Generation of Topically Transgenic Rats by In utero Electroporation and In vivo Bioluminescence Screening
Summary
Genes can be manipulated during development of the cortex or the hippocampus of the rat via in utero electroporation (IUE) at E16, to enable fast and targeted modifications in neuronal connectivity for later studies of behavior or neuropathology in adult animals. Postnatal in vivo imaging for control of IUE success is performed by bioluminescence of activating co-transfected luciferase.
Abstract
In utero electroporation (IUE) is a technique which allows genetic modification of cells in the brain for investigating neuronal development. So far, the use of IUE for investigating behavior or neuropathology in the adult brain has been limited by insufficient methods for monitoring of IUE transfection success by non-invasive techniques in postnatal animals.
For the present study, E16 rats were used for IUE. After intraventricular injection of the nucleic acids into the embryos, positioning of the tweezer electrodes was critical for targeting either the developing cortex or the hippocampus.
Ventricular co-injection and electroporation of a luciferase gene allowed monitoring of the transfected cells postnatally after intraperitoneal luciferin injection in the anesthetized live P7 pup by in vivo bioluminescence, using an IVIS Spectrum device with 3D quantification software.
Area definition by bioluminescence could clearly differentiate between cortical and hippocampal electroporations and detect a signal longitudinally over time up to 5 weeks after birth. This imaging technique allowed us to select pups with a sufficient number of transfected cells assumed necessary for triggering biological effects and, subsequently, to perform behavioral investigations at 3 month of age. As an example, this study demonstrates that IUE with the human full length DISC1 gene into the rat cortex led to amphetamine hypersensitivity. Co-transfected GFP could be detected in neurons by post mortem fluorescence microscopy in cryosections indicating gene expression present at ≥6 months after birth.
We conclude that postnatal bioluminescence imaging allows evaluating the success of transient transfections with IUE in rats. Investigations on the influence of topical gene manipulations during neurodevelopment on the adult brain and its connectivity are greatly facilitated. For many scientific questions, this technique can supplement or even replace the use of transgenic rats and provide a novel technology for behavioral neuroscience.
Introduction
The development of the in utero electroporation (IUE) method which allows a modulation of gene expression in the developing brain, has been a break-through since it enabled studying neurodevelopment with relative ease.1-7 Changes in expression levels of a target gene in a specific brain region during embryonic and/or perinatal development in rodents were demonstrated to critically influence neuronal proliferation, migration, arborization, and connectivity.8-10
Schizophrenia is a complex mental illness with acute and chronic symptoms that has been related to neurodevelopmental abnormalities11, 12 and therefore many of the identified candidate genes for schizophrenia are investigated for potential modulating effects on neurodevelopment, like for example for the disrupted-in-schizophrenia-1 (DISC1) gene13-15.
Brain development is regulated by genetic factors and their interactions with environment which play roles in pre-, peri- and postnatal developmental periods. One major genetic risk factor for various behavioral disorders is the DISC1 16 gene. DISC1 knockdown leads to migration defects in mice 13, 17, and manipulation of DISC1 expression in the developing cortex by IUE has been shown to impact behavior of adult mice18.
Manipulating brain gene expression by IUE has several advantages19 over the generation of transgenic animal lines. First, gene expression within areas of interest is achieved within weeks to months rather than several generations of breeding transgenic rodent lines. Second, compensatory mechanisms during early development that may shield phenotypes in germline-engineered animals 20 are avoided. Third, through targeting only a specific cell population or specific area of the brain, migration or proliferation differences can be directly compared with the non-mutant or control contralateral side if unilateral electroporations are chosen. On the other hand, IUE does not have the accuracy of promoter-driven cre/lox-induced timing of expression and only a subpopulation of the cells in a certain area is targeted leading to a mosaic kind of gene expression pattern.
For many experimental applications in adult rodents, a transient transfection of a limited number of cells in a brain region may be sufficient, or even desired, so that the major advantage of stable, germline-transgenic rodents is negligible. In fact, IUE is useful to investigate whether some abnormally developed cells may affect a whole network of cells or circuitry. Another advantage may be the ability to demonstrate non cell-autonomous effects of a gene due to the mosaic nature of the hit. Furthermore, the generation of transgenic and knockout rats is still in its infancy and the use of IUE in this species for studying aberrant brain development consequences is of high interest.
So far, a major obstacle of using IUE for investigating the intervention consequences in those animals as adults is the lack of monitoring electroporation success. So far, GFP-co-transfected fluorescent neurons in the live newborn rat pups could not be detected under a suitable binocular fluorescence microscope or with the fluorescence imaging of the IVIS Spectrum.
To overcome this obstacle, we co-transfected a luciferase reporter gene and performed bioluminescence live imaging of pups by 3-dimensional (3D) quantitation of the IUE brain area.
