Simultaneous EEG Monitoring During Transcranial Direct Current Stimulation
Summary
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that has shown initial therapeutic effects in several neurological conditions. The main mechanism underlying these therapeutic effects is the modulation of cortical excitability. Therefore, online monitoring of cortical excitability would help guide stimulation parameters and optimize its therapeutic effects. In the present article we review the use of a novel device that combines simultaneous tDCS and EEG monitoring in real time.
Abstract
Transcranial direct current stimulation (tDCS) is a technique that delivers weak electric currents through the scalp. This constant electric current induces shifts in neuronal membrane excitability, resulting in secondary changes in cortical activity. Although tDCS has most of its neuromodulatory effects on the underlying cortex, tDCS effects can also be observed in distant neural networks. Therefore, concomitant EEG monitoring of the effects of tDCS can provide valuable information on the mechanisms of tDCS. In addition, EEG findings can be an important surrogate marker for the effects of tDCS and thus can be used to optimize its parameters. This combined EEG-tDCS system can also be used for preventive treatment of neurological conditions characterized by abnormal peaks of cortical excitability, such as seizures. Such a system would be the basis of a non-invasive closed-loop device. In this article, we present a novel device that is capable of utilizing tDCS and EEG simultaneously. For that, we describe in a step-by-step fashion the main procedures of the application of this device using schematic figures, tables and video demonstrations. Additionally, we provide a literature review on clinical uses of tDCS and its cortical effects measured by EEG techniques.
Introduction
Transcranial direct current stimulation (tDCS) is a technique that uses weak and direct electric currents delivered continuously through the scalp to induce changes in cortical excitability 1, 2. Using motor evoked potentials as a marker of motor cortex excitability, Nitsche and Paulus3 demonstrated that the direction of the tDCS effects over the brain is polarity-specific: cathodal stimulation induces a decrease in cortical excitability, whereas anodal stimulation induces an increase in cortical excitability. This effect on cortical excitability can last for over an hour following stimulation. These tDCS-induced changes in cortical excitability can result in significant behavioral effects. One important issue is the variability of tDCS effects on behavior. There are several reasons to explain this variability. Studies on fMRI 4 and electroencephalography (EEG) 5,6 reveal that although tDCS has the most activating effect on the underlying cortex, the stimulation evokes widespread changes in other regions of the brain. In addition, it has been shown that tDCS effects depend on the state of baseline cortical activity 7. Therefore, given these sources of variability, the use of better surrogates to measure the effects of tDCS is desirable.
In this context, we propose the use of concomitant EEG monitoring to provide real-time data on the impact of tDCS on cortical excitability for several reasons. First, to optimize the stimulation parameters of tDCS. Second, to provide insights into new targets for therapies. Third, to assure safety during brain stimulation, especially in children. Fourth, to aid in the early detection and treatment of seizures in patients with intractable epilepsy i.e. closed-loop system. Finally, this device might also have a potential application in brain-computer interface systems.
Due to the critical role of monitoring cortical excitability changes related to non-invasive brain stimulation, the purpose of this article is to demonstrate how to combine the use of tDCS with EEG by means of a novel device (StarstimÒ – Neuroelectrics Instrument Controller, v 1.0; Rev 2012-08-01, Neurolelectrics, Barcelona, Spain). It should be noted that this article does not provide details of tDCS application. For a complete understanding of the application of this technique we recommend reading the article on tDCS from DaSilva et al. 11
Protocol
1. Materials
- Check that if all materials are available (Figure 1) before starting the following steps.
- There are 3 sizes of neoprene caps, depending on the size of the subjects’ head (small, medium and large). The cap has 27 holes representing EEG positions based on the 10/20 system: prefrontal (F8, AF8, Fp2, Fpz, Fp1, AF7, F7), frontal (F4, Fz, F3), central (C3, C1, Cz, C2, C4), parietal (P7, P3, Pz, P4, P8), temporal (T7, T8) and occipital (PO7, O1, Oz, O2, PO8).
- The electrodes have 2 different uses; they can be used for the EEG (six channels) and for tDCS (two channels for sponge-electrodes, the anode and the cathode). In some circumstances, more than two sites of stimulation can be used. In this case four sponge-electrodes will be required and consequently, only 4 channels will remain for EEG recordings.
