Ex Vivo Red Blood Cell Hemolysis Assay for the Evaluation of pH-responsive Endosomolytic Agents for Cytosolic Delivery of Biomacromolecular Drugs
Summary
A hemolysis assay can be used as a rapid, high-throughput screen of drug delivery systems’ cytocompatibility and endosomolytic activity for intracellular cargo delivery. The assay measures the disruption of erythrocyte membranes as a function of environmental pH.
Abstract
Phospholipid bilayers that constitute endo-lysosomal vesicles can pose a barrier to delivery of biologic drugs to intracellular targets. To overcome this barrier, a number of synthetic drug carriers have been engineered to actively disrupt the endosomal membrane and deliver cargo into the cytoplasm. Here, we describe the hemolysis assay, which can be used as rapid, high-throughput screen for the cytocompatibility and endosomolytic activity of intracellular drug delivery systems.
In the hemolysis assay, human red blood cells and test materials are co-incubated in buffers at defined pHs that mimic extracellular, early endosomal, and late endo-lysosomal environments. Following a centrifugation step to pellet intact red blood cells, the amount of hemoglobin released into the medium is spectrophotometrically measured (405 nm for best dynamic range). The percent red blood cell disruption is then quantified relative to positive control samples lysed with a detergent. In this model system the erythrocyte membrane serves as a surrogate for the lipid bilayer membrane that enclose endo-lysosomal vesicles. The desired result is negligible hemolysis at physiologic pH (7.4) and robust hemolysis in the endo-lysosomal pH range from approximately pH 5-6.8.
Introduction
Although there are many potential high-impact therapeutic targets inside the cell, the intracellular delivery of agents poses a significant challenge. Frequently, drugs, especially biologics, are internalized by cells and trafficked into vesicles that either lead to degradation of their contents through the endo-lysosomal pathway, or are shuttled back out of the cell via exocytosis.1 In the latter process, the internal pH of the vesicles is acidified to approximately 5-6, which is the optimal pH for activity of enzymes that function in this compartment, such as lysozyme.2
Recently, a number of materials have been specifically engineered to leverage the acidification of endosomes to facilitate cytosolic delivery of their cargo. One example of this approach uses synthetic, polymer micelle nanoparticles whose core is zwitterionic and charge-neutral at physiologic pH (i.e. 7.4). However, at pH 6.0 – 6.5, the polymers become protonated and acquire a net positive charge that destabilizes the micelle core, and the exposed polymer segments interact with and disrupt the endosomal membrane. This activity has been shown to promote the endosomal escape of peptide and nucleic acid-based therapeutics, allowing them to access their cytosolic targets.3,4 Other examples of methods developed to mediate endosomal escape that disrupt the membrane barrier include ‘fusogenic’ peptides or proteins that can mediate membrane fusion or transient pore formation in the phospholipid bilayer.5 Homopolymers of anionic alkyl acrylic acids such as poly(propylacrylic acid) are another well-studied approach, and in these polymers, the protonation state of pendant carboxylic acid dictates transition into a hydrophobic, membrane-disruptive state in endo-lysosomal pH ranges.6,7
One useful model system for screening endosomolytic behavior is the ex vivo pH-dependent hemolysis assay.8 In this model system, the erythrocyte membrane serves as a surrogate for the lipid bilayer membrane that enclose endo-lysosomal vesicles. This generalizable model has been used by others to evaluate the endosomolytic behavior of cell-penetrating peptides and other polymeric gene delivery systems.8-11 In this experiment, human red blood cells and test materials are co-incubated in buffers at defined pHs that mimic extracellular (7.4), early endosomal (6.8), and late endo-lysosomal (< 6.8) environments. The amount of hemoglobin released during the incubation period is quantified as a measure of red blood cell lysis, which is normalized to the amount of hemoglobin released in positive control samples lysed with a detergent.
From screening a small library of potentially endosomolytic test materials, one can infer that samples that produce no hemolysis at pH 7.4, but significantly elevated hemolysis at pH < 6.5, will be the most effective and cytocompatible candidates for cytosolic drug delivery. Materials that fit these criteria would be expected to remain inert and not indiscriminately destroy lipid bilayer membranes (i.e. that could cause cytotoxicity) until being exposed to a drop in the local pH following internalization into endo-lysosomal compartments.