As an example for demonstrating the applicability of this method in a later functional assay testing the neurodevelopmental genetic manipulation, a co-injection of plasmids containing human DISC1, luciferase, and GFP into the lateral ventricle of rat embryos3 followed by electroporation with a tweezer electrode was performed. While fluorescence signals could not be detected in postnatal stages in vivo, a solid bioluminescence signal derived from luciferin metabolism by co-transfected luciferase gene was detected up to five weeks after birth. 3D-measurements of the electroporated brain area allowed quantification whereby pups with insufficient or misplaced electroporation were identified from the outset, thus, enabling the assignment of IUE animals (gene of interest and scrambled control) to experimental groups with matched electroporated brain areas of low variability. The use of adult IUE rats in behavioral paradigms was demonstrated as an example of the usefulness of this protocol.
Protocol
All animal experiments were authorized by the responsible Landesministerium für Natur, Umwelt und Verbraucherschutz (LANUV NRW; 87-51.05.2010.A301) in accordance with National and European legislation.
1. In utero Electroporation
This method has been described in detail in JoVE for the rat by Walantus et al.3, as well as Rice et al.4 and is here summarized only briefly. A litter size of 6-8 pups yields a good outcome. There should be at least two non-electroporated embryos in order to increase the overall survival rate (see below).
- Prepare DNA-Mixture that contains 1.5 μg/μl of target-Plasmid (shRNA: pENTR-U6; Invitrogen, Eugene, OR / DISC1 overexpression: pCAX21), 0.5 μg/μl Luciferase containing Plasmid (pCAX), 0.5 μg/μl GFP containing plasmid (pCAGGS22) in 1x PBS solution stained light blue with Fast Green Dye.
- Prepare injection needles out of glass capillaries with a Needle Pipette Puller. And sterilize surgical instruments either by autoclaving or incubation with an alcohol-based disinfectant (kodan Tinktur forte).
- Administer a pre-operative dose of buprenorphine (0.05 mg/kg) 15 min before surgery to a pregnant rat 16 days after fertilization (E16). Then anesthetize the animal in an isoflurane chamber.
- Upon anesthesia, place the rat in a supine position on a 37 °C-warmed operation table with breathing mask connected to the anesthesia device, using oxygen setting at 0.4 L/min and isoflurane at 1.8%.
- After shaving the abdomen, disinfect the shaved area three times with kodan Tinktur Forte (an alcohol-based disinfectant).
- Cover the rat with sterile cloths, exposing only the shaved operation field.
- Perform de utero electroporation.
- Cut the abdomen with a scissor along the linea alba (~2 cm).
- Expose the uterine horns carefully with a ring forceps.
- Take care to keep the uterus wall wet with warmed sterile PBS during the whole surgery.
- Inject DNA solution with a thin glass needle into one of the lateral ventricle of the embryos.
- Place the 7 mm electrode around the head of the embryo. To hit a cell population of upper cortical layers, perform the IUE at E1623 and position the positive electrode on the hemisphere above the injected ventricle with a slight dorsal/lateral tendency. To target hippocampal cells change the positive electrode placement to the opposite side than the injected ventricle with lateral to slightly dorsal direction (Figure 1).
- Perform electroporation by five 50 msec pulses at 55 V with 950 msec breaks with a square wave pulse electroporator.
- Spare the first embryo at the vaginal end of each uterus horn in order to increase the chances of survival of all embryos.
- Put the uterus horns back into the mother rat.
- Stich the abdominal wall up with an absorbable Vicryl surgical suture material.
- Close the skin with the Vicryl suture material or with suture clips.
- Place the mother rat back into the home cage and keep it warm for 2-3 hr.
- Hold the rats alone in their home cage in the animal facility room and feed ad libitum. They give birth between E22-24.
- Keep the rat pups with their mother for three weeks and separate them afterwards by gender.
2. Bioluminescence Live Imaging of the Enzymatic Luciferase Reaction
This method is used to analyze the position of the in utero transfected cells. Co-electroporated firefly luciferase cDNA is translated into active luciferase, which upon metabolizing D-luciferin to oxyluciferin, emits a photon (Figure 2). The resulting luminescence can be detected in the brains of positive electroporated young rats in an IVIS Spectrum up to the age of around 35 days postnatally (Figure 4).
In the present study, the luciferase assay and bioluminescence imaging was performed starting at P7. This time point was chosen to allow mother and pups to recover from birth stress. When initially working with the pups at P0, pup survival was severely affected in that the pups were found dead or eaten by the mother.
Initially, rat pups with successful electroporation are identified by a 2D-bioluminescence picture with an exposure time of three minutes. Subsequently, positive pups are used for creating 3D images in order to specify the location of the electroporated area.
- Dilute D-luciferin sodium salt in PBS to a concentration of 15 mg/ml and sterilize it by filtration through a sterile syringe filter.
- Weigh the pups.
- Take the pup in one hand with the abdomen on top and stretch the abdomen slightly. Inject 10 μl/g of body weight of luciferin solution intraperitoneal. For older and more agile ones, pre-anaesthetize the pups with isoflurane in the induction chamber before injecting the luciferin.
