Label-free Isolation and Enrichment of Cells Through Contactless Dielectrophoresis
Summary
Contactless dielectrophoresis (cDEP) achieves sorting and enrichment of particles via their intrinsic dielectric properties. Fluidic electrode channels replace metallic electrodes traditional to DEP, suiting cDEP to non-damaging sterile characterization and sorting of biological particles. We demonstrate how to prepare a cDEP microdevice and conduct cell characterization and sorting experiments.
Abstract
Dielectrophoresis (DEP) is the phenomenon by which polarized particles in a non-uniform electric field undergo translational motion, and can be used to direct the motion of microparticles in a surface marker-independent manner. Traditionally, DEP devices include planar metallic electrodes patterned in the sample channel. This approach can be expensive and requires a specialized cleanroom environment. Recently, a contact-free approach called contactless dielectrophoresis (cDEP) has been developed. This method utilizes the classic principle of DEP while avoiding direct contact between electrodes and sample by patterning fluidic electrodes and a sample channel from a single polydimethylsiloxane (PDMS) substrate, and has application as a rapid microfluidic strategy designed to sort and enrich microparticles. Unique to this method is that the electric field is generated via fluidic electrode channels containing a highly conductive fluid, which are separated from the sample channel by a thin insulating barrier. Because metal electrodes do not directly contact the sample, electrolysis, electrode delamination, and sample contamination are avoided. Additionally, this enables an inexpensive and simple fabrication process.
cDEP is thus well-suited for manipulating sensitive biological particles. The dielectrophoretic force acting upon the particles depends not only upon spatial gradients of the electric field generated by customizable design of the device geometry, but the intrinsic biophysical properties of the cell. As such, cDEP is a label-free technique that avoids depending upon surface-expressed molecular biomarkers that may be variably expressed within a population, while still allowing characterization, enrichment, and sorting of bioparticles.
Here, we demonstrate the basics of fabrication and experimentation using cDEP. We explain the simple preparation of a cDEP chip using soft lithography techniques. We discuss the experimental procedure for characterizing crossover frequency of a particle or cell, the frequency at which the dielectrophoretic force is zero. Finally, we demonstrate the use of this technique for sorting a mixture of ovarian cancer cells and fluorescing microspheres (beads).
Introduction
Biological sample enrichment and particle sorting is often necessary for subsequent analysis.1 For instance, isolation of rare cells from body fluids has important applications in cancer detection and individualized medicine. 2,3 The most commonly used enrichment techniques are fluorescent activated cell sorting (FACS)4 and magnetic activated cell sorting (MACS),5 which rely upon expressed surface markers to differentiate cells. Other strategies include hydrodynamic6 or inertial7,8 sorting, optical tweezers,9 acoustophoresis,10 and dielectrophoresis.11,12 Dielectrophoresis is the movement of a polarized particle in the presence of a non-uniform electric field.13 DEP has been used for a wide range of applications,2,14 including sorting cells based on viability,15 characterizing bioelectrical properties of cells,16 and sorting upon induced changes in biophysical properties of cells.17,18 Traditional DEP utilizes planar electrodes patterned within a microfluidic channel to apply a voltage and induce a non-uniform electric field.13 While this is a powerful technique, challenges can arise, such as electrode delamination and electrolysis. Insulator-based dielectrophoresis (iDEP)19 has addressed the challenges of fouling, electrode delamination, and spatial degradation of the electrode field through patterning insulating structures that induce non-uniformities in a DC electric field. iDEP has been used to selectively separate live and dead bacteria cells,19 isolate bacterial spores,20 and manipulate DNA,21 among other applications. Joule heating can be a challenge because it can occur as a result of the high DC voltages often required. To ameliorate these challenges, contact-free DEP microdevices have been developed.22-24
The technique presented here utilizes contactless dielectrophoresis (cDEP), so named for the lack of direct contact between metallic electrodes and the sample channel.22 Unique to this strategy is the replacement of metallic electrodes with fluid electrode channels filled with a highly conductive solution. These fluid electrodes are capacitively coupled across a thin insulating barrier to the sample channel via an AC voltage. Eliminating sample contact with electrodes reduces issues associated with DEP-based methods such as electrolysis and bubble formation, sample contamination, and electrode delamination. As a result, cDEP is especially useful for biological samples because it supports viability of the cells in the sample. Importantly, cDEP can maintain sample sterility. A chip can be prepared in a cell culture hood and the experiment can be conducted without requiring sample contact to metal electrodes or requiring that the sample be open to the environment. A simple reservoir can be fixed to the chip outlet to facilitate sterile sample recovery. Additionally, the fabrication of fluid electrodes from the same biocompatible polymer material (PDMS) as the sample channel reduces the high cost incurred with custom patterning of metal electrodes, the time required for fabrication, and limits the need for specialized cleanroom equipment to the initial patterning of the reusable silicon wafer stamp.
