Fabrication of Spatially Confined Complex Oxides
Summary
We describe the use of pulsed laser deposition (PLD), photolithography and wire-bonding techniques to create micrometer scale complex oxides devices. The PLD is utilized to grow epitaxial thin films. Photolithography and wire-bonding techniques are introduced to create practical devices for measurement purposes.
Abstract
Complex materials such as high Tc superconductors, multiferroics, and colossal magnetoresistors have electronic and magnetic properties that arise from the inherent strong electron correlations that reside within them. These materials can also possess electronic phase separation in which regions of vastly different resistive and magnetic behavior can coexist within a single crystal alloy material. By reducing the scale of these materials to length scales at and below the inherent size of the electronic domains, novel behaviors can be exposed. Because of this and the fact that spin-charge-lattice-orbital order parameters each involve correlation lengths, spatially reducing these materials for transport measurements is a critical step in understanding the fundamental physics that drives complex behaviors. These materials also offer great potential to become the next generation of electronic devices 1-3. Thus, the fabrication of low dimensional nano- or micro-structures is extremely important to achieve new functionality. This involves multiple controllable processes from high quality thin film growth to accurate electronic property characterization. Here, we present fabrication protocols of high quality microstructures for complex oxide manganite devices. Detailed descriptions and required equipment of thin film growth, photo-lithography, and wire-bonding are presented.
Introduction
The first and one of the most important steps towards high quality devices is the growth of epitaxial oxide thin films. A single crystal substrate is used as a “template” to deposit the target materials. Among different deposition methods, pulsed laser deposition (PLD) is one of the best ways to acquire good quality thin films 4,5. The growth processes involve heating the substrate to around 800 °C in an oxygen environment and using laser pulses to hit the target material and generate a flux to be deposited onto the substrate. The typical system is shown in Figure 1.
While unpatterned films have been shown to reveal exotic new physics 6, reducing film dimension provides more opportunities to explore new phenomena and device fabrication. Photolithography can be used to shrink the in-plane sample dimension down to the order of 1 μm. The detailed protocol of the photolithography process will be discussed below. This technique is compatible with most widely used substrates which allows for investigations of confinement effects on epitaxial films held at different strain states.
Since many complex oxides have interesting characteristics at low temperatures and/or high magnetic fields, the electronic connection between the device and measurement equipment is very important. High quality contacts can be formed by evaporating Au contact pads in a 4-probe geometry and with the use of a wire bonder to make connections between the pads and measurement device. When done correctly, these connections can easily withstand extreme measurement environments within wide temperature ranges of 4 K to 400 K and magnetic field ranges of up to ± 9 T.
Protocol
1. Sample Growth Fabrication
- Clean a 5 mm x 5 mm x 0.5 mm single crystal substrate having a miscut angle <0.1 degree such as SrTiO3 or LaAlO3 with acetone and then water in an ultrasonic cleaner for 10 min each. To get a TiO2 termination on SrTiO3, etch the substrate in 10% hydrogen fluoride for 30 sec and rinse in water for 1 min, followed by an anneal at 1,100 °C for 10 hr. After cleaning, mount the substrate on a heater suitable for ultra-high vacuum conditions.
- Mount the heater into the PLD vacuum chamber and open the chamber oxygen source to fill the chamber with 2 x 10E-5 Torr oxygen. Raise the heater temperature to 800 °C and allow it to anneal for 20 min. Temperature can be monitored using a computer controlled pyrometer or a thermocouple.
- To begin film deposition, start the excimer pulsed laser using a laser fluence of 1 to 2 J/cm2 and laser frequency of 1 or 2 Hz. The laser pulses will hit the target material and generate a plume flux. The flux will penetrate through the oxygen environment and deposit onto the substrate.
- Reflection High Energy Electron Diffraction (RHEED) can be used to monitor unit cell growth and confirm surface quality 7. This technique allows for very clear thickness monitoring.
- When the film is of the desired thickness, turn off the laser and decrease the heater temperature at 5 °C/min. Once the heater is cooled to room temperature, turn off the oxygen source and remove the sample.
- Ex situ annealing can be used on oxide materials to remove oxygen deficiencies that may be present after growth or after long periods in vacuum. Place the sample in a tube furnace under 1 atm of flowing oxygen. Raise the temperature from 20 °C to 700 °C at 5 °C/min, anneal for 2 hr, and then decrease temperature from 700 °C to 20 °C at 2 °C/min. An important note is to never post-anneal at higher temperatures than those used during film growth when filling oxygen vacancies as this can adversely affect surface quality and may negatively influence crystal quality.