In this protocol, erythrocytes are isolated from a human donor and co-incubated at pH 5.6, 6.2, 6.8, or 7.4 with experimental endosomolytic drug delivery agents. Intact erythrocytes are pelleted, and the supernatants (containing hemoglobin released from lysed erythrocytes) are analyzed for the characteristic absorbance of hemoglobin via a plate reader (Figure 1).
Protocol
1. Preparation and Sterilization of Buffers and Test Agents
- 150 mM NaCl buffer: Dissolve 4.383 g NaCl crystals in 500 ml of nanopure water.
- pH Buffers: Prepare phosphate buffers at pH 5.6, 6.2, 6.8, and 7.4 by mixing appropriate amounts of monobasic and dibasic sodium phosphate. If samples are to be tested at lower pH values (i.e. pH < 5.6) then a more appropriate buffer, such as citrate buffer, should be used. Buffer recipes are readily available, and an example reference has been provided here.12
- Sterilize all buffers noted above through a bottle-top vacuum filtration apparatus and re-check buffer pH’s.
- 20% Triton X-100 (positive control): Mix 20 ml pure Triton X-100 in 80 ml of nanopure water. Vortex vigorously and sonicate to dissolve. Leave at room temperature overnight before use.
2. Preparation of Erythrocytes
- Obtain 25 ml of blood from an anonymous human donor, drawn directly into K2-EDTA-coated Vacutainer tubes to prevent coagulation.
NOTE: All procedures must be pre-approved by the appropriate Institutional Review Board (IRB), and venipuncture and blood collection must be performed by a trained phlebotomist in order to minimize the risk to the donor. Standard phlebotomy procedures have been published elsewhere.13
- Centrifuge blood at 500 x g for 5 min, and mark levels of hematocrit (red, lower layer) and plasma (yellowish, upper layer) on tube.
- Aspirate plasma gently via a micropipettor, add into bleach, and discard into biohazardous waste.
- Fill hematocrit tube to marked line (original level of plasma) with 150 mM NaCl solution. Cap and invert a few times to gently mix. Centrifuge at 500 x g for 5 min.
- Repeat step 2.3-2.4 to wash blood cells again. Then aspirate supernatant and replace with PBS at pH 7.4. Invert to mix.
- Split blood evenly into four tubes, corresponding to each pH that will be tested. Label the tubes according to each pH to be tested (5.6, 6.2, 6.8, 7.4).
- Centrifuge blood tubes at 500 x g for 5 min. Mark levels on tubes, then aspirate supernatant.
- Fill each tube to marked line with buffer of appropriate pH (as indicated in 2.6).
- Label four 50 ml conical tubes (one per pH to test), and pipet 49 ml of PBS of appropriate pH into each conical tube.
- Add 1 ml of erythrocytes (same pH) into corresponding tube for a 1:50 dilution. Visually inspect the diluted blood, which should be turbid and will settle if left undisturbed. If no pellet forms, cells have lysed.
3. Lysis Assay 96 Well Plate Setup and Quantification
- Prepare stock solutions of all experimental drug delivery agents, at 20x the desired final concentration to be tested (Assay will take 10 μl of drug delivery agent + 190 μl diluted red blood cells, leading to a 1/20 dilution of the original drug delivery agent into the final test mixture). Stocks of 20, 100, and 800 μg/ml are suggested, resulting in final test concentrations of 1, 5, and 40 μg/ml, respectively.
- Pipet 10 μl of each stock solution into V-bottom 96-well plates. For optimal results, load each sample in triplicate or quadruplicate.
NOTE: For ease, it is recommended that a separate 96-well plate should be prepared for each pH to be tested, with each sample (at each concentration) loaded at n=3-4 per plate.
- For positive control wells, add 10 μl of 20% Triton X-100.
- For negative control wells, add 10 μl of phosphate buffer. Use buffer at the same pH to be tested.