Movement of particles due to DEP depends on the characteristics of the particle and the medium, as well as the spatial gradients of the electric field. A particle- and frequency-dependent factor, called the Clausius-Mossotti (CM) factor, takes a value in the range -0.5 to 1, and determines the direction of the DEP force. The frequency at which the CM factor is exactly zero is called the crossover frequency. This is the point at which no dielectrophoretic force is exerted on a particle and the CM factor changes sign. A single crossover frequency for inert solid microspheres occurs when the CM factor changes from negative to positive. 25 For mammalian cells in low conductivity buffer on the order of 0.01 S/m, a first crossover frequency indicating a transition from nDEP to pDEP exists near 10-100 kHz, and is influenced by the size, shape, cytoskeleton, and membrane properties of the cell.26,27 A second crossover frequency at a shift from the pDEP to nDEP regime is on the order of 10 MHz, and is influenced by the nucleus-cytoplasm ratio, cytoplasm conductivity, and endoplasmic reticulum.27 DEP force can be applied without the presence of fluid flow, but here we utilize a flowing fluid to achieve continuous sorting of suspended particles. The combined influence of the dielectrophoretic force and the Stokes’ drag force dictate the translational motion of a particle.
We have developed devices for operation in two frequency ranges. Higher frequency devices (100-600 kHz) have operated using pDEP and achieved batch sorting of cells, such as prostate tumor initiating cells (TICs), murine ovarian surface epithelial (MOSE) cells, MDA-MB-231 breast cancer cells, or live THP-1 cells by selectively trapping cells of interest on insulating posts located in the sample channel.28-31 Lower frequency (5-100 kHz) devices operate continuously, and when operated at a frequency at which one population experiences pDEP while the background population experiences nDEP, can redirect particle trajectories to achieve sorting.32-34 These low frequency devices have been used to sort cancer cells from red blood cells, determine the changes in dielectric properties of a progressive MOSE cell line, and to elucidate the effects of non-toxic sphingolipid treatments on reversing aggressive characteristics of aggressive MOSE cells. Additionally, cDEP microdevices can be designed to operate at increased throughput, currently up to 1 ml/hr.31,35
As described, the flexibility and low cost of the fabrication process enables custom-designed device geometries, which allow the presented experimental procedure to be relevant for a wide range of applications. The long-term goal of cDEP is to realize label-free cell sorting and enrichment on a clinical level, with sample recovery for subsequent culture or processing. The technique presented here is a simple and inexpensive method, from fabrication to experimentation, which increases the accessibility of DEP. We demonstrate the preparation of a cDEP chip and the experimental protocol to achieve characterization and enrichment of ovarian cancer cells from fluorescent microspheres.
Protocol
1. Fabricating a Prototype cDEP Microfluidic Device: Overview
- Follow previously reported procedures22 using photoresist and Deep Reactive Ion Etching (DRIE) to etch the desired channel design (Figure 1) into a silicon wafer (Figure 2). Deposit a thin layer of Teflon to improve the release of the microdevice from the wafer.
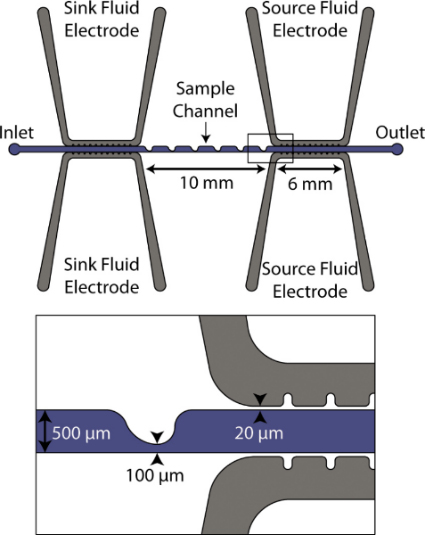
Figure 1. Schematic of a low frequency continuous sorting device. The sample channel runs left to right and is 500 μm wide with sawtooth constrictions to 100 μm. The two pairs of fluidic electrode channels compose the source and sink electrodes, respectively, and are separated from the sample channel by a 20 μm thick PDMS barrier.
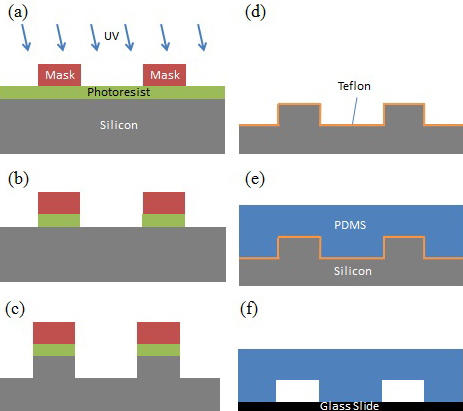
Figure 2. The fabrication process of a cDEP device. (a) For 60 sec, UV light selectively reacts with photoresist exposed through a mask pattern of the cDEP device geometry. (b) Photoresist is removed using developer. (c) Deep Reactive Ion Etching (DRIE) is used to etch 50 μm deep features, and 5 min of wet etching by tetramethylammonium hydroxide (TMAH) 25% at 70 °C is used to reduce roughness on the side walls. (d) A Teflon coating is added to improve device release from the wafer. (e) PDMS is poured onto the re-usable wafer stamp after degassing and cured for 45 min at 100 °F. (f) PDMS is removed from the wafer. PDMS and a clean glass slide are exposed to air plasma for 2 min and bonded together.