2. Photolithography Fabrication
- Ultrasonically clean the sample in acetone and then water for 10 min each. An optical microscope can be used to check that the sample surface is clean of large particulates. (Figure 2a)
- Spin coat a layer of 1 micron thick photoresist. Typical spin speed and duration are around 6,000 rpm and 80 sec though these numbers are dependent on specific photoresist used. Place the sample on a heat plate at 115 °C for 2 min to cure the photoresist. Check the photoresist quality under an optical microscope. The coating should appear uniform with no bubbling.
- Use a mask aligner to expose the sample under a predefined lithography mask with UV light for 9 sec with an exposure dose around 90 mJ/cm2. Again these numbers will be specific to the photoresist used. When positive photoresist is used, the part of the photoresist which is covered by the mask will not change its chemical property while the part of the PR which is uncovered by the mask will change its property and can be dissolved in the chemical developer. Heat the photoresist and sample at 110 °C for 80 sec to further cure the exposed photoresist.
- Rinse the sample in a developer solution for 25-35 sec. Take out the sample immediately and rinse in water for 30 sec. If positive photoresist is used, the part of photoresist which is uncovered by the mask will be washed away while the part which is covered will remain. Note that the duration of the developing step is crucial to accurately control photoresist dimensions and quality (Figure 2b).
- Prepare a solution of potassium iodide, hydrochloric acid and water of ratio 1:1:1. Use plastic tweezers to rinse the sample in the acid for approximately 10 sec. The unprotected part of the thin film will be etched away. Immediately rinse the sample in pure water for 60 sec. Check with an optical microscope to see if the thin film has been totally etched. If not, add 2 to 3 more seconds of acid etch and immediately rinse with pure water, then check again with an optical microscope. Repeat this procedure until all the unprotected film is etched away. This process is governed by the etchant strength and film thickness. Typical etch rates for many manganites are about 1-4 nm/second for the 1:1:1 solution ratio described above.
- Rinse the sample in acetone for 20 sec to remove the remaining photoresist. Check the quality of sample with microscope (Figure 2c and 2d).
3. Wire-bonding Connection
- Using a photo-mask, repeat the steps 2.1-2.3 above using a lithography mask that will leave open regions on the wires suitable for contact pads. Evaporate 5 nm Ti and 100 nm Au onto the sample and rinse in acetone. This will remove the photoresist and leave only the desired contact pad geometry (Figure 3a).
- Use GE varnish to mount the sample onto the sample puck. Allow 15 min to cure.
- Fix the sample position on wire bonder stage and use the wire bonder to connect Al wires from the sample puck to Ti/Au contacts (Figure 3b). Then perform electrical measurements.
Representative Results
This paper focuses mostly on the photolithography and wire-bonding aspects of sample preparation. More details on film growth procedures can be found in our other recent publications 8.
Photolithography is an important method to control dimensionality in complex oxides for the purpose of investigating electron correlation lengths and electronic phase separation 9-13. Figure 2 shows optical images of partial steps during the process. It is necessary to point out that among all these steps, the precise control of developing and etching time is the most crucial to successfully fabricate a device. For example, one more second of developing time could cause the unexposed photoresist to be washed away. On the other hand, several more seconds of acid etching could cause the oxides film to be over-etched and completely removed, thus damaging the desired structure, as shown in Figure 4.
Figure 3 shows a ready-to-measure sample. Electrical voltage and current can be applied to the prototype devices for a wide range of electronic measurements across a wide range of temperatures and magnetic fields.
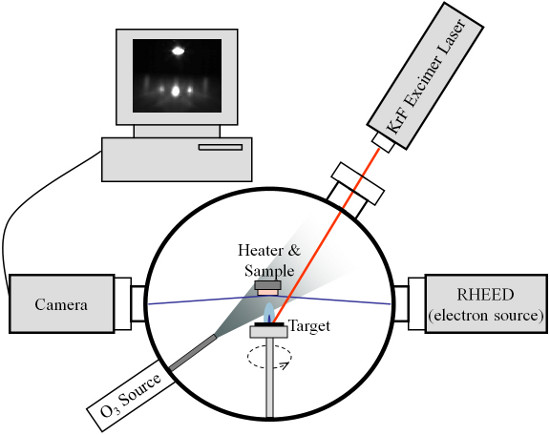
Figure 1. Schematic of the Pulsed Laser Deposition (PLD) system. The KrF excimer laser is used to generate the target plume. The heater is used to control the sample temperature. The O3 source is used to supply background oxygen pressure. The RHEED gun, camera and computer are used to monitor the growth dynamics and surface structure.