- Pipet 190 μl of diluted erythrocytes (see 2.10) to each well, making sure cell stock solution remains homogenous during transfer. Hint: Use multi-channel pipette to simplify this task.
- Incubate plates at 37 °C for one hour (Optional: Use an orbital shaker or rocker).
- Centrifuge plates for 5 min at 500 x g to pellet intact erythrocytes. Note: when removing the plate from the centrifuge and transporting it to the next step, handle with care and be certain not to disrupt the cell pellet.
- Using a multichannel pipet, transfer 100 μl of supernatant from each well into a clear, flat-bottomed 96-well plate. Note: if the cell pellet is accidentally disturbed for any sample(s), one can re-centrifuge the plate and then proceed with supernatant transfer.
- Measure absorbance of supernatants with a plate reader. Note that a range of wavelengths can be used (400 – 541 nm).
NOTE: Different plate readers may have different saturation points and sensitivities, so the choice of a wavelength for measurements depends on whether or not hemolysis data from experimental samples can be reliably normalized against maximum hemolysis as induced by detergent treatment. This requires accurate measurement of absorbance values of the positive control samples.
- Using Microsoft Excel or a similar data analysis software, find the average of the background absorbance readings from the negative control samples set up for each pH (step 3.4). Subtract this background absorbance value from all other samples that were measured at that pH.
- After background subtraction, find the average absorbance of the positive control detergent-treated samples (step 3.3). Then normalize all experimental data points to this mean absorbance value, which should represent 100% hemolysis. Finally, multiply each well value by 100% to calculate % hemolysis that occurred in each individual well relative to the detergent control.
Representative Results
Typically, the agents that exhibit ideal pH-dependent hemolytic behavior have the highest potential for cytosolic delivery of drugs, nucleic acids, or other bioactive molecules. This is exemplified by Agent #1 as portrayed in Figure 2, which exhibits minimal hemolysis at pH 7.4, but a sharp increase in hemolytic behavior at endosomal pH ranges (< 6.5). Some agents may exhibit significant levels of hemolytic behavior at physiological pH ranges (Agent #2 at 40 μg/ml; Figure 2), suggesting that these agents may not be hemocompatible and could potentially be cytotoxic at these concentrations.
In most cases, hemolysis is also dose-dependent, as increasing concentrations of the test materials correspond with higher levels of hemolysis, especially at the lower pH ranges tested (5.6 – 6.2).
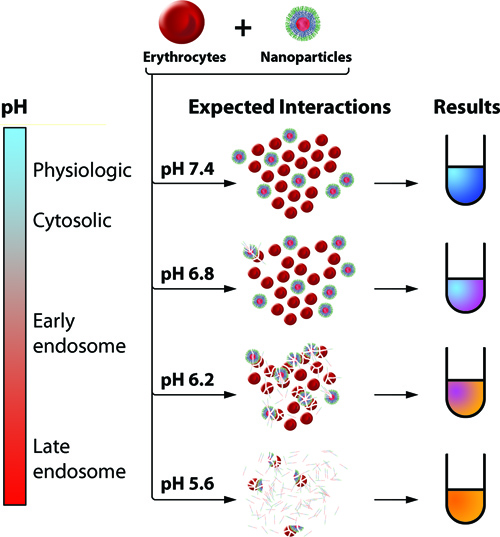
Figure 1. Schematic Diagram of Red Blood Cell Hemolysis Assay. Human erythrocytes are isolated and incubated with experimental endosomolytic drug delivery agents in a series of buffers simulating the pH range from physiologic (7.4) to late endosomes/lysosomes (5.6). The optimal drug delivery agents will not disrupt the erythrocytes at physiologic pH but will exhibit robust hemolysis in more acidic conditions. To assess percent hemolysis, the release of hemoglobin into the surrounding medium is measured via absorbance on a plate reader.