- Wrap the wafer with aluminum foil to prevent spill-over. Mix polydimethylsiloxane (PDMS) in 10:1 ratio of elastomer to curing agent, de-gas for approximately 20 min, and pour onto the wafer. Heat for 45 min at 100 °C to avoid damaging the Teflon coating of the stamp. Allow the device to cool down.
- After cooling, remove aluminum foil, trim PDMS using a surgical blade, and punch access holes at channel inlet/outlet using a blunt puncher suited to the size of tubing. Here, a 1.5 mm blunt puncher is used.
- Clean a glass microscope slide using a rinse of soap and water, ethanol, DI water, and drying with air, respectively. Clean the PDMS device using Scotch Magic tape. Examine under microscope to be sure channels are clean. Expose the slide and PDMS device to air plasma for 2 min. Firmly press channel side of device to glass slide, avoiding formation of air bubbles between the device and the slide (Figure 3).
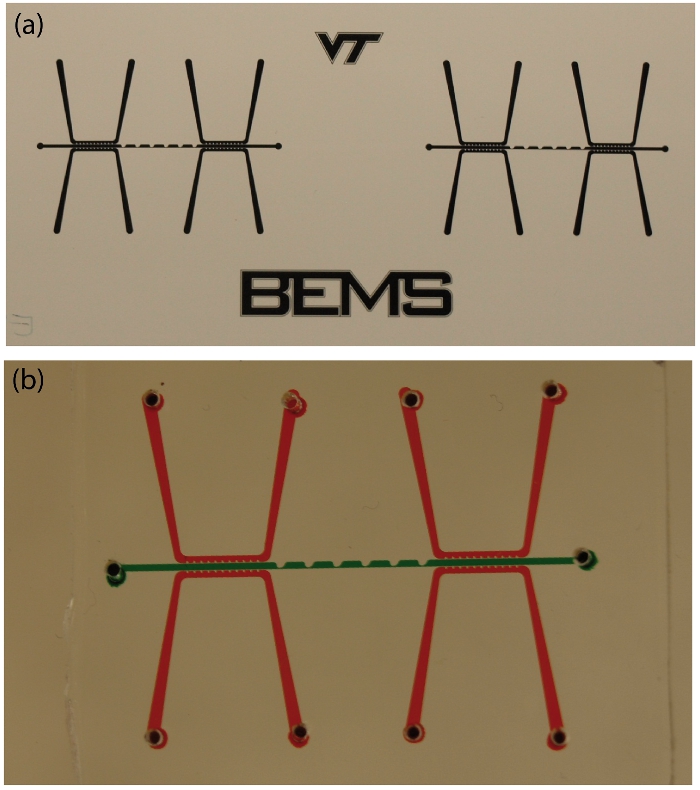
Figure 3. (a) The mask used for photolithography during the silicon wafer stamp fabrication, and (b) the finished PDMS-glass device with the sample and fluid electrode channels filled with green and red food coloring, respectively. See Figure 1 for dimensions. Click here to view larger figure.
2. Preparing a Cell Suspension in DEP Buffer
- Prepare a low conductivity (100 μS/cm) buffer, which will be referred to as "DEP buffer" (8.5% sucrose [w/v], 0.3% glucose [w/v], 0.725% RPMI [v/v]).16
- Suspend cells in DEP buffer at 3 x 106 cells/ml. Here, cancerous late-stage mouse ovarian surface epithelial (MOSE-L) cells are used.
Note: Cell viability analysis by the trypan blue dye exclusion method showed a slight decrease in viability (from 95.1-91.6%) of early-stage MOSE (MOSE-E) cells suspended in DEP buffer at room temperature after 5 hr. It is predicted that a similar slight decrease in viability occurs for MOSE-L cells, indicating that cells should not be stored in DEP buffer long-term.
- Dye cells using a fluorescent membrane-permeable dye, such as the enzymatically-activated Calcein AM.
- Measure conductivity of the dyed cell suspension. The conductivity should be approximately 100 μS/cm. If the conductivity measurement is too high, spin the sample down, resuspend in DEP buffer at 3 x 106 cells/ml and repeat the conductivity measurement.
3. Loading the cDEP Microfluidic Chip
- Place the microfluidic chip under vacuum for 30 min.
- Meanwhile, cut a piece of flexible tubing to span the distance from the syringe pump to the microscope stage where the microfluidic chip will be located. Here, a 6 inch length of tubing is required. Fit tubing to needle tip on syringe.
- Estimate the required length for outlet tubing by dividing the target collected sample volume by the cross-sectional area of the tube. Cut the require length of tubing.
- Draw cell suspension into syringe. Check that no bubbles are located in the tubing or syringe.
- Upon removing chip from vacuum, insert the outlet tubing and prime the sample channel quickly to avoid trapped air bubbles in the channels. Gently tap the syringe when priming to avoid rupturing the thin (20 μm) insulating barrier, though it has been observed that the barrier can withstand a constant high throughput near 5 ml/hr without rupturing.
- To prime the electrode channels, quickly place empty pipette tips in one outlet of each fluidic electrode channel. Pipette 200 μl of PBS containing a small amount of rhodamine B and gently tap to introduce the fluid into the opposite end of the electrode channel. Maintain gentle pressure until PBS with rhodamine B visibly progresses up the tip of the empty pipette tip. Repeat for each electrode channel. Rhodamine B is used only to fluorescently visualize the fluidic electrode channels.