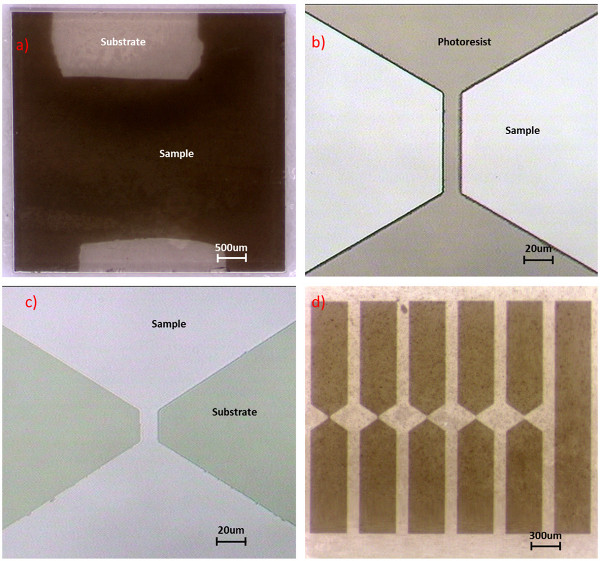
Figure 2. Photolithography images. a) optical image of an as-grown sample, light areas are regions that were left without film during growth as they lay under heater clamps, and slight inhomogeneity of color is caused by discoloration on the back of the substrate and not a result of film non-uniformity; b) typical image of developed photoresist on top of sample; c) typical image of sample after acid etching; d) full set of devices etched from a single film allow for measuring confinement effects on 6 wire widths.
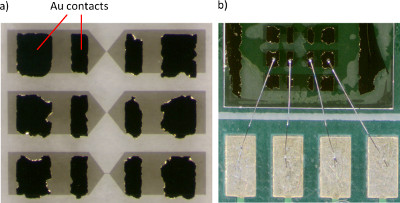
Figure 3. a) typical contacts for 4-probe transport; b) single device connections wire bonded from wire pads to resistivity puck. Click here to view larger figure.
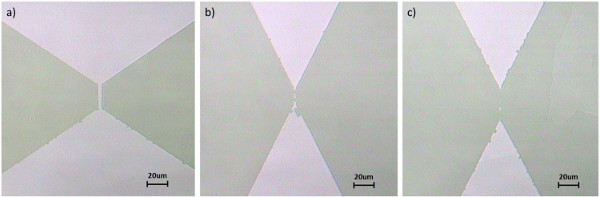
Figure 4. Effects of overetching. 50 nm films etched for a) 15 sec; b) 21 sec and c) 25 sec. Click here to view larger figure.
Discussion
Unlike single element semiconducting materials such as Si, the fabrication of complex materials can be more difficult due to the fact that the complex structure and multiple elements must all be taken into consideration. The use of photolithography to fabricate complex oxide devices is relatively low cost and fast to prototype as opposed to other confinement techniques. There are however some important limitations to understand. Photolithography has a spatial limitation to creating structures of about 1 micron so is not suitable for truly nanoscale device fabrication. Also important is the fact that edge roughness arising from the chemical etching process can be on the order of 50 nm.
Other techniques such as electron beam lithography (EBL) and focused ion beam (FIB) milling can be used to create much smaller structures than those possible with photolithography. These are generally limited to > 50 nm and > 20 nm structures respectively 14,15. These techniques also have limitations. EBL can take hours to days to develop a structure so is much slower than photolithography and may still result in edge roughness arising from the etching process. FIB milling is also much slower than photolithography and involves risking structure stoichiometry changes from implanted ions. Moreover, re-deposition of etched material when using FIB milling can negatively influence the device. A possible direction for overcoming the problems of chemical and plasma etching or ion bombardment is to completely remove that step in the processing. Self-assembled growth of nano-structures offers a promising way to avoid issues such as roughness and ion implantation. The goal is to use different growth techniques to controllably fabricate stoichiometric, small structures such as nano-rods and nano-pillars 16,17, and measure their properties. However this is still a fairly young technique in complex oxides and needs further development before it becomes viable for regular use across all materials.