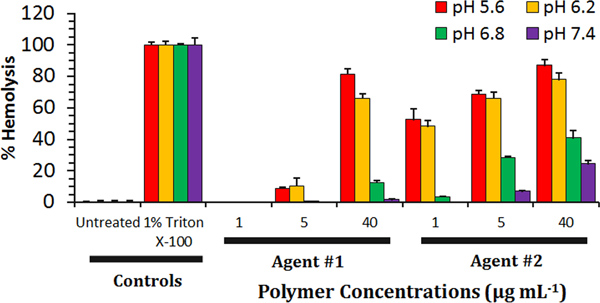
Figure 2. Representative Results of a Hemolysis Assay Demonstrating Behavior of Two Experimental Endosomolytic Agents. First, the average A450 of the vehicle (PBS) control was subtracted from the other samples tested at that pH. Afterward, experimental samples were normalized to the A450 of Triton X-100-treated erythrocyte samples and multiplied by 100%. Based on these control samples, the ability of experimental transfection agents to lyse erythrocytes can be calculated. Typically, ideal endosomolytic agents exhibit dose-dependent and pH-dependent hemolytic behavior. Ideal agents (such as Agent #1) exhibit minimal hemolysis at pH 7.4, but a sharp increase in hemolytic behavior at endosomal pH ranges (< 6.5). Some agents exhibit substantial hemolytic behavior at physiological pH ranges (Agent #2 at 40 μg/ml), suggesting that these agents may be cytotoxic at these concentrations. Error bars indicate standard deviation of 4 independent measurements.
Discussion
pH-responsive polymers or other agents designed for endosomolytic function can be rapidly and effectively screened based on lysis of red blood cells at pH values encountered in the endosome (Figure 1; pH 6.8 – early endosome, pH 6.2 – late endosome, pH 5.6 – lysosome).14-17 pH-dependent hemolysis has been used to screen the ability of carriers to mediate endosomal release of biomacromolecular therapeutics (e.g. peptides, siRNA, ODNs, proteins), and results of this assay can be predictive of performance as an intracellular drug delivery vehicle.3,4,8,18,19 Thus, this assay represents an effective screen to gauge the ability of polymeric drug carriers to mediate intracellular drug delivery based on their pH-dependent membrane disruption.
As noted in the procedure, hemolysis is detected through spectrophotometric measurement of the supernatants of red blood cells treated with experimental agents. Therefore, in addition to hemoglobin, it is likely to contain other erythrocyte-derived cytosolic components, including proteins and carbohydrates. While these other components may contribute a small amount of signal to the spectrophotometric measurement, 100% hemolysis was calibrated to the erythrocyte lysate resulting from treatment with Triton X-100. Assuming all erythrocytes from the same donor contain similar levels of hemoglobin and other biomolecules, we can safely conclude that the ‘contaminating’ components will not contribute any artifacts to the hemolysis measurement, especially if the same blood sample is used for all tests. However, this highlights the possibility that, for any given blood donor, there are likely to be small day-to-day variations in blood composition and hematocrit, and therefore, internal control samples (steps 3.3-3.4, 3.10-3.11) should be analyzed for all experiments.
One should also always closely examine the raw absorbance data. Though it cannot be appreciated in the normalized example data shown in Figure 2, detergents such as Triton X-100 effectively destabilize erythrocyte membranes regardless of pH, and one should expect to see very little sample to sample variability in positive controls. The absorbance spectrum of Triton X-100 does not include peaks in the 400-600 nm range recommended for this assay, and therefore, should not interfere with the normalization of experimental data.20 Furthermore, for negative control samples, one should not observe significant hemolysis after the 1 hr incubation in any of the buffers used (i.e. even the most acidic buffer does not typically generate hemolysis on this timeframe). Background readings on negative control samples should closely approximate background absorbance readings taken on the fresh buffers.