- After priming, fill each pipette tip reservoir with PBS with rhodamine B. Avoid bubble formation in the pipette tip reservoirs, and remove any visible bubble by pipetting gently up and down. Detach the outlet tubing and discard appropriately, then insert a fresh outlet tubing reservoir to collect the target volume.
4. Characterizing Crossover Frequency of Cells using cDEP Chip
- Position the chip on the stage of an inverted microscope equipped with digital camera (Figure 4).
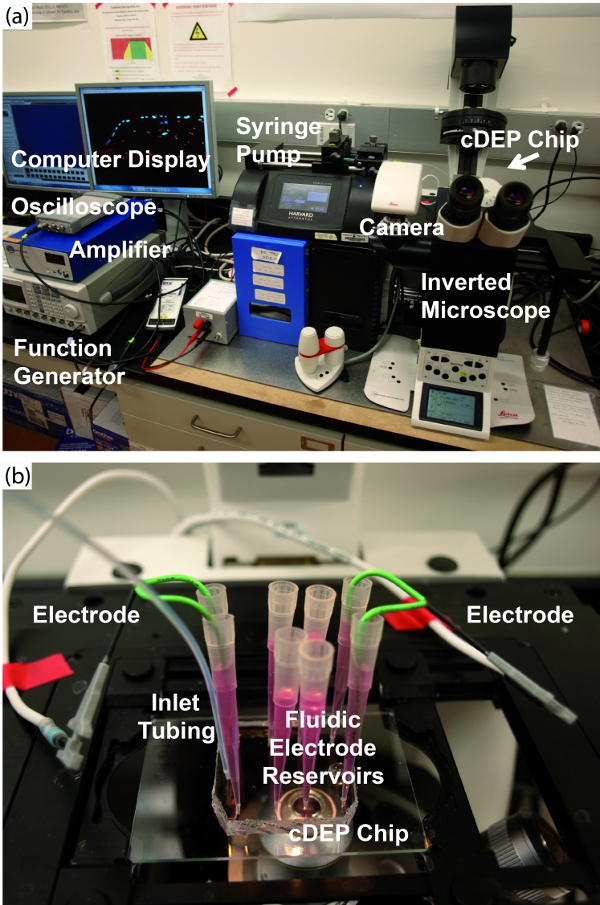
Figure 4. (a) Overall experimental setup for cDEP experiments. The cDEP chip is located on the platform of the inverted microscope. A camera sends images to the computer for recording. The syringe pump maintains a constant inlet flow. The function generator generates a signal which is stepped up by the high voltage amplifier and connected to the cDEP chip by wire electrodes. The oscilloscope monitors the signal sent to the chip. (b) Detail view of the cDEP chip and fluidic and electronic connections. Click here to view larger figure.
- Mount syringe on syringe pump and insert wire electrodes into fluidic electrode channel reservoirs.
- Pump fluid through at 1 ml/hr to ensure good contact between the syringe pump and the syringe. Visually inspect the channel length to ensure unimpeded flow of cells and the absence of bubbles or leaks. Reduce flow rate to 0.005 ml/hr.
Note: Throughput as high as 1 ml/hr31,35 has been achieved in devices of other geometries. - For safety, test the electronics by powering on the function generator, high voltage amplifier, and oscilloscope, and adjusting to desired voltage and frequency. Here these parameters are 200 V and 20 kHz. Upon verification of acceptable performance, power off. Connect electrodes to high voltage amplifier.
Note: Observe appropriate electrical safety procedures when operating the electronics at the high voltages used for these experiments. - Upon achieving steady flow, apply the desired voltage at the desired frequency. In this experiment, voltage is 200 VRMS at frequencies between 5-60 kHz.
- Record video files of the cell response with image processing techniques (step 5) to quantify cell distribution in the channel and to characterize the crossover frequency. To do this, maintain a constant voltage and vary the frequency systematically. At the start and end of the experiment, as well as randomly between experimental runs, record the control cell distribution, the distribution of the cells without applying any electrical field. Estimate the amount of time required for cells to flow from the inlet to the monitored location (here, 5 min). After setting a new frequency, wait this amount of time, then begin recording (here, a 2 min video of cell distribution).
5. Image Processing for Characterizing Crossover Frequency
- Using a computational script, analyze each video frame by frame to determine the relative y-position (where the z-direction is the vertical depth of the channel, the y-direction is the channel width, and x-direction is the channel length) of each fluorescing cell or particle at a specified x-coordinate downstream from the region of high DEP force in the channel. Create a graph of the relative distribution.
- Compare the centerline of the distribution at each voltage and frequency with the centerline of the control distribution. For a given voltage and varying frequencies, determine the crossover frequency, where the centerline matches the y-position of the control.
6. Varying the Experiment to Perform Cell Sorting or Enrichment
- Suspend desired mixture in DEP buffer at a total concentration of 3 x 106 particles/ml. Here, this mixture is MOSE-L cells with 4 μm fluorescing beads in DEP buffer. Perform the previously described steps to prepare a cDEP device.