- The variation of the tDCS electrodes size leads to a variation of focal effects 11. With a decrease of electrode dimension, a more focal stimulation can be achieved. On the other hand, by increasing electrode size it is possible to have a functionally ineffective electrode. The most commonly used proportions are 25 cm2 (5 cm x 5 cm) or 35 cm2 (5 cm x 7 cm). In this paper, sponge-electrodes of 25 cm2 will be used.
- All the electrodes have to be connected to the Control Box device through the wires. This device has to be charged periodically using the Control Box Battery charger. For safety reasons, it is not possible to charge the Control Box during active stimulation.
- The USB for Bluetooth connection is needed to pair the Control Box to the laptop/computer (see below).
2. Skin Preparation
- Inspect the skin for any pre-existing lesions – avoid electrical stimulation/EEG recording over damaged skin or over skull lesions.
- To increase conductance, move hair away from the site of electrical stimulation/EEG registering and place plastic hair clips to keep hair away, clean the surface of the skin to remove any signs of lotion, dirt, grease, etc. and allow it to dry.
3. Head Measurements
- Find and mark the localization of the Vertex or Cz (Figure 2), by measuring the distance of nasion to inion and marking halfway using a skin marker 11.
4. Electrodes Positioning in the Cap
- Put saline solution on the tDCS sponge-electrodes. The sponge-electrodes should be soaked with saline solution 11 before wearing the head cap. For a 25-35 cm2 sponge, approximately 6 ml of solution per side should suffice. It is important to periodically refill the sponge-electrode with saline solution in the case of a prolonged stimulation protocol.
- The EEG and the tDCS electrodes have to be fixed in the cap before the subject is physically wearing it.
- For further details on general tDCS electrodes preparation and positioning see 11.
5. Wearing the Cap and Fixing the Control Box on it
- Make sure the subject is seated comfortably.
- Place the cap in a way that the Vertex (measured on the head) matches the Cz point on the cap. Important: this is only valid for average size heads. Three different cap sizes are available, if necessary.
- Fill the EEG electrodes with gel using a curved syringe.
- Connect EEG and tDCS electrodes to the Control Box wires. The Control Box has to be fixed to the posterior part of the cap. Use channels 1 and 2 for stimulation and the remaining ones (3 to 8) for EEG recording. Their position in the cap will depend on the desired experimental approach for both recording and stimulation (Table 1). As a demonstration, the classical left anodal tDCS set up will be displayed: anode = M1; cathode = Supraorbital contralateral. For this montage, connect the anode (red sponge-electrode) to the C3 and the cathode (black sponge-electrode) to Fp2.
- Put the reference electrodes to one of the mastoids making sure they do not touch one another and attached them to the wires (CMS, Common Mode Sense and DRL, Driven Right Leg) from the Control Box.
6. Stimulation and Recording Set Up
- In order to configure parameters of stimulation and check recording, the software needs to be properly installed according to the manufacturer’s instruction.
- Press “STIMULATION” in the horizontal bar on upper screen (Figure 3).
- Select the option “EDIT” in the upper screen and choose “tDCS” or “sham” out of other electrical stimulation techniques, such as “transcranial alternating current stimulation (tACS)” and “transcranial random noise stimulation” (tRNS) (Figure 3a). The details of such approaches is out of the scope of this paper and are better discussed elsewhere 12, 13.
- Choose the total duration of the electrical stimulation, usually 20 min (Figure 3b) and at intensity of 2mA. Note: the device is capable of stimulating electrically and recording EEG signals for up to 1 hr, if needed.
- Choose the electrode positioning according to channels (Figure 3c).
- Configure tDCS and EEG channels (Figure 3d) according to the experimental approach (Table 1). The reference electrodes are labeled as DRL and CMS. Be sure to select the right function for each channel. Important: label the active stimulation electrode as “anode” or “cathode” and its reference as “return” (Figure 3d).
- In the bar menu located in the lower part of the screen choose the duration of the ramp down and ramp up period, usually 30 sec (Figure 3e). During this step you will also select the duration of pre- and post EEG recordings (Figure 3f). The EEG recording is not dependent on the stimulation and can be programmed to start before, during or after the end of the tDCS.
- To check electrode impedance press “STIMULATION” in the upper part of the screen and then “MOUNT” in the left side of the screen and then “START IMPEDANCE CHECK” (Figure 4).