Ideally, researchers will employ complementary strategies to characterize their experimental drug delivery systems. The hemolysis assay is advantageous for initial endosomolytic agent screening on naturally-occurring biomembranes, but should be considered as only one of the many tools in the drug delivery researcher’s armamentarium for testing cytosolic delivery agents. For example, one potential shortcoming of the hemolysis assay is that it utilizes the red blood cell membrane as a biological model for endosomal membranes. However, the make-up and lipid content of endosomal membranes varies by cell-type and may not be accurately recapitulated by the blood cell membrane.1 A variety of other, complementary assays have been developed to mimic endosomal membrane composition and behavior.21,22 One alternative to is to utilize liposomes containing fluorescence resonance energy transfer (FRET)-quenched fluorophores, which become unquenched following destabilization of the liposomes and release of the fluorophores to the surrounding media. In studies that employ this method, the quantification of unquenched fluorophores has been found to correlate with the ability of a vehicle to mediate endosomal escape of its payload.21,23 Microscopy-based measurements can provide a more robust but lower-throughput method that is complementary to the hemolysis assay. For example, it is common to assess colocalization of the carrier or the drug itself with dye-labeled lysosomes (e.g. LysoTracker by Life Technologies), or the trafficking pathways can be characterized using pH-sensitive dyes conjugated the carrier or drug (e.g. pHrodo by Life Technologies).24-26
Once an endosomolytic delivery system has been confirmed to achieve cytosolic cargo delivery, the hemolysis assay can also provide information on the mechanism through which endosomal escape occurs. For example, gene delivery vehicles based on polyethyleneimine (PEI) lack an inherent ability to disrupt phospholipid membranes at neutral or acidic pH’s.11,27 Instead, PEI achieves cytosolic gene delivery through a ‘proton sponge’ effect. After internalization, PEI buffers the endosome by “absorbing” protons that are pumped across the endosomal membrane to acidify these compartments. Eventually, this leads to buildup of excess protons and their counter-ions inside the endosome. This results in a rise in osmotic pressure, water influx, vesicle swelling, and endosomolysis. Therefore, the success of proton sponge effect necessitates the accumulation of a critical concentration of PEI into an endosome.27 Delivery vehicles that achieve endosomal disruption through the ‘proton sponge’ effect will not physically disrupt red blood cells or liposomes, and their efficacy in achieving intracellular drug delivery must be assessed through osmotic pressure calculations, or in vitro microscopy, or functional studies.
In conclusion, the hemolysis assay described here is a reliable model for screening pharmaceutical agents designed for intracellular delivery of biologic drugs. This assay provides a high throughput means of drug delivery vehicle screening, enabling the rapid development of formulations that deliver biologics with intracellular targets.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge funding through the Department of Defense Congressionally Directed Medical Research Programs (#W81XWH-10-1-0445), National Institutes of Health (NIH R21 HL110056), and American Heart Association (#11SDG4890030).
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| BD Vacutainer – K2EDTA Vacutainer Tubes | Fisher Scientific | 22-253-145 | For blood collection |
| BD Vacutainer Blood Collection Needles, 20.5-gauge | Fisher Scientific | 02-665-31 | For blood collection |
| BD Vacutainer Tube Holder / Needle Adapter | Fisher Scientific | 22-289-953 | For blood collection |
| BD Brand Isopropyl Alcohol Swabs | Fisher Scientific | 13-680-63 | For blood collection |
| BD Vacutainer Latex-Free Tourniquet | Fisher Scientific | 02-657-6 | For blood collection |
| Hydrochloric acid (conc.) | Fisher Scientific | A144-500 | For adjustment of pH of D-PBS. |
| Triton X-100 | Sigma-Aldrich | T8787 | Positive control |
| Dulbecco’s PBS | Invitrogen | 14190 | |
| Nalgene MF75 Sterile Disposable Bottle-Top Filter Unit with SFCA Membrane | Fisher Scientific | 09-740-44A | |
| BD 96-well plates, flat-bottomed, tissue culture-treated polystyrene | Fisher Scientific | 08-772-2C | For plate-reading at the end of the assay. |
| BD 96-well plates, round-bottomed, tissue culture-treated polystyrene | Fisher Scientific | 08-772-17 | For incubation of red blood cells with experimental agents. |
References
- Alberts, B., et al. . Molecular Biology of the Cell. , (2002).
- Boasson, E. H. On the Bacteriolysis by Lysozyme. The Journal of Immunology. 34, 281-293 (1938).
- Convertine, A. J., Benoit, D. S., Duvall, C. L., Hoffman, A. S., Stayton, P. S. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control. Release. 133, 221-229 (2009).