- To sort or enrich cells from a mixture, determine the crossover frequencies of the target cells and the background particles as previously described. Operate the experiment at a frequency between the two crossover frequencies of the target cells and the background particles so that the two populations of particles experience DEP forces in opposite directions. To sort MOSE-L cells and 4 μm beads, operate the microdevice above 11.90±0.63 kHz. the first crossover frequency of MOSE-L cells recently reported by Salmanzadeh et al.33
- To collect the contents of a sorted sample, remove the outlet tubing upon collecting the target volume, by placing a glove finger over the outlet opening and gently tugging on the tubing. Pour the collected target volume into a reservoir for further analysis. Note: Other sample recovery methods could include attaching a permanent reservoir to the chip and removing the sample by simple pipetting.
Representative Results
DEP should be observed qualitatively in the sample channel to confirm the device is operational when the electric field is present. One method to test for the presence of DEP is to adjust the frequency to values far from the crossover frequency where strong pDEP and nDEP are predicted (roughly >40 kHz to observe pDEP and <10 kHz to observe nDEP for most mammalian cancer cells in a buffer with conductivity of 100 μS/cm). It should be noted that inert microspheres exhibit pDEP below their crossover frequency and nDEP above their crossover frequency. For the sawtooth feature geometry presented here, pDEP should be seen for cells at the higher test frequency, indicated by the occurrence of pearl chaining and focusing of cells or particles at the sawtooth feature edge of the channel, while at the lower test frequency, nDEP should be apparent as cells are restricted to the region nearer the straight edge of the channel. At these low frequencies, cells may lyse due to electroporation, so the sample may appear to contain fewer cells, and those that are visible may appear enlarged or blurry if using an enzymatically-activated fluorescing dye. Verifying the frequencies at which pDEP and nDEP occur allows the determination of the crossover frequency as described in the protocol. For mammalian cancer cells, such as MOSE-L used here, we observed nDEP at 5 kHz (Figure 5a) and strong pDEP at 60 kHz (Figure 5b). MOSE-L cells had a diameter of 17.7±3.3 μm (n = 268 cells) and the beads have a 4 μm nominal diameter. A full analysis of the dielectric properties of MOSE-L cells compared to MOSE cells in earlier stages of progression can be found in the recent work by Salmanzadeh et al.33
If pDEP and nDEP are not observed at these extremes, various problems could be occurring. The sample conductivity may be too high due to contaminants or cell lysing. Insufficient electric field may be generated due to bubbles trapped in the fluid electrode channels, which prevent conduction of current to the portion of the channels bordering the sample channel. Unsteady flow may be caused by a bubble trapped in the syringe or inlet tubing, bubbles resulting from not keeping the device under vacuum for a sufficient time or not quickly filling the device upon removing from vacuum, leakage due to delamination of poorly bonded PDMS to the glass slide, tears in the barrier membrane due to excessive force exerted when filling the channels, or flow rates at the extremes of the pump operating range.
In addition to determining the crossover frequency of cells, this technique can be used to sort a mixture, illustrated here by a mixture of MOSE-L cells and beads. This example takes advantage of the negative dielectrophoresis (nDEP) exhibited by 4 μm beads at frequencies where MOSE-L cells experience positive dielectrophoresis (pDEP). We operated the device at 200 VRMS and 10 kHz (Figure 6a) and observed that both cells and beads were mixed as they experienced nDEP. At 50 kHz we observed movement of MOSE-L cells to the top of the channel, while beads were restricted to the lower portion of the channel, enabling separation of the mixture into two components (Figure 6b).
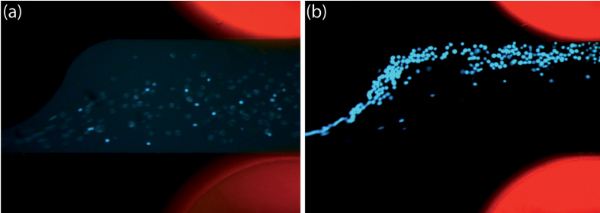
Figure 5. The position of MOSE-L cells is manipulated by changing the frequency of the applied electric field. The photos are taken at the final sawtooth feature in the sample channel and the fluidic electrodes are located at the corners on the right side. They are filled with PBS containing rhodamine B, which fluoresces to visualize the channels. (a) MOSE-L cells experience nDEP at 200 VRMS and 5 kHz. (b) MOSE-L cells experience pearl chaining and pDEP at 200 VRMS and 60 kHz. Click here to view larger figure.
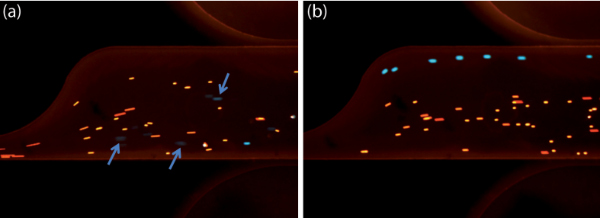
Figure 6. A mixture of MOSE-L cells (green) and 4 μm beads (red) flow through the final sawtooth feature in the sample channel of a low frequency cDEP device. (a) At 200 V and 10 kHz cells and beads are mixed. Arrows point to faintly appearing cells that likely have died due to the low frequency conditions. (b) At 200 V and 50 kHz cells experience pDEP and move to the feature edge of the channel, while beads experience nDEP and remain confined to the region nearer the straight edge. Click here to view larger figure.