- Turn on the isoflurane influx of the XGI-8 Gas Anesthesia System within the IVIS Spectrum with 3% isoflurane.
- Put the snout of the animal into the glass nose cones of the Anesthesia System.
- Hold the animal in a prone position until it is in deep anesthesia (2-3 min). Then reduce the isoflurane influx to 1.5%.
- Choose a 2D-bioluminescence measurement to select positive pups from the whole litter. Use the following settings
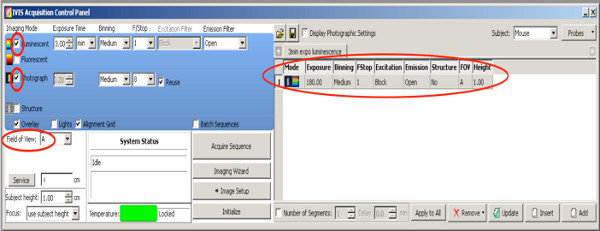
Click here to view larger figure.- Set a checkmark on Photograph with medium binning and F/Stop at 8, the camera takes a photo from above after starting the measurement.
- Set Excitation filter: block.
- Set Emission filter: open.
- Set Binning to medium.
- Set F/Stop at 1.
- Set Stage level A.
- Set luminescence exposure time to 180 sec.
- For creation of 3D pictures in order to better quantify the electroporated area from the positive pups, use the following DLIT settings for firefly luciferase.
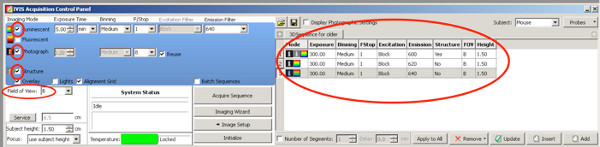
Click here to view larger figure.- Set a checkmark on Photograph; the camera takes a photo from above after starting the measurement.
- Set a checkmark on Structure, the surface of the animal is scanned by the IVIS prior the bioluminescence measurement.
- Use following Emission filters and exposure time settings until the age of two weeks:
Emission filter 1: 590 nm, exposure time 300 sec
Emission filter 2: 600 nm, exposure time 240 sec
Emission filter 3: 620 nm, exposure time 180 sec
Emission filter 4: 640 nm, exposure time 120 sec
For rats older than P20
Emission filter 1: 600 nm, exposure time 300 sec
Emission filter 2: 620 nm, exposure time 300 sec
Emission filter 3: 640 nm, exposure time 300 sec

Click here to view larger figure.
Due to the decrease in signal strength in older animals, the exposure time is enlarged for the three best emission filters. - Set Stage level B
- Set Binning to medium.
- Set F/Stop at 1
- After measurement, mark the rats by an earhole code to differentiate them from each other and to match them to the IVIS Live Imaging pictures
- At the end of the measurement procedure, turn off isoflurane influx and keep the rat on the warmed plate for some minutes before returning it to its cage.
3. Analysis of Bioluminescence Images
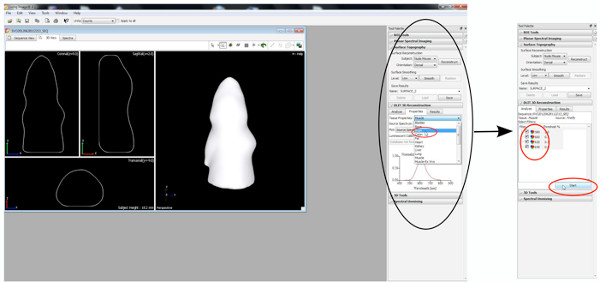
The generation of 3D images, 3D movies and the quantification of the volume of the signal source is made by the Living Image software pre-installed on the IVIS Spectrum.
- Generation of 3D images
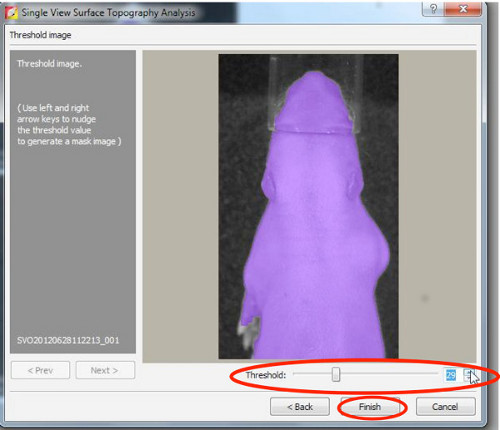
- First, reconstruct a surface topography, therefore set threshold between 20-30%.