7. Start the Device
- The subject should be relaxed, comfortable and awake during the procedure.
- Press “LAUNCH” in the lower part of the screen (Figure 5a).
- Check if the vertical gray bar is moving forward before (Figure 5b), during (5c) and after (5d) the tDCS.
- Re-check electrode impedances (Figure 5e).
- Press “Abort” to suspend the stimulation at any moment, if needed (Figure 5f).
8. Record EEG Data
- Press “EEG” in the upper screen to check if the EEG signals are visible and without any artifacts (Figure 6, yellow bracket). The signals can be filtered from 2 to 15 Hz in order to clarify the EEG traces.
- EEG recording will start automatically as soon as the icon LAUNCH is pressed.
- During the stimulation the ongoing EEG can be checked in three different panels, localized at the vertical menu bar (Figure 6).
- Time domain (Figure 6): see the data as it is being received, choosing different time and voltage scales.
- Spectrum (Figure 7): select a channel and visualize the power spectrum online i.e. the screen shows the power of each EEG frequency by real-time Fast Fourier Transform (FFT) analysis.
- Spectrogram (Figure 8): visualize the power spectrogram online by getting the information about the frequency content of the recorded EEG as a function of time (time-frequency analysis).
- In any of the aforementioned options the researcher is able to filter the EEG activity (Figure 6, yellow rectangle) into specific frequency bands (Table 2). Most studies addressing the effects of tDCS on EEG activity have used this approach for data analysis (Table 3).
Representative Results
tDCS is currently being investigated as a therapeutic instrument for varied neurological conditions, which includes major depression 14, 15, post-traumatic stress disorder 16, craving for food 17, marijuana 18, alcohol 19 and smoking 20, as well as pain 21, tinnitus 22, migraine 23, epilepsy 24, Parkinson’s disease 25, 26, stroke rehabilitation 27, 28 and cognitive dysfunction 6, 29. Table 1 shows the evidence-based tDCS electrode montages to be used as treatment for different clinical conditions.
In most cases, clinical improvement after tDCS is mainly attributed to its cortical effects. There are several ways to quantify cortical changes and the most frequently used ones are functional magnetic resonance imaging (fMRI), TMS-indexed cortical excitability and the electroencephalography (EEG). In comparison with fMRI, EEG has poorer spatial resolution, but superior temporal resolution 30, reflecting timing of neuronal activity more accurately. In addition, as compared with TMS-indexed cortical excitability, EEG provides a greater spatial resolution. For instance, using the tDCS/EEG device, it is possible to detect ongoing changes on the raw EEG in response to tDCS. Figure 9 shows the attenuation of cortical activity, mainly on the parietal region, after the tDCS was turned on (channels C3 and C4). Note that during stimulation it is not possible to record brain activity in the same channels used for stimulation.
The effects of tDCS on EEG have been recently studied by several authors (see Table 3), but only one has applied tDCS and EEG concomitantly 31. Most of the studies showed significant EEG changes upon tDCS by analyzing the EEG power spectrum in response to active versus sham-tDCS. Using power spectrum analysis, EEG signals can be decomposed into a sum of pure frequency components using FFT analysis. In this way, the signals can be analyzed in terms of its power spectrum, which provides information on the signal’s power at each frequency (Table 2).
Figure 7 shows a representative example of an ongoing EEG activity during tDCS (red bracket on the bottom) and after FFT analysis (red circle). The first peak activity corresponds to theta (5-7 Hz) and the second to alpha (8-10 Hz) band frequencies. The amplitude of EEG peaks is measured in μV2.
Another example comes from the study by Maeoka et al. 36, in which the authors found a local decrease in alpha and an increase in beta band amplitudes after anodal stimulation of the dorsolateral prefrontal cortex combined with emotional stress.
Figure 10 shows an illustrative example of the effects of tDCS on quantitative EEG (power spectrum). The size of frontal alpha amplitude was significantly higher in response to active-tDCS when compared to sham-tDCS of the left dorsolateral prefrontal cortex.
Therefore, using the automatic FFT analysis (Figure 7) the investigator is able to determine and measure the amplitude of the predominant EEG frequency activities (delta, theta, alpha, beta, gamma) during and after tDCS. Depending on the region of stimulation and other experimental conditions, the amplitude of specific EEG frequency bands is expected to change after tDCS (Table 3). Indeed, adding the FFT analysis function to the EEG recording during tDCS offers a unique opportunity to understand the cortical neuromodulatory effects in real time.