- Duvall, C. L., Convertine, A. J., Benoit, D. S., Hoffman, A. S., Stayton, P. S. Intracellular Delivery of a Proapoptotic Peptide via Conjugation to a RAFT Synthesized Endosomolytic. 7, 468-476 (2010).
- Varkouhi, A. K., Scholte, M., Storm, G., Haisma, H. J. Endosomal escape pathways for delivery of biologicals. Journal of Controlled Release. 151, 220-228 (2011).
- Plank, C., Oberhauser, B., Mechtler, K., Koch, C., Wagner, E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. Journal of Biological Chemistry. 269, 12918-12924 (1994).
- Ratner, A. J., et al. Epithelial Cells Are Sensitive Detectors of Bacterial Pore-forming Toxins. Journal of Biological Chemistry. 281, 12994-12998 (2006).
- Saar, K., et al. Cell-penetrating peptides: A comparative membrane toxicity study. Analytical Biochemistry. 345, 55-65 (2005).
- Kichler, A., Leborgne, C., Coeytaux, E., Danos, O. Polyethylenimine-mediated gene delivery: a mechanistic study. The Journal of Gene Medicine. 3, 135-144 (2001).
- Behr, J. -. P. The Proton Sponge: a Trick to Enter Cells the Viruses Did Not Exploit. CHIMIA International Journal for Chemistry. 51, 34-36 (1997).
- Dawson, R. M. C., Elliot, D. C., Elliot, W. H., Jones, K. M. . Data for Biochemical Research. , (1986).
- Ernst, D. J. . Applied Phlebotomy. , (2005).
- Bulmus, V., et al. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. Journal of controlled release : official journal of the Controlled Release Society. 93, 105-120 (2003).
- Lackey, C. A., et al. Hemolytic Activity of pH-Responsive Polymer-Streptavidin Bioconjugates. Bioconjugate Chemistry. 10, 401-405 (1999).
- Murthy, N., Campbell, J., Fausto, N., Hoffman, A. S., Stayton, P. S. Bioinspired pH-responsive polymers for the intracellular delivery of biomolecular drugs. Bioconjugate chemistry. 14, 412-419 (2003).
- Murthy, N., Robichaud, J. R., Tirrell, D. A., Stayton, P. S., Hoffman, A. S. The design and synthesis of polymers for eukaryotic membrane disruption. Journal of controlled release : official journal of the Controlled Release Society. 61, 137-143 (1999).
- Yu, H., et al. Overcoming endosomal barrier by amphotericin B-loaded dual pH-responsive PDMA-b-PDPA micelleplexes for siRNA delivery. ACS nano. 5, 9246-9255 (2011).
- Nelson, C. E., et al. Sustained local delivery of siRNA from an injectable scaffold. Biomaterials. 33, 1154-1161 (2012).
- Miozzari, G. F., Niederberger, P., Hütter, R. Permeabilization of microorganisms by Triton X-100. Analytical Biochemistry. 90, 220-233 (1978).
- Chen, H., Zhang, H., McCallum, C. M., Szoka, F. C., Guo, X. Unsaturated Cationic Ortho Esters for Endosome Permeation in Gene Delivery. Journal of Medicinal Chemistry. 50, 4269-4278 (2007).
- Roth, C. M. Quantitative Measurements and Rational Materials Design for Intracellular Delivery of Oligonucleotides. Biotechnology Progress. 24, 23-28 (2008).
- Blumenthal, R., Seth, P., Willingham, M. C., Pastan, I. pH-dependent lysis of liposomes by adenovirus. Biochimie. 25, 2231-2237 (1986).
- Moore, N. M., Sheppard, C. L., Barbour, T. R., Sakiyama-Elbert, S. E. The effect of endosomal escape peptides on in vitro gene delivery of polyethylene glycol-based vehicles. The Journal of Gene Medicine. 10, 1134-1149 (2008).
- Panyam, J., Zhou, W. Z., Prabha, S., Sahoo, S. K., Labhasetwar, V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. The FASEB Journal. 16, 1217-1226 (2002).