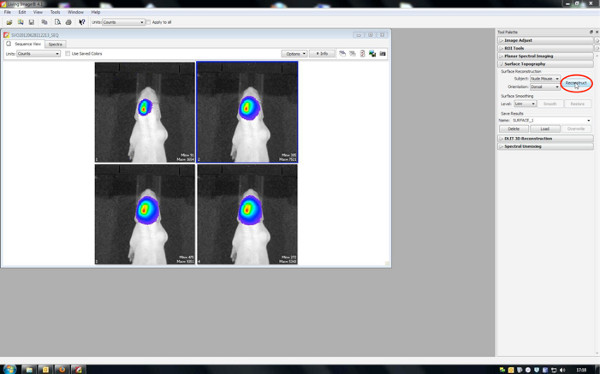
Click here to view larger figure.
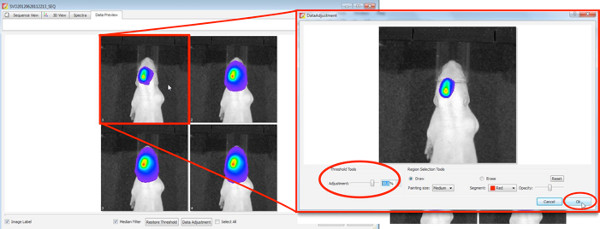
- Start DLIT 3D reconstruction with an image threshold of 10% for each wavelength.
Click here to view larger figure.
Click here to view larger figure.
Click here to view larger figure. - Create 3D movies by using the animate button within the 3D toolbar.
- Choose different orientations of the 3D image, as well as zooming views as key frames and press the 'Record' button, recording the changeover from one position/orientation to another.
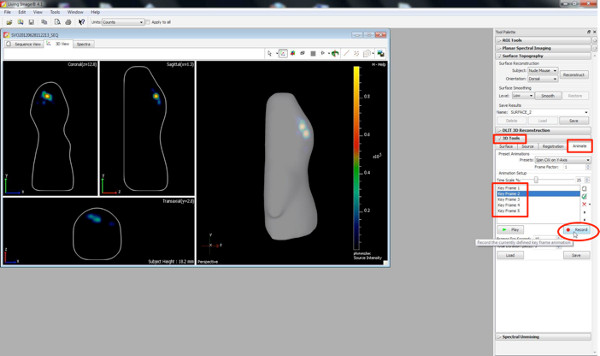
Click here to view larger figure.
- First, reconstruct a surface topography, therefore set threshold between 20-30%.
4. Behavioral Testing
Behavioral testing was performed in order to determine whether IUE-mediated gene manipulations in the rat might initiate long-term effects that persist into adulthood. In the present particular case, the effect of transient, unilateral full length human DISC1 cortical overexpression after IUE was investigated by testing locomotion in an open field (OF), with and without a low dose of amphetamine, as a specific test for dopamine-related behavior 24. In a similar procedure performed by Niwa et al. in IUE mice using DISC1 knockdown, IUE mice but not controls showed hypersensitivity to amphetamine18.
Rats that were in utero electroporated with a DISC1 overexpressing vector were held under laboratory conditions with 12 hr light from 7 am to 7 pm and were fed ad libitum. At 3 months of age, rats underwent behavioral testing.
For quantifying locomotion as a readout of amphetamine effects, an open-field of a Tru Scan activity system situated in a sound- and light-isolated chamber was used. This system measures the duration time and distance the animal moves, time and distance spent in the margin or center of the open field, as well as rearing behavior25.
- On the first day, test after saline injection
- Weigh the animals.
- Inject intraperitoneally 1 μl/g body weight of a saline solution (1x PBS).
- Right after the injection, put the animal into the open-field and start the measurement of the TruScan system. Record for 15 min and subdivide data into 3 x 5 min parts.
- Put the animal back into its home cage.
- Second day, test on amphetamine injection
- Weigh the animals.
- Inject intraperitoneally 1 μl/g body weight of a 0.5 mg/ml amphetamine solution.
- Right after the injection put the animal into the open-field and start the measurement of the TruScan system. Record for 15 min and subdivide data into 3 x 5 min parts.
- Return the animal to its home cage.
- Analyze locomotion and rearing behavior generated by specific Tru Scan software. Create Graphs with GraphPad (Prism) and calculate statistics by SPSS Statistics software.
Representative Results
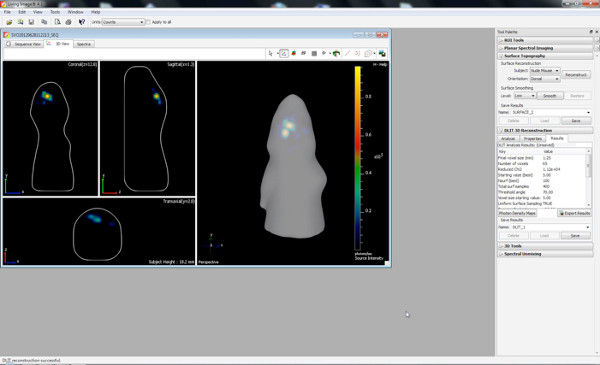
Figure 3 shows live imaging measurements of three rat pups at P7 after the injection of 150 mg luciferin/kg body weight. Differences of signal strength indicating the variability in the efficiency of the IUE are visible. Strong bioluminescence signals were recorded until P36 (Figure 4). In Figure 5, the ability to define cortical (Figures 5A and 5C) and hippocampal (Figures 5B and 5D) electroporation by bioluminescence imaging are depicted. Correlation of the bioluminescence signal (Figure 7A) with its fluorescence signal after skull removal (Figure 7B) and the corresponding GFP-electroporated cells in cryosectioned brain (Figure 8) at P14 are depicted in Figures 6-8. Of note, there was no detection of a fluorescence signal in live rats at any time point.
