrical or non-specific noise of less than 1 pA are desirable for successful recording.
- Data are analyzed with Clampfit 10.0 software for example to determine frequency and amplitude of single channel events. Voltage dependence is determined by plotting a current voltage relationship.
Representative Results
The first step of our protocol allows for isolation of purified mitochondria as shown by Western blot in Figure 1. In Figure 2 is shown an example of a brain-derived submitochondrial vesicle patch recording. Using the inside-out patch configuration we demonstrate channel activity modulated by ATP. The control (CTL) recording (left) shows multi-conductance channel activity with a peak conductance of 600 pS on average. that was immediately decreased upon addition of 1 mM ATP to the bath. The overall effect of ATP is to decrease the conductance of SMVs patches in a concentration-dependent manner. To measure the efficiency of F1FO ATPase after isolation, we measured the movement of H+ ions into the SMVs in response to ATP hydrolysis as shown in Figure 3, measuring the decrease in fluorescence intensity of the SMV-excluded H+ indicator ACMA. ATP-sensitive H+ ion sequestration into SMVs is enhanced after addition of ATP. This assay can be used to measure the efficiency of H+ ion sequestration. Less efficient SMVs will accumulate H+ ions more slowly or to a smaller peak value. For example H+ ion sequestration is attenuated by the addition of Bcl-xL inhibitors and FCCP (Alavian et al., 2011).
| Final Concentration | |
| Sucrose | 250 Mm |
| Hepes | 20 Mm |
| EDTA | 1 Mm |
| BSA | 0.5% |
Table 1. Isolation Buffer.
| Ficoll | 22 ml 20% > |
| Sucrose | 12 ml 1 M |
| EDTA | 18.75 μl 0.1 M |
| Tris-HCl | 375 μl 1 M (pH 7.4) |
| Top Layer 7.5% of Ficoll | |
| Ficoll stock | 10 ml |
| Isolation Buffer | 6 ml |
| Bottom Layer 10% of Ficoll | |
| Ficoll stock | 15.5 ml |
| Isolation Buffer | 2.5 ml |
Table 2. Ficoll Stock.
| KCl | 120 mM |
| NaCl | 8 mM |
| EGTA | 0.5 mM |
| Hepes | 10 mM |
| pH | 7.3 |
Table 3. Internal Solution.
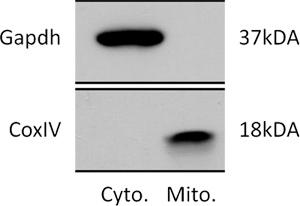
Figure 1. Representative immunoblot of lysate purified from Cytosol and Mitochondria. The upper panel shows an immunoblot using an antibody against the cytosolic protein Gapdh. The bottom panel shows an immunoblot using an antibody against the mitochondrial innner membrane protein CoxIV.
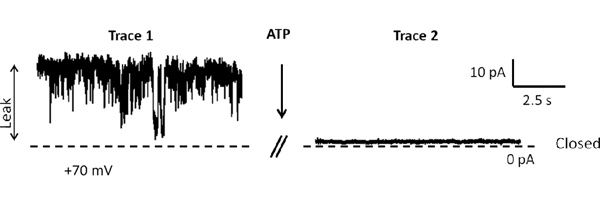
Figure 2. Two representative patch clamp recording before and after addition of 1 mM ATP. Holding potential is +70 mV. The dashed line represents 0 pA. Each trace is recorded for 10 sec and there is 10 sec between traces.
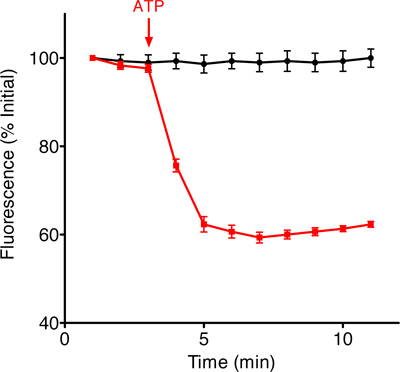
Figure 3. Example traces of fluorescence intensity changes of the ACMA indicator over time in the presence of SMVs and in the absence (black trace) and presence (red trace) of ATP.
Discussion
The methods described herein enable the isolation of pure mitochondria at the end of step 1 and submitochondrial vesicles (SMVs) after step 2 from whole brain without distinction of cell phenotypes.SMVspurified by this method are essentially free of contamination by other subcellular organelles as shown in Figure 1 and our previous work (Alavian KN et al.8) and retain their structural and functional integrity prior to freezing. After freezing and thawing, isolated mitochondria or SMVs are used for recordings and biochemical assays, but not for respiratory studies.