Discussion
DEP is a powerful technique for determining dielectric properties of particles and directing particle motion towards sorting, isolation, or enrichment applications. Due to the detrimental effect of direct electrode contact with a sample, others have taken approaches similar to the previously presented method to avoid contact. For example, Bashir et al. developed contact-free DEP devices by using a microfluidic PDMS device separated from printed circuit board electrodes by a thin glass coverslip, and this technique is also made available in video format.23,36
Here, we have shown the fabrication of a cDEP chip and fluidic electrodes using a single PDMS substrate, and the experimental protocol for separating ovarian cancer cells from a mixture of cells and fluorescent beads. The presented technique has been successfully used for a variety of more complex and physiologically relevant applications, including sorting live and dead cells,28 tumor initiating cells from prostate cancer cells,30 cancer cells from dilute red blood cells,31,32 and differentiating among stages of breast cancer37 and ovarian cancer.29 cDEP has also been used for mixing particles.38 These applications suggest that by using the simple technique presented, diverse purposes can be accomplished simply by altering the design of the channel geometry.
DEP is useful on the microscale for manipulation of particles.13 For spherical particles, the translational dielectrophoretic force depends upon the size and electrical properties of the particle and its suspending medium, as well as the gradient of the electric field squared:
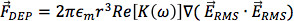
where εm is the permittivity of the suspending medium, r is the radius of the particle, and Rc[k(ω)]is the real part of the Clausius-Mossotti (CM) factor. The CM factor is a measure of the relative polarizability of the particle compared to the suspending medium and determines the direction of the dielectrophoretic force. It is described as
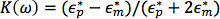
where 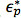 and
and 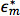 are the complex permittivities of the particle and medium, respectively. The complex permittivity,
are the complex permittivities of the particle and medium, respectively. The complex permittivity, 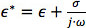 , depends upon conductivity (σ) and frequency (ω). The CM factor of spherical particles is theoretically bound between -0.5 and 1. If the CM factor is negative, the particles experience nDEP because the medium is more polarizable than the particle, so particles move away from regions of high electric field gradients. If the CM factor is positive, the particles are more polarizable than the medium and they experience pDEP in which they move toward regions of high electric field gradients.
, depends upon conductivity (σ) and frequency (ω). The CM factor of spherical particles is theoretically bound between -0.5 and 1. If the CM factor is negative, the particles experience nDEP because the medium is more polarizable than the particle, so particles move away from regions of high electric field gradients. If the CM factor is positive, the particles are more polarizable than the medium and they experience pDEP in which they move toward regions of high electric field gradients.
For biological particles that are nonhomogeneous in structure, such as cells, the Clausius-Mossotti factor can be determined from an effective value for the particle permittivity:
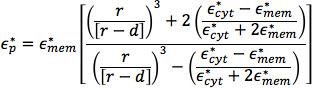
where 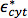 and
and 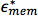 are the complex permittivity of the effective complex permittivity of the interior of the cell, such as the cytoplasm, and the plasma membrane, respectively; r is the radius of the cell, and d is the thickness of the plasma membrane.26
are the complex permittivity of the effective complex permittivity of the interior of the cell, such as the cytoplasm, and the plasma membrane, respectively; r is the radius of the cell, and d is the thickness of the plasma membrane.26
When DEP occurs with particles suspended in a fluid, the motion of the particle relative to the fluid will generate a drag force on the particle. This drag force must be considered when determining the overall force acting on the particle. For the situations of interest here, viscous forces dominate and particles are assumed spherical, small, and moving with relatively low velocity, so Stokes’ drag law provides a good approximation for the drag force:
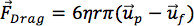
where η is the viscosity of the fluid, up is the velocity of the particle, and uf is the velocity of the fluid, which may also be moving. Given the dielectrophoretic force, known fluid and particle properties, and a known flow velocity, the balance between the drag force and the dielectrophoretic force can be solved to estimate the particle velocity. The shear rate that the cells experience should be below the threshold at which cell lysing can occur.
Characterization of the electrical properties of particles is necessary to predict and control how they will respond under DEP them. In this work, we specifically utilized low frequency cDEP with MOSE-L cells to demonstrate the protocol for determining first crossover frequency of cells, and then showed continuous sorting of polystyrene beads and MOSE-L cells based on their opposing DEP responses.