Finally, EEG signals can be analyzed with a technique called a time-frequency based, or spectrogram image. This technique has been considered promising for research purposes; however, this type of EEG analysis is still not fully validated for diagnostic intentions and should be interpreted with caution for this purpose 8.
Figure 8 shows an illustrative example of an EEG spectrogram processed by the same device.
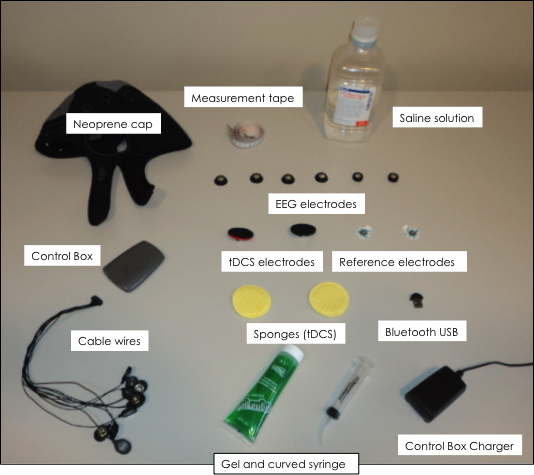
Figure 1. List of required materials for simultaneous EEG monitoring during tDCS: neoprene cap, Control Box, cables, electrodes, measurement tape, saline solution and Bluetooth USB.
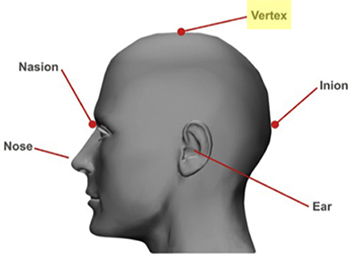
Figure 2. Localization of vertex (Cz) on the scalp 11: Measure the distance of nasion to inion and mark halfway using a skin marker.
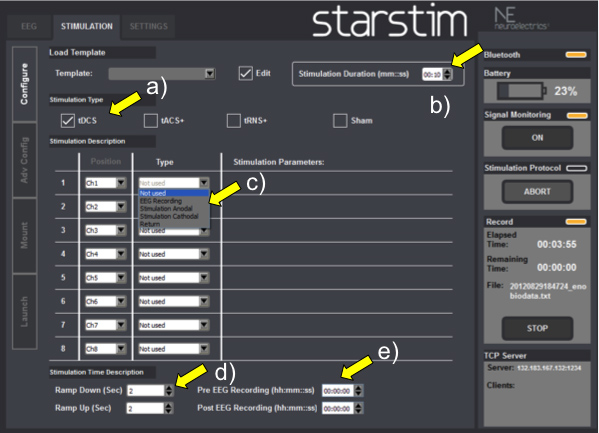
Figure 3. Stimulation Screenshot: a) Electrical stimulation mode (tDCS, tACS, tRNS, sham); b) Total duration of electrical stimulation; c) Electrode positioning according to channels; d) tDCS and EEG channel configuration; e) tDCS ramping duration; f) EEG recording durations.
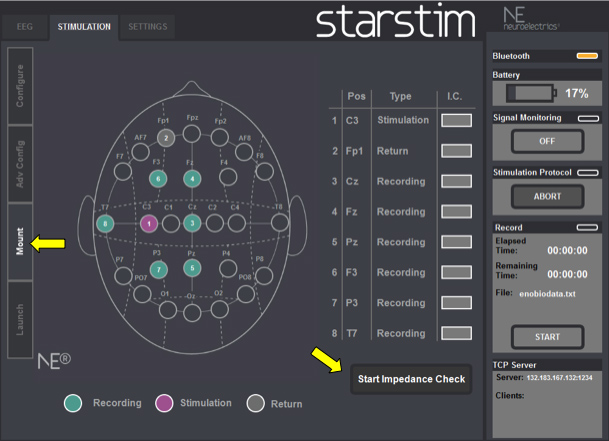
Figure 4. Mount Screenshot: Check electrodes impedance before stimulation begins.