The electric connection between the sample and instrument can also be achieved in different ways. Other than wire bonding, indium and silver paint are often used to create electrical connections. However, both indium and silver paint methods have issues such as large contact areas (around 1 mm2) and can require high temperature curing (~100 °C) or soldering (> 200 °C) which may induce oxygen deficiencies in oxide films. Thus, wire bonding has the advantage of a small contact area (around 100 μm2) which is stable under large temperature ranges and repeated usage.
The series of methods presented here enable the fabrication of small complex oxides structures from thin films. These methods allow for the investigation of strongly correlated systems both for basic physics research and in the quest for new functionality and application.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This effort was wholly supported by the US DOE, Office of Basic Energy Sciences, Materials Sciences and Engineering Division.
Materials
| Reagent/Material | |||
| SrTiO3(001) & LaAlO3(100) substrates | CrysTec GmbH | ||
| Microposit S1813 Photoresist | Shipley | ||
| CD-26 Developer | Shipley | 38490 | |
| GE varnish | Lakeshore | VGE-7031 | |
| Equipment | |||
| Reflected High Energy Electron Diffraction (RHEED) | Staib Instruments | 35 kV TorrRHEED | |
| Mask Aligner | ABM | Model 85-3 (350 W) Lightsource | |
| Resistivity Puck | Quantum Design | P102 | |
| Wire Bonder | Kulicke & Soffa | 04524-0XDA-000-00 |
References
- Ahn, C. H., Triscone, J. -. M., Mannhart, J. Electric field effect in correlated oxide systems. Nature. 424, 1015-1018 (2003).
- Basov, D. N., Averitt, R. D., Van der Marel, D., Dressel, M., Haule, K. Electrodynamics of correlated electron materials. Reviews of Modern Physics. 83, 471-541 (2011).
- Waser, R., Aono, M. Nanoionics-based resistive switching memories. Nat. Mater. 6, 833-840 (2007).
- Willmott, P. R., Huber, J. R. Pulsed laser vaporization and deposition. Rev. Mod. Phys. 72, 315-328 (2000).
- Eres, H. M. C., G, Recent advances in pulsed-laser deposition of complex oxides. Journal of Physics: Condensed Matter. 20, 264005 (2008).
- Ding, J. F., Jin, K. X., Zhang, Z., Wu, T. Dependence of negative differential resistance on electronic phase separation in unpatterned manganite films. Applied Physics Letters. 100, 62402-62404 (2012).
- Ichimiya, A., I, P. C. . Reflection High Energy Electron Diffraction. , (2004).
- Guo, H., Sun, D., et al. Growth diagram of La0.7Sr0.3MnO3 thin films using pulsed laser deposition. arXiv. , 1210.5989 (2012).
- Ward, T. Z., Gai, Z., Guo, H. W., Yin, L. F., Shen, J. Dynamics of a first-order electronic phase transition in manganites. Physical Review B. 83, 125125 (2011).
- Ward, T. Z., Liang, S., et al. Reemergent Metal-Insulator Transitions in Manganites Exposed with Spatial Confinement. Physical Review Letters. 100, 247204 (2008).
- Ward, T. Z., Zhang, X. G., et al. Time-Resolved Electronic Phase Transitions in Manganites. Physical Review Letters. 102, 87201 (2009).
- Zhai, H. -. Y., Ma, J. X., et al. Giant Discrete Steps in Metal-Insulator Transition in Perovskite Manganite Wires. Physical Review Letters. 97, 167201 (2006).
- Wu, T., Mitchell, J. F. Creation and annihilation of conducting filaments in mesoscopic manganite structures. Physical Review B. 74, 214423 (2006).
- Altissimo, M. E-beam lithography for micro-/nanofabrication. Biomicrofluidics. 4, 26503-26506 (2010).
- Watt, F., Bettiol, A. A., Van Kan, J. A., Teo, E. J., Breese, M. B. H. Ion Beam Lithography and Nanofabrication: A Review. International Journal of Nanoscience. 4, 269-286 (2005).
- Urban, J. J., Yun, W. S., Gu, Q., Park, H. Synthesis of single-crystalline perovskite nanorods composed of barium titanate and strontium titanate. J. Am. Chem. Soc. 124, 1186-1187 (2002).
- Wang, Y., Fan, H. J. The origin of different magnetic properties in nanosized Ca0.82La0.18MnO3: Wires versus particles. Applied Physics Letters. 98, 142502 (2011).