In vivo bioluminescence imaging enables approximate discrimination of different electroporated brain areas by 2D (Figures 5A and 5B) which is greatly improved with the DLIT program of the IVIS Spectrum software generating 3D images (Figures 5C and 5D). In the shown examples, electroporation of the prefrontal cortex and hippocampal could be distinguished. It should be noted that while aiming to electroporate the hippocampus, some progenitor cells for the cortex lying dorsal of the hippocampus can also be hit (Figure 7).
Rats unilaterally in utero electroporated with pCAX vector into the cortex and subsequently overexpressing full length human DISC1 were investigated for both spontaneous and amphetamine-induced hyperactivity as adults. Rats electroporated with pCAX-DISC1 were hypersensitive to a low dose of amphetamine. These rats moved significantly more after amphetamine treatment than after to saline injection, whereas control animals did not (Figure 10).
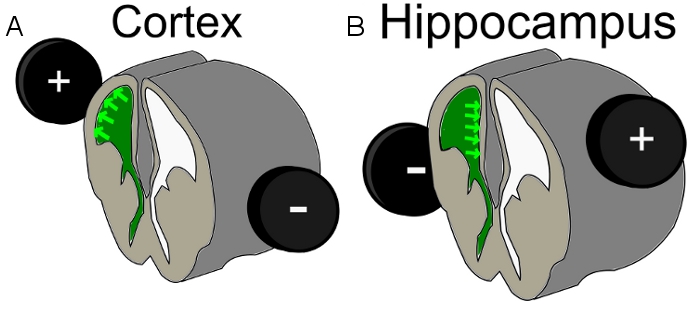
Figure 1. Scheme of electrode position for A) cortex electroporation and B) hippocampal electroporation; green = injected DNA-Mix within the ventricle.
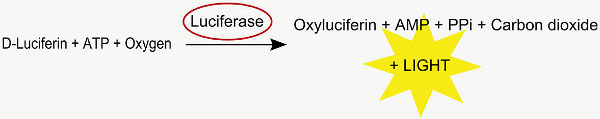
Figure 2. Luciferase reaction.
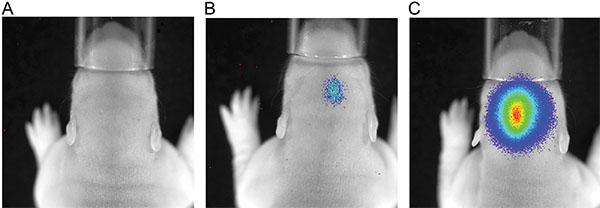
Figure 3. Luminescence measurement of P7 rats after injection of 150 mg/kg body weight luciferine; exposure time 180 sec; A) rat with no luminescence signal; B) rat with a weak bioluminescence signal; C) rat with a strong luminescence signal.
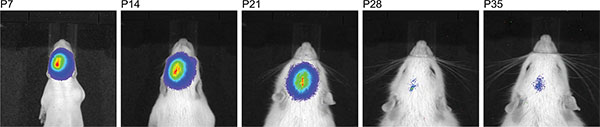
Figure 4. Time line of consecutive bioluminescence measurements of the same rat after injection of 150 mg/kg luciferine demonstrating a large time window where IUE success can be detected.
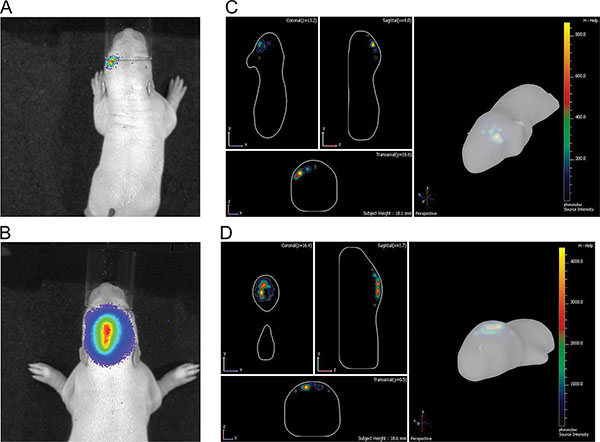
Figure 5. Illustration of differences between cortical and hippocampal electroporation at P7. A) & B) 2D pictures; C) & D) 3D pictures; A) & C) from cortex; B) & D) from hippocampus. Click here to view larger figure.