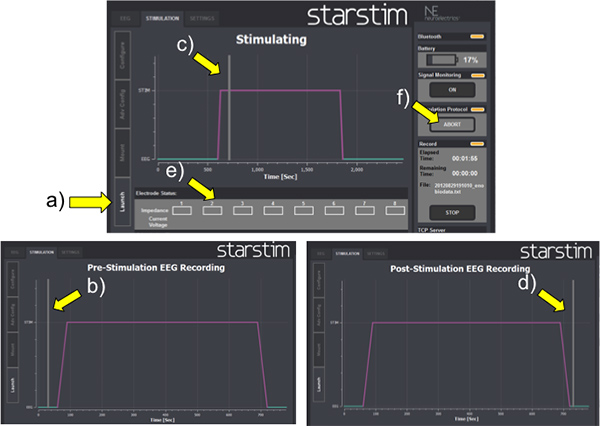
Figure 5. Launch Screenshot: a) LAUNCH button; b) Vertical gray bar before tDCS; c) Vertical gray bar during tDCS; d) Vertical gray bar after tDCS; e) Impedance re-checking; f) ABORT button.
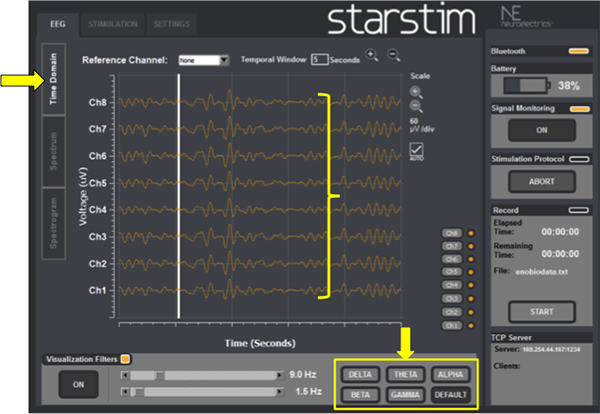
Figure 6. EEG Time domain: check the baseline ongoing EEG activity and select EEG band frequencies if needed (yellow arrow at the right bottom).
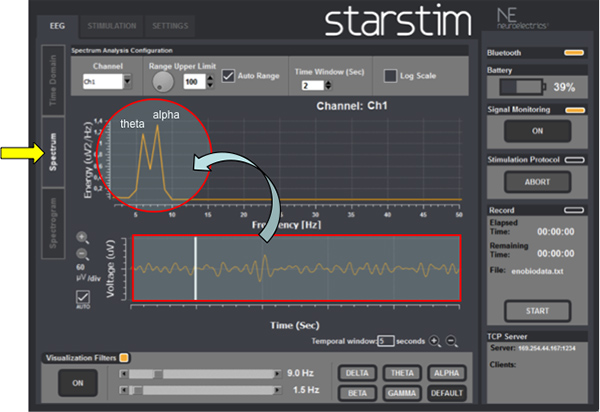
Figure 7. EEG power spectrum: check the predominant EEG frequency band (red circle) after automatic Fast Fourier Transform (FFT) analysis over the raw ongoing EEG activity (red rectangle on the bottom).
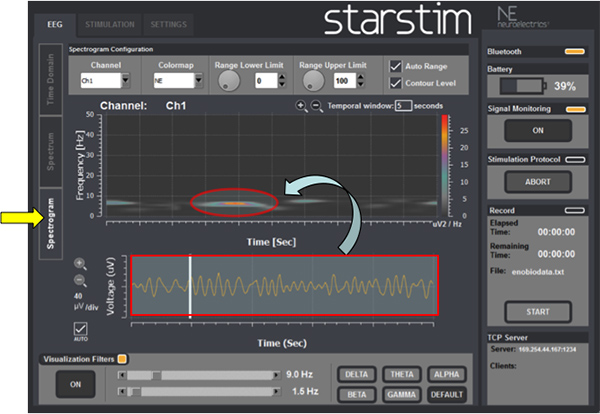
Figure 8. EEG spectrogram: EEG signals (red rectangle on the bottom) can also be transformed into images (red circle) using a technique called time-frequency based.
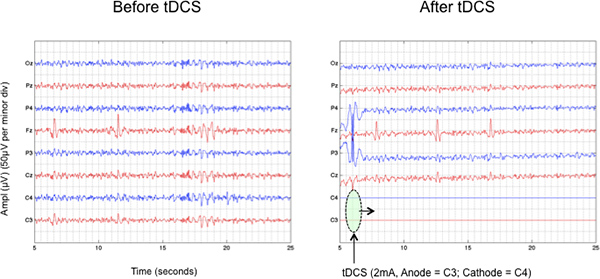
Figure 9. Attenuation of parietal EEG activity in response to anodal tDCS (Anode = C3; Cathode = C4). Note that during stimulation it not possible to record brain activity in the same channels used for stimulation. Click here to view larger figure.