Altering the geometry of the cDEP device will change the spatial gradients of the electric field, allowing devices to be designed for high frequency or low frequency operation, and for high selectivity and efficiency of sorting for a specified cell type. Additionally, high throughput devices can be developed by fabricating wider channels,30 channels in parallel,30 or by multilayer fabrication35, in which the electrode channels are vertically stacked above and below a relatively deep sample channel. Thin membranes form the barrier between layers. Preliminary testing with devices fabricated in polymethylmethacrylate (PMMA) and polycarbonate (PC) thin films have demonstrated DEP response of MOSE-L cells. Current efforts are underway to refine multi-layer high-throughput devices and to improve the peripheral system towards an eventual plug-and-play platform. To expand from the basic experimental technique presented, the applications and specifications of the device can be tailored to fit particular demands, such as sorting versus characterization, or adding sample reservoirs and a semi-automated system to the device.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work supported has been supported in part by the National Science Foundation under Grant No. EFRI 0938047, and by the Virginia Tech Institute for Critical Technology and Applied Science (ICTAS). The authors would like to express appreciation to Dr. Eva Schmelz and Dr. Paul Roberts for their kind gift of MOSE-L cells. The authors acknowledge Angela Anderson for her assistance with cell culture, Caitlan Swaffar for her help with editing this document and preparing experiments, and all Bioelectromechanical Systems lab members.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| Polydimethylsiloxane (PDMS) | Dow Corning, Midland, MI,USA | Sylgard 184 | |
| Glass slides | The Microscope Depot | 76079 | 2×3 inch-ground edges |
| Microbore PTFE Tubing | Cole-Parmer Instrument Co, Vernon Hills, IL, USA | EW-06417-31 | Thin walled 20 gauge, 0.032″ID x 0.056″OD, 100 ft/roll |
| Luer-slip plastic syringes | National Scientific company | S7510-1 | |
| Needle tip | Howard Electronic instruments | JG20-1.0 | 20 Gauge 1.0″, ID=0.025″ OD=0.036″ |
| D(+)-Sucrose | Fisher Scientific, Fair Lawn, NJ | S3-500 | |
| D(+)-glucose, reagent ACS, anhydrous | Acros Organics N.V., Fair Lawn, NJ | AC410955000 | |
| RPMI-1640 Medium | Quality Biological Inc. | 112-025-101 | |
| Calcein AM, Molecular Probes | Invitrogen Corp. (life technologies), Carlsbad, CA, USA | C3100MP | excitation wavelength 488/emission wavelength 516 |
| Rhodamine B, O | Science Lab | SLR1465-100G | excitation wavelength 540/emission wavelength 625 |
| Phosphate buffered saline (10X) | Gbiosciences, St. Louis, MO | RC-147 | |
| Leica, inverted light microscope | Leica Microsystems, Bannockburn, IL, USA | Leica DMI 6000B | |
| Leica DFC420, color camera | Leica Microsystems, Bannockburn, IL, USA | Leica DFC420 | |
| Function generator | GW Instek, Taipei, Taiwan | GFG-3015 | |
| Wideband power amplifier | Amp-Line Corp., Oakland Gardens, NY, USA | AL-50HF-A | |
| HFHV Output Transformer | AL-T50-V25/300-F100K-600K | ||
| High voltage amplifier | Trek | Model 2205 | |
| USB Modular Oscilloscope, 100 MHz | AgilentTechnologies | U2701A | |
| Expanded Plasma Cleaner | Harrick Plasma | PDC-001/002 (115/230V) | air plasma |
| Scotch Magic tape | 3M | any available width is sufficient | |
| 1.5 mm puncher | Harris Uni-Core | Z708836-25EA | |
| .25% Trypsin-EDTA | Invitrogen | 25200-056 | |
| FluoSpheres Sulfate Microspheres | Invitrogen | F8858 | 4.0 μm, red fluorescent (excitation wavelength 580/emission wavelength 605) |
| AZ 9260 photoresist | AZ Electronic Materials | ||
| AZ 400 K developer | AZ Electronic Materials | ||
| Tetramethylammonium hydroxide (TMAH) 25% | provided by Virginia Tech cleanroom | ||
| Teflon coating | applied using DRIE machine | ||
| Silicon wafer | University Wafer | 452 | 100 mm diameter, 500 μm thickness, one side polished (SSP) |
| Deep Reactive Ion Etching (DRIE) | Alcatrel | AMS SDE 100 |
References
- Butler, M. . Cell culture and upstream processing. , (2007).
- Pratt, E. D., Huang, C., Hawkins, B. G., Gleghorn, J. P., Kirby, B. J. Rare Cell Capture in Microfluidic Devices. Chemical Engineering Science. 66, 1508-1522 (2011).
- Salmanzadeh, A. D., Davalos, R. V. Isolation of rare cells through their dielectrophoretic signature. Journal of Membrane Science and Technology. 3, e112 (2013).
- Bonner, W. A., Hulett, H. R., Sweet, R. G., Herzenberg, L. A. Fluorescence activated cell sorting. The Review of Scientific Instruments. 43, 404-409 (1972).
- Adams, J. D., Kim, U., Soh, H. T. Multitarget magnetic activated cell sorter. Proc. Natl. Acad. Sci. USA. 105, 18165-18170 (2008).
- Chabert, M., Viovy, J. L. Microfluidic high-throughput encapsulation and hydrodynamic self-sorting of single cells. Proc. Natl. Acad. Sci. U.S.A. 105, 3191-3196 (2008).
- Di Carlo, D., Irimia, D., Tompkins, R. G., Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. U.S.A. 104, 18892-18897 (2007).
- Di Carlo, D. Inertial microfluidics. Lab Chip. 9, 3038-3046 (2009).
- Grover, S. C., Skirtach, A. G., Gauthier, R. C., Grover, C. P. Automated single-cell sorting system based on optical trapping. J. Biomed. Opt. 6, 14-22 (2001).
- Lin, S. C. S., Mao, X. L., Huang, T. J. Surface acoustic wave (SAW) acoustophoresis: now and beyond. Lab Chip. 12, 2766-2770 (2012).
- Pethig, R. Review Article-Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics. 4, (2010).