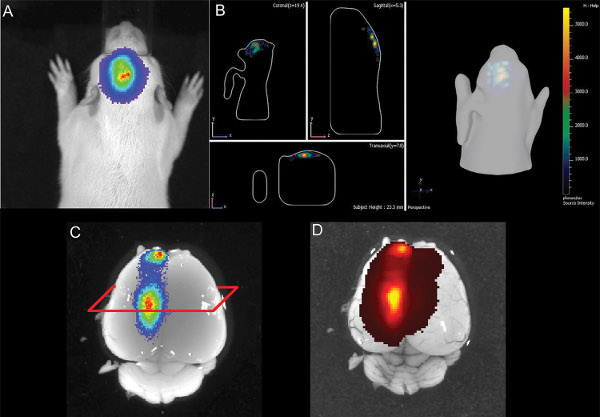
Figure 6. Illustration of E16 hippocampal electroporation of a rat pup at P14 A) 2D-picture of bioluminescence B) 3D illustration of the bioluminescence signal C) dissected brain with bioluminescence signal D) brain with GFP epi-fluorescence signal. Click here to view larger figure.
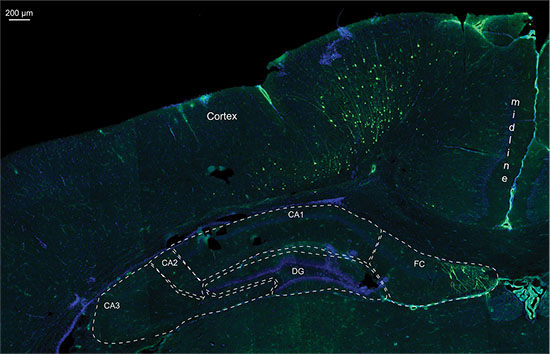
Figure 7. Detail of a fluorescence picture from a 20 μm cryo section of the same P14 rat brain electroporated with GFP containing plasmid and luciferase vector, nuclei staining with DAPI; CA1-3 = Cornu Ammonis 1-3; DG = Dentate Gyrus; FC = Fasciolarum cinereum.
Video 1. 3D animation of a hippocampus electroporated rat at P14.
Figure 8. Amphetamine test scheme: first day saline injection before the 15 min trial, 24 hr later amphetamine injection before testing in the open field chamber.
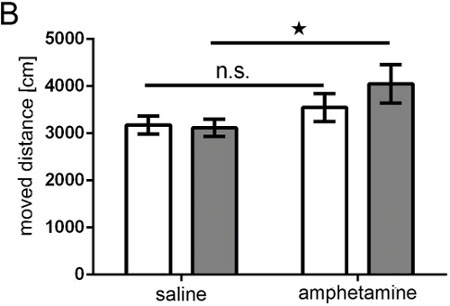
Figure 9. Amphetamine test. Bar graph showing moved distance (in cm) of the animal recorded by a TrueScan system over 15 min. White bars = control group; grey bars = DISC1 overexpressing group; control group n = 10; DISC1 overexpression group n = 11; ANOVA: geno*treatment p = 0.043; T-Test for total time saline vs amphetamine n.s.= not significant pcontrol = 0.172, pDISC1 = 0.001.
Discussion
Our study demonstrates that IUE is suited to generate adult rats with neurons expressing a transgene in a selective area of the brain and that, as a result of this intervention, these animals exhibit changes in behavior indicating functionality of the performed manipulation. In this study, as an example, rats overexpressing DISC1 unilaterally in a small part of the prefrontal cortex showed hypersensitivity towards amphetamine (Figure 9).
Selecting rats for electroporation success by in vivo bioluminescence imaging was effective in controlling for the inherent variability of IUE cell transfection and was applied to generate groups with a homogenous IUE area of low inter-subject variability for later investigations.
In this study, we were unable to select electroporated pups from the litter by detection of the co-electroporated GFP-induced fluorescence in the newborn animal, even though at the same time and in the same animal a bioluminescence signal of the equally co-electroporated luciferase could be detected after luciferin injection (Figure 6), and the GFP expressing neurons were still present in the brain at an age of six months. We conclude that, in the rat, the luciferase/luciferin reaction is well-suited to differentiate animals with successful electroporated brains (Figure 3).
The quantitative monitoring of IUE success relates to the strength of the bioluminescence signal which is measured by the counts of photons within the same exposure time (Figure 3) and corresponds to the enzymatic activity of co-expressed luciferase. Small bioluminescence signals are detectable by 100-200 counts of photons, and, at a radiance of ~ 1×104 photons/sec/cm2/steradian show 1,000-2,000 GFP-stained cells in the histology in the 6 months old rat brain. The highest signal displayed a radiance of up to ~5×106 photons/sec/cm2/steradian and ~64,000 counts.