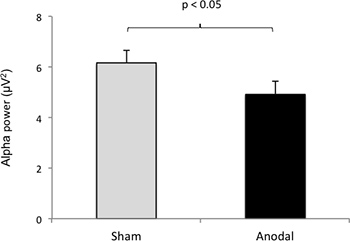
Figure 10. tDCS effects on EEG power spectrum: Note differences in frontal alpha (a) and beta (b) amplitude in response to active-tDCS when compared to sham-tDCS over the left dorsolateral prefrontal cortex.
| Disease | Auteurs | Anode electrode positioning | Cathode electrode positioning |
| Depression | Boggio et al., 2008; Loo et al., 2012 | DLPFC | Supraorbital |
| Pain | Fregni et al., 2006 | M1 | Supraorbital |
| Stroke | Lindenberg et al., 2010 | M1 | M1 |
| Boggio et al., 2007 | M1 (affected side) | Supraorbital | |
| Supraorbital | MI (non-affected side) | ||
| Tinnitus | Fregni et al., 2006 | LTA | Supraorbital |
| Parkinson | Benninger et al., 2010 | M1/DLPFC | Mastoid |
| Fregni et al., 2006 | M1 | Supraorbital | |
| Migraine | Antal et al., 2011 | V1 | Oz |
| Alcohol abuse | Boggio et al., 2008 | R/L – DLPFC | L/R – DLPFC |
Table 1. tDCS electrode montages in different clinical conditions. Legends: LTA, left temporoparietal area; V1, Visual cortex; DLPFC, Dorsolateral prefrontal cortex; M1, Motor cortex, R, Right; L, Left.
| Bands | Symbol | Frequency (Hz) | Best recording site | More prominent during… |
| Delta | δ | 1-4 | Frontal (adults), Posterior (children) | Deep stages of sleep (3 and 4) |
| Theta | θ | 5-7 | Diffuse in the scalp | Drowsiness |
| Alpha | α | 8-12 | Posterior regions | Awakens, with eyes closed |
| Beta | β | 13-30 | Frontal | Mental effort, deep sleep |
| Gamma | γ | 31-45 | Somato-sensory cortex | Short term memory tasks and tactile stimulation |
Table 2. EEG frequency bands.
| Auteurs | Anode electrode positioning | Cathode electrode positioning | EEG Channels (number) | Main Findings |
| Ardolino et al., 2005 | Fp1 | C4 | 4 | Bilateral increase of frontal delta and theta bands. |
| Keeser et al., 2011 | F3 | Fp2 | 25 | Decrease in frontal and prefrontal delta band. |
| Marshall et al., 2011 | F3/F4 | Mastoids | 7 | – Non-REM sleep: frontal decrease of delta band. – REM sleep: global increase of gamma band. |
| Wirth et al., 2011 | F3 | Right shoulder | 52 | Global decrease in delta band. |
| Zaehle et al., 2011 | F3 | Mastoids | 32 | – Anodal: local increase of theta and alpha bands. – Cathodal: local decrease of theta and alpha bands. |
| Jacobson et al., 2011 | Between T4-Fz | Fp1 | 27 | Decrease in right frontal theta band. |
| Polania et al., 2011 | C3 | Fp3 | 62 | – Global synchronization of all studied bands. |
| Maeoka et al., 2012 | F3 | Fp2 | 128 | Local increase in beta and decreased alpha bands. |
Table 3. Studies analyzing the effects of tDCS on EEG recordings.
Discussion
Safety issues
Initially, subjects should be screened for any contraindications for tDCS 11. Check also for skin lesions or diseases, since there is evidence of tDCS induced lesions according to skin integrity. If tDCS is strongly indicated over a lesioned area, it is possible to do it at lower intensity, i.e. 0.5-1.0 mA. However, it is not guaranteed that this will prevent skin irritations or lesions. Thus, the condition of the skin under the electrodes should be inspected before and after tDCS 2.