- Gascoyne, P. R. C., Wang, X. B., Huang, Y., Becker, F. F. Dielectrophoretic separation of cancer cells from blood. Ieee T. Ind. Appl. 33, 670-678 (1997).
- Pohl, H. A. . Dielectrophoresis : the behavior of neutral matter in nonuniform electric fields. , (1978).
- Salmanzadeh, A., Davalos, R. V. Isolation of rare cells through their dielectrophoretic signature. Journal of Membrane Science. , (2012).
- Markx, G. H., Talary, M. S., Pethig, R. Separation of Viable and Nonviable Yeast Using Dielectrophoresis. J. Biotechnol. 32 (94), 29-37 (1994).
- Flanagan, L. A., et al. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells. 26, 656-665 (2008).
- Labeed, F. H., Coley, H. M., Thomas, H., Hughes, M. P. Assessment of multidrug resistance reversal using dielectrophoresis and flow cytometry. Biophys. J. 85, 2028-2034 (2003).
- Hoettges, K. F., et al. Dielectrophoresis-activated multiwell plate for label-free high-throughput drug assessment. Anal. Chem. 80, 2063-2068 (2008).
- Lapizco-Encinas, B. H., Simmons, B. A., Cummings, E. B., Fintschenko, Y. Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis. 25, 1695-1704 (2004).
- Davalos, R. V., et al. Performance impact of dynamic surface coatings on polymeric insulator-based dielectrophoretic particle separators. Anal. Bioanal. Chem. 390, 847-855 (2008).
- Gallo-Villanueva, R. C., Rodriguez-Lopez, C. E., Diaz-De-La-Garza, R. I., Reyes-Betanzo, C., Lapizco-Encinas, B. H. DNA manipulation by means of insulator-based dielectrophoresis employing direct current electric fields. Electrophoresis. 30, 4195-4205 (2009).
- Shafiee, H., Caldwell, J. L., Sano, M. B., Davalos, R. V. Contactless dielectrophoresis: a new technique for cell manipulation. Biomed Microdevices. 11, 997-1006 (2009).
- Park, K., Suk, H. J., Akin, D., Bashir, R. Dielectrophoresis-based cell manipulation using electrodes on a reusable printed circuit board. Lab Chip. 9, 2224-2229 (2009).
- Jen, C. P., Maslov, N. A., Shih, H. Y., Lee, Y. C., Hsiao, F. B. Particle focusing in a contactless dielectrophoretic microfluidic chip with insulating structures. Microsyst Technol. 18, 1879-1886 (2012).
- Hughes, M. P. AC electrokinetics: applications for nanotechnology. Nanotechnology. 11, 124-132 (2000).
- Pethig, R. Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics. 4, 022811 (2010).
- Pethig, R., Ferrari, M. a. u. r. o., Ozkan, M. i. h. r. i. m. a. h., Heller, M. i. c. h. a. e. l. . J. .. Ch. 4. BioMEMS and Biomedical Nanotechnology. 4, 103-126 (2007).
- Shafiee, H., Sano, M. B., Henslee, E. A., Caldwell, J. L., Davalos, R. V. Selective isolation of live/dead cells using contactless dielectrophoresis (cDEP). Lab Chip. 10, 438-445 (2010).
- Salmanzadeh, A., et al. Dielectrophoretic differentiation of mouse ovarian surface epithelial cells, macrophages, and fibroblasts using contactless dielectrophoresis. Biomicrofluidics. 6, (2012).
- Salmanzadeh, A., et al. Isolation of prostate tumor initiating cells (TICs) through their dielectrophoretic signature. Lab Chip. 12, 182-189 (2012).
- Salmanzadeh, A., Sano, M. B., Shafiee, H., Stremler, M., Davalos, R. V. Isolation of Rare Cancer Cells from Blood Cells Using Dielectrophoresis. , (2012).
- Sano, M. B., Caldwell, J. L., Davalos, R. V. Modeling and development of a low frequency contactless dielectrophoresis (cDEP) platform to sort cancer cells from dilute whole blood samples. Biosens. Bioelectron. 30, 13-20 (2011).
- Salmanzadeh, A., et al. Investigating Dielectric Properties of Different Stages of Syngeneic Murine Ovarian Cancer Cells. Biomicrofluidics. , (2013).
- Salmanzadeh, A., Sano, M. B., Shafiee, H., Stremler, M. A., Davalos, R. V. . , 590-593 (2012).
- Sano, M. B., Salmanzadeh, A., Davalos, R. V. Multilayer contactless dielectrophoresis: Theoretical considerations. Electrophoresis. 33, 1938-1946 (2012).
- Millet, L. J., Park, K., Watkins, N. N., Hsia, K. J., Bashir, R. Separating beads and cells in multi-channel microfluidic devices using dielectrophoresis and laminar. J. Vis. Exp. (48), e2545 (2011).
- Henslee, E. A., Sano, M. B., Rojas, A. D., Schmelz, E. M., Davalos, R. V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis. 32, 2523-2529 (2011).
- Salmanzadeh, A., Shafiee, H., Davalos, R. V., Stremler, M. A. Microfluidic mixing using contactless dielectrophoresis. Electrophoresis. 32, 2569-2578 (2011).