In the Sprague Dawley rat strain used, we observed a weakening of the bioluminescence signal longitudinally with increasing age and the signal disappeared beyond the age of P35 (Figure 4). At this point, we do not know whether either the transient, plasmid vector-based expression of luciferase decreases, or if the bioluminescence signal weakens due to increasing brain mass, or both are the causes for the disappearing signal. For the present functional assay in the adult rats, the selection for behavioral studies was merely made based on the location of the signal, but not by bioluminescence signal strength.
Even though 3D quantitative bioluminescence monitoring allowed differentiation between different electroporated areas (Figure 5), its accurateness was limited for cells located in the depth dimension of the brain. Figure 6 shows an example of a hippocampal electroporation where the bioluminescence measurement in the 2D and the 3D picture indicated a good positioning of the electroporation. In the dissected post mortem brain, a GFP-fluorescence signal was detected at about the same position as the bioluminescence signal, indicating correct targeting of the hippocampus. But histology shows that also cells in the cortex dorsal of the hippocampus had been targeted (Figure 7). This indicates that the bioluminescence assay is a useful tool to detect positive, IUE pups and also to have an idea of the electroporated area, but, ultimately, imaging cannot replace post mortem histology to exactly localize positively targeted cells.
Our demonstration indicates promise for the application of the IUE technology to generate subtle targeted manipulations of cortical or hippocampal brain regions to simulate aberrances in cortical migration or other neurodevelopmental defects that may influence the adult animal. While bilateral electroporation26 has the advantage of a likely bigger effect on behavior, there is also more mortality of embryos. Unilateral electroporation was chosen in order to compare the two hemispheres with one as an internal control, as well as for showing that even IUE manipulating in an unilateral, small region is sufficient to change behavior. IUE-induced changes in connectivity or architecture between neurons may thus be induced without evoking a lesion and the required match of the to-be-IUE-manipulated region with the appropriate behavioral test is dependent on the scientific question.
Trouble shooting
Reduced litter size There are several suggestions regarding increasing the survival of the IUE pups. First, the use of very thin glass capillaries during electroporation in order to minimize tissue lesion is recommended. Second, do not electroporate the first embryo at the vaginal end of each uterus horn: death of the first-born embryo increases the chances of an abort of all other embryos. Third, after birth, mother rats often kill part of their progeny due to perinatal stress. In order to reduce additional stress, do not start with the live imaging right after birth, but wait for seven days.
GFP-fluorescence detection of the pups
At one week after birth, no signal of fluorescence by either using live binocular fluorescence microscopic imaging or fluorescence imaging with the IVIS Spectrum (epifluorescence and transfluorescence modes; for GFP excitation/emission: 465/520 nm and 500/540 nm). It is possible that both, the limited transmission of short wavelength excitation and emission light through tissue like the skull and the high autofluorescence background of the skin prevent using fluorescence under the described conditions in the rat. As shown in Figure 6, the luciferase signal in the living animal can also be detected in the dissected brain (without skull) and there, also a fluorescence signal is detectable (Figure 6D).
Differentiation of bioluminescence in closely spaced brain areas
Even in the 3D illustration the location of the bioluminescence area cannot be predicted to 100%. Especially cells on top of or below of the predicted area can also be accidentally targeted and transfected. The exact position has to be controlled by post mortem (fluorescence) histology (see Figure 7).
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors thank Tracy Young-Pearse and Atsushi Kamiya for providing plasmids.
This work was funded by NEURON-ERANET DISCover to O.R. and C.K. (BMBF 01EW1003), DFG (Ko 1679/3-1; GRK1033) to C.K., and (DE 792/2-4) to M.A.S.S.