Impedance and electrodes
Electrode impedances should be as low as possible. This reduces the risk for internal and external noise interference or distorted signals. Impedances should also be rechecked whenever there is any artifact present in the signal 37.
All electrodes must be of good quality with intact surfaces. Reusable electrodes with inconsistent surfaces can create uneven current densities. All surface electrodes should be applied with sufficient conductive gel to ensure low impedances, and the impedances should be checked for artifacts 37.
Closed-loop systems
A closed-loop system is a system capable of diagnosing electrophysiological abnormalities and treating them promptly 8, 10. An illustrative example is the EEG spike detector for an oncoming seizure. This principle has been successfully applied in patients with severe epilepsy. Morrell and colleagues 9 treated 191 subjects with intractable epilepsy using a brain implanted stimulator and observed a significant reduction in seizure frequency as well as improvements in quality of life. Despite the success, invasive procedures are associated with risks and complications such as local infection or unwanted mood or cognitive effects and therefore an alternative, non-invasive approach is desirable. Hence, the present device may represent an interesting option for those patients who need rapid neurophysiological diagnosis and prompt treatment, such as epileptic patients.
The closed-loop system application might not be restricted to patients with epilepsy only. A number of recent studies have suggested that EEG alterations may be markers of various neuropsychiatric diseases 30. Using a combination of tDCS and EEG could also be useful for optimizing the parameters of stimulation. Such algorithms are still undeveloped, but the combination of findings from EEG and tDCS studies may help in such development.
Compared to TMS, which is another non-invasive brain stimulation technique, tDCS is considered much more suitable for therapeutic purposes mainly because of its low cost and relative portability. In addition, having a system that uses a head cap with pre-determined electrode locations can standardize location of stimulation and improve results. Another advantage of this device is the possibility to stimulate more than one site at the same time, which has been found to be clinically superior than conventional stimulation according to some authors 38, 39.
Although the device shows clear advantages, some limitations need to be addressed in order to improve the device for the future. First, the device cannot stimulate and record EEG signals in the same location simultaneously (See Figure 9). Second, the number of channels available to record EEG is low. The usual recommendation is to use at least 16-channels for an adequate EEG study 40 and even more channels for electro-oculography to detect eye movement artifacts. Indeed, in the past few years there has been a tendency to increase the number of channels in EEG/tDCS studies (Table 3). Although the low number of channels might affect sensibility in detecting dynamic changes in cortical excitability, such system may still be useful for finding algorithms for specific electrode locations.
Divulgations
The authors have nothing to disclose.
Acknowledgements
P.S. received funding support from CAPES, Brazil. This work was partially supported with a grant from CIMIT. The authors are also grateful to Uri Fligil for his technical assistance and to Olivia Gozel and Noelle Chiavetta for their help in editing this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Material | |||
| Neoprene HeadCap | Neuroelectrics | NE019 | 1 |
| Neoprene Headband | Neuroelectrics | NE020 | 1 |
| Frontal dry electrode front-end | Neuroelectrics | NE021 | 4 |
| Gel electrode front-end | Neuroelectrics | NE022 | 8 |
| Gel Bottle 60cl | Neuroelectrics | NE016 | 1 |
| Stimulation electrode Pi cm2 | Neuroelectrics | NE024 | 8 |
| Saline solution bottle 100ml | Neuroelectrics | NE033 | 1 |
| Sponge electrode fron-end 25 cm2 | Neuroelectrics | NE026 | 4 |
| Adhesive Electrode Front-end | Neuroelectrics | NE025 | 25 |
| USB Bluetooth Dongle | Neuroelectrics | NE031 | 1 |
| USB card with software | Neuroelectrics | NE015 | 1 |
| Curved Syringe | Neuroelectrics | NE014 | 1 |
| microUSB NECBOX charger | Neuroelectrics | NE013 | 1 |
| Electrode cable | Neuroelectrics | NE017 10 | 1 |
| Material Name | |||
| StarStim NECBOX | Neuroelectrics | NE012 | 1 |
References
- Fregni, F., Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 3, 383-393 (2007).
- Nitsche, M. A., Cohen, L. G. Transcranial direct current stimulation: State of the art. Brain Stimul. 1, 206-223 (2008).
- Nitsche, M. A., Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 57, 1899-1901 (2001).
- Kwon, Y. H., Ko, M. H. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci. Lett. 435, 56-59 (2008).