This method moreover can be used to isolate mitochondria from liver using the same steps. Because the mitochondria and SMVs tend to form clusters when plated into the electrophysiological recording chamber, attention should be given to loading the sample, as an excessive clumping or excessive dilution may decrease the possibility of seal formation. Moreover, attention should be given to several technical details before starting the isolation. It is crucial to use clean tools and to keep them on ice. All procedures should be performed on ice and all centrifuges set to 4 °C. Preparing the Ficoll gradient properly is critical for a successful experiment. Creating a perfect separation between the two concentrations of Ficoll enhances the purity of subcellular fractions.
A limitation of this technique is imposed by the final amount of protein, which can be small if sample from a specific brain region is desired for example mitochondria from substantia nigra pars compacta or striatum. Pooling samples from several animals may be necessary to enhance the total amount of protein harvested.
Mitochondria or SMVs are aliquoted at 1 mg/ml and stored at -80 °C and they are stable for recordings for several months, however, it is preferable not to re-freeze the mitochondrial sample after thawing. Vesicles purified by this method are useful to study the ATP synthase, using different approaches such as patch clamp recording, biochemical techniques and other functional studies. With this preparation one can analyze the effects of drugs or small molecules on F1FO ATPase activity and structure.
Divulgations
The authors have nothing to disclose.
Materials
| Name | Company | Catalogue number |
| Potter-Elvehjem Tissue Grinder withPTFEPestle | Krackeler Scientific, Inc. | 1-7725T-5 |
| Eppendorf Centrifuge 5424 | Eppendorf | 5424 000.410 |
| 4639 Cell Disruption Vessel | Parr Instrument Company | 4639 |
| Ficoll | Sigma-Aldrich | F5415 |
| Polycarbonate centrifuge tubes | Beckman Coulter | P20314 |
| SW-50.1 rotor | Beckman Coulter | |
| L8-70M Ultracentrifuge | Beckman Coulter | |
| Digitonin | Sigma-Aldrich | D5628 |
| Lubrol PX (C12E9) | Calbiochem | 205534 |
| Axopatch 200B | Axon Instruments | |
| Digidata 1440A | Molecular Device | |
| pClamp10.0 | Molecular Device | |
| Manipulator | Sutter Instrument | |
| Borosilicate glass capillary | World Precision Instruments | 1308325 |
| Flaming/Brown Micropipette Puller Model P-87 | Sutter Instrument |
References
- Cheng, W. C., Berman, S. B., Ivanovska, I., Jonas, E. A., Lee, S. J., Chen, Y., Kaczmarek, L. K., Pineda, F., Hardwick, J. M. Mitochondrial factors with dual roles in death and survival. Oncogene. 25, 4697-4705 (2006).
- Duchen, M. R., et al. Mitochondria and calcium in health and disease. Cell Calcium. 44, 1-5 (2008).
- Lemasters, J. J. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. J. Gastroenterol. Hepatol. 22, S31-S37 (2007).
- Cox, G. B., Jans, D. A., Fimmel, A. L., Gibson, F., Hatch, L. Hypothesis. The mechanism of ATP synthase. Conformational change by rotation of the beta-subunit. Biochim. Biophys. Acta. 768, 201-208 (1984).
- Cox, G. B., Fimmel, A. L., Gibson, F., Hatch, L. The mechanism of ATP synthase: a reassessment of the functions of the b and a subunits. Biochim. Biophys. Acta. 849, 62-69 (1986).
- Cory, S., Huang, D. C., Adams, J. M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 22, 8590-8607 (2003).
- Vander Heiden, M. G., Thompson, C. B. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis. Nat. Cell Biol. 1, 209-216 (1999).
- Alavian, K. N., Li, H., Collis, L., Bonanni, L., Zeng, L., Sacchetti, S., Lazrove, E., Nabili, P., Flaherty, B., Graham, M., Chen, Y., Messerli, S. M., Mariggio, M. A., Rahner, C., McNay, E., Shore, G. C., Smith, P. J. S., Hardwick, J. M., Jonas, E. A. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 13 (10), 1224-1233 (2011).
- Brown, M. R., Sullivan, P. G., Dorenbos, K. A., Modafferi, E. A., Geddes, J. W., Steward, O. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. Journal of Neuroscience Methods. 137, 299-303 (2004).
- Chan, T. L., Greenawalt, J. W., Pedersen, P. L. Biochemical and ultrastructural properties of a mitochondrial inner membrane fraction deficient in outer membrane and matrix activities. J. Cell Biol. 45 (2), 291-305 (1970).
- Young, H. K. o., Delannoy, M., Hullihen, J., Chiu, W., Pedersen, P. L. Mitochondrial ATP Synthasomes. J. Biol. Chem. 278 (14), 12305-12309 (2003).