Materials
| Reagent name | |||||||||||||||||||||||||||||||
| Dulbecco's Phosphate-Buffered Saline (PBS) | Invitrogen | 14190-250 | without calcium, without magnesium | ||||||||||||||||||||||||||||
| D-luciferin, sodium salt | SynChem OHG, Germany | BC218 | CAS number: 103404-75-7 substrate for firefly-luciferase | ||||||||||||||||||||||||||||
| Fast Green FCF | Sigma Aldrich, USA | F7258-25G | CAS: 2353-45-9 | ||||||||||||||||||||||||||||
| D-Amphetamine | Sigma Aldrich, USA | A 5880 | CAS: 51-63-8 | ||||||||||||||||||||||||||||
| kodan Tinktur forte | Schülke & Mayr GmbH, Germany | 104 005 | |||||||||||||||||||||||||||||
| Material / product | |||||||||||||||||||||||||||||||
| Glass capillaries | Sutter Instrument | Novato, California, USA | borosilicate glass O.D.:1 mm, I.D.: 0.78 mm | ||||||||||||||||||||||||||||
| Needle Pipette Puller | David Kopf Instruments | Tujunga, California, USA | |||||||||||||||||||||||||||||
| Tweezer electrode | Nepa Gene CO., LTD. | Shioyaki, Ichikawa, Chiba, Japan | 7 mm in diameter platinum disc electrodes (CUY650P7) | ||||||||||||||||||||||||||||
| Surgical Scissors – sharp | Fine Science Tools | Heidelberg, Germany | Straight, 12 cm (14002-12) | ||||||||||||||||||||||||||||
| Ring Forceps | Fine Science Tools | Heidelberg, Germany | 2.2 mm ID, 3 mm OD (11021-12) | ||||||||||||||||||||||||||||
| Square wave pulse electroporator (CUY21SC) | Nepa Gene CO., LTD. | Shioyaki, Ichikawa, Chiba, Japan | (CUY21SC) | ||||||||||||||||||||||||||||
| Vicryl surgical suture material | Ethicon | Norderstedt, Germany | 3-0; 2 Ph. Eur; | ||||||||||||||||||||||||||||
| Wound Clip Applicator | Fine Science Tools | Heidelberg, Germany | Reflex 9 mm (12032-09) | ||||||||||||||||||||||||||||
| Syringe filter | VWR | Darmstadt, Germany | 0.45 μm cellulose acetate | ||||||||||||||||||||||||||||
| IVIS Spectrum | Caliper Life Science / PerkinElmer | Waltham, MassachusettsUSA | |||||||||||||||||||||||||||||
| XGI-8 Gas Anesthesia System | PerkinElmer | Waltham, Massachuset tsUSA | |||||||||||||||||||||||||||||
| Open-field | Coulbourn Instruments | Allentown, USA | (40 x 40 x 39 cm) | ||||||||||||||||||||||||||||
| Tru Scan activity system | Coulbourn Instruments | Allentown, USA | |||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
References
- Saito, T., Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Biologie du développement. 240, 237-246 (2001).
- Fukuchi-Shimogori, T., Grove, E. A. Neocortex patterning by the secreted signaling molecule FGF8. Science. 294, 1071-1074 (2001).
- Walantus, W., Elias, L., Kriegstein, A. In Utero Intraventricular Injection and Electroporation of E16 Rat Embryos. J. Vis. Exp. (6), e236 (2007).
- Rice, H., Suth, S., Cavanaugh, W., Bai, J., Young-Pearse, T. L. In utero Electroporation followed by Primary Neuronal Culture for Studying Gene Function in Subset of Cortical Neurons. J. Vis. Exp. (44), e2103 (2010).
- Takahashi, M. Manipulating gene expressions by electroporation in the developing brain of mammalian embryos. Differentiation. 70, 155 (2002).
- Tabata, H., Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neurosciences. 103, 865-872 (2001).
- Nakahira, E., Yuasa, S. Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J Comp Neurol. 483, 329-340 (2005).
- Young-Pearse, T. L., et al. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 27, 14459-14469 (2007).
- Niwa, M., et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 65, 480-489 (2010).
- Sapir, T., et al. Accurate balance of the polarity kinase MARK2/Par-1 is required for proper cortical neuronal migration. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 28, 5710-5720 (2008).
- Weinberger, D. R. From neuropathology to neurodevelopment. Lancet. 346, 552-557 (1995).
- Murray, R. M., Lewis, S. W. Is schizophrenia a neurodevelopmental disorder?. British Medical Journal. 295, 681-682 (1987).
- Kamiya, A., et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 7, 1167-1178 (2005).
- Miyoshi, K., et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 8, 685-694 (2003).
- Mao, Y., et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 136, 1017-1031 (2009).
- Millar, J. K., et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 9, 1415-1423 (2000).
- Steinecke, A., Gampe, C., Valkova, C., Kaether, C., Bolz, J. Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience. 32, 738-745 (2012).
- Niwa, M., et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 65, 480-489 (2010).
- Taniguchi, Y., Young-Pearse, T., Sawa, A., Kamiya, A. In utero electroporation as a tool for genetic manipulation in vivo to study psychiatric disorders: from genes to circuits and behaviors. The Neuroscientist : a Review Journal Bringing Neurobiology, Neurology and Psychiatry 18. , 169-179 (2012).
- Bai, J., et al. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 6, 1277-1283 (2003).
- Osumi, N., Inoue, T. Gene transfer into cultured mammalian embryos by electroporation. Methods. 24, 35-42 (2001).
- Momose, T., et al. Efficient targeting of gene expression in chick embryos by microelectroporation. Development, Growth & Differentiation. 41, 335-344 (1999).
- LoTurco, J., Manent, J. B., Sidiqi, F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex. 19, 120-125 (2009).
- Featherstone, R. E., Kapur, S., Fletcher, P. J. The amphetamine-induced sensitized state as a model of schizophrenia. Progress in Neuro-psychopharmacology & Biological Psychiatry. 31, 1556-1571 (2007).
- Pum, M., Carey, R. J., Huston, J. P., Muller, C. P. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology. 193, 375-390 (2007).
- dal Maschio, M., et al. High-performance and site-directed in utero electroporation by a triple-electrode probe. Nature Communications. 3, 960 (2012).