- Ardolino, G., Bossi, B., Barbieri, S., Priori, A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J. Physiol. 568, 653-663 (2005).
- Marshall, L., Kirov, R., Brade, J., Mölle, M., Born, J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One. 6, 16905 (2011).
- Brunoni, A. R., Nitsche, M. A. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 5, 175-195 (2012).
- Nuwer, M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 49, 277-292 (1997).
- Morrell, M. J. RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 77, 1295-1304 (2011).
- Berényi, A., Belluscio, M., Mao, D., Buzsáki, G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science. 337, 735-737 (2012).
- DaSilva, A. F., Volz, M. S., Bikson, M., Fregni, F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. (51), e2744 (2011).
- Antal, A., Boros, K., Poreisz, C., Chaieb, L., Terney, D., Paulus, W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 1, 97-105 (2008).
- Terney, D., Chaieb, L., Moliadze, V., Antal, A., Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 28, 14147-14155 (2008).
- Boggio, P. S., Rigonatti, S. P. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 11, 249-254 (2008).
- Loo, C. K., Alonzo, A., Martin, D., Mitchell, P. B., Galvez, V., Sachdev, P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br. J. Psychiatry. 200, 52-59 (2012).
- Boggio, P. S., Rocha, M. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry. 71, 992-999 (2010).
- Goldman, R. L., Borckardt, J. J. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite. 56, 741-746 (2011).
- Boggio, P. S., Zaghi, S. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend. 112, 220-225 (2010).
- Boggio, P. S., Sultani, N. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 92, 55-60 (2008).
- Fregni, F., Liguori, P. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J. Clin. Psychiatry. 69, 32-40 (2008).
- Fregni, F., Gimenes, R. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 54, 3988-3998 (2006).
- Fregni, F., Marcondes, R. Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur. J. Neurol. 13, 996-1001 (2006).
- Antal, A., Kriener, N., Lang, N., Boros, K., Paulus, W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia. 31, 820-828 (2011).
- Fregni, F., Otachi, P. T. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann. Neurol. 60, 447-455 (2006).
- Benninger, D. H., Lomarev, M. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 81, 1105-1111 (2010).
- Boggio, P. S., Nunes, A. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 25, 123-129 (2007).
- Lindenberg, R., Renga, V., Zhu, L. L., Nair, D., Schlaug, G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 75, 2176-2184 (2010).
- Fregni, F., Boggio, P. S. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 166, 23-30 (2005).
- Shafi, M. M., Westover, M. B., Fox, M. D., Pascual-Leone, A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci. 35, 805-825 (2012).
- Wirth, M., Rahman, R. A. Effects of transcranial direct current stimulation (tDCS) on behaviour and electrophysiology of language production. Neuropsychologia. 49, 3989-3998 (2011).
- Keeser, D., Padberg, F. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage. 55, 644-657 (2011).
- Zaehle, T., Sandmann, P., Thorne, J. D., Jäncke, L., Herrmann, C. S. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioral and electrophysiological evidence. BMC Neurosci. 12, 979-984 (2011).
- Polanía, R., Nitsche, M. A., Paulus, W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum. Brain Mapp. 32, 1236-1249 (2011).
- Maeoka, H., Matsuo, A., Hiyamizu, M., Morioka, S., Ando, H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: a study using electroencephalographic power spectrum analysis. Neurosci. Lett. 512, 12-16 (2012).
- Isley, M. R., Edmonds, H. L., Stecker, M. American Society of Neurophysiological Monitoring. Guidelines for intraoperative neuromonitoring using raw (analog or digital waveforms) and quantitative electroencephalography: a position statement by the American Society of Neurophysiological Monitoring. J. Clin. Monit. Comput. 23, 369-390 (2009).
- Faria, P., Leal, A., Miranda, P. C. Comparing different electrode configurations using the 10-10 international system in tDCS: a finite element model analysis. Conf Proc. IEEE Eng. Med. Biol. Soc. , 1596-1599 (2009).
- Dmochowski, J. P., Datta, A., Bikson, M., Su, Y., Parra, L. C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural. Eng. 8, 046011 (2011).
- Flink, R., Pedersen, B. Guidelines for the use of EEG methodology in the diagnosis of epilepsy. International League Against Epilepsy: commission report. Acta Neurol. Scand. 106, 1-7 (2002).

