Quantitative In vitro Assay to Measure Neutrophil Adhesion to Activated Primary Human Microvascular Endothelial Cells under Static Conditions
Summary
Neutrophil adherence to the activated endothelium at sites of infection is an integral component of the host’s inflammatory response. Described in this report is a neutrophil binding assay that allows for the in vitro quantitation of primary human neutrophil binding to endothelial cells activated by inflammatory mediators under static conditions.
Abstract
The vascular endothelium plays an integral part in the inflammatory response. During the acute phase of inflammation, endothelial cells (ECs) are activated by host mediators or directly by conserved microbial components or host-derived danger molecules. Activated ECs express cytokines, chemokines and adhesion molecules that mobilize, activate and retain leukocytes at the site of infection or injury. Neutrophils are the first leukocytes to arrive, and adhere to the endothelium through a variety of adhesion molecules present on the surfaces of both cells. The main functions of neutrophils are to directly eliminate microbial threats, promote the recruitment of other leukocytes through the release of additional factors, and initiate wound repair. Therefore, their recruitment and attachment to the endothelium is a critical step in the initiation of the inflammatory response. In this report, we describe an in vitro neutrophil adhesion assay using calcein AM-labeled primary human neutrophils to quantitate the extent of microvascular endothelial cell activation under static conditions. This method has the additional advantage that the same samples quantitated by fluorescence spectrophotometry can also be visualized directly using fluorescence microscopy for a more qualitative assessment of neutrophil binding.
Introduction
Since the vascular endothelium is in direct contact with the circulating blood, it is uniquely situated to initiate a rapid inflammatory response during infection or injury. Endothelial cells (ECs) express pattern recognition receptors that recognize a variety of conserved bacterial components and danger molecules, and receptors for host inflammatory mediators such as TNFα. Activation of these receptors induces ECs to secrete cytokines (e.g. IL-6, IL-8, CXCL1 and CCL2), and to upregulate adhesion molecules (e.g. E/P-selectin, VCAM-1 and ICAM-1) at their cell surface 1,2. These molecules all facilitate the localization of leukocytes to sites of infection and injury in order to clear the host of the infectious agents and initiate tissue repair 3,4.The neutrophil response to infection involves a well-coordinated interplay between the vascular endothelium and the responding neutrophils. Upon EC activation, IL-8 is secreted and forms an intravascular gradient on the endothelium that allows neutrophils to home in to the site of infection or injury 5,6. E/P-selectins mediate neutrophil capture and rolling through relatively weak associations with glycomolecules on the neutrophil cell surface. These interactions, along with IL-8 binding to its cognate receptors, facilitate the robust, integrin-mediated attachment and eventual arrest of neutrophils on the endothelial cell surface 7-10. After arrest, neutrophils can migrate out of the vasculature to the specific sites of infection to directly eliminate pathogens, generate neutrophil extracellular traps to prevent the spread of pathogens, promote wound healing and release additional factors that recruit other leukocytes such as monocytes, macrophages and dendritic cells 11-17.
Described in this report is an in vitro method to quantitate neutrophil adherence to microvascular ECs after activation by the host inflammatory mediator TNFα. This assay is designed to assess the activation of ECs, and not neutrophils. Primary human neutrophils are first isolated using density gradient separation, and are then labeled with calcein acetoxymethyl (AM). Esterases within the live cells hydrolyze calcein AM to the highly fluorescent calcein molecule with an excitation of 492-495 nm and emission of 513-516 nm 18. The fluorescently-labeled neutrophils are then incubated with EC monolayers, and non-adherent neutrophils are subsequently removed. The fluorescence of the remaining, bound neutrophils is then measured using a fluorescence spectrophotometer, and calculated as a percent of total neutrophil fluorescence input per well. This method has the additional advantage that the bound calcein-labeled neutrophils used in spectrophotometry can be directly visualized using fluorescence microscopy to give a more qualitative read out of EC activation. Since this assay is performed under static conditions, only the very initial events that occur in the neutrophil adhesion cascade will be assessed. This is confirmed in this report using E-selectin blocking antibodies to show that neutrophil adherence to TNFα-treated human lung microvascular EC (HMVEC-Lung) monolayers is drastically reduced when the interaction with E-selectin is disrupted.
In addition to TNFα, we have successfully used this assay to determine the extent of human umbilical vein EC (HUVEC) activation by the Toll-like receptor 1/2 agonists peptidoglycan-associated lipoprotein (PAL), murein lipoprotein (MLP) and Pam3Cys and HMVEC-Lung activation by Pam3Cys 19,20. In addition, we successfully used this assay with kinase inhibitors and after RNAi-mediated knockdown of surface and cytoplasmic proteins in HMVEC-Lung, suggesting that this methodology is compatible with a variety of biochemical and screening assays 20. In summary, this assay provides an easy to use, reproducible, more functional way to access the extent of EC activation by inflammatory mediators in vitro.
Protocol
1. Plating and Maintenance of Microvascular Endothelial Cells
- Thaw and grow your microvascular endothelial cells according to the manufacturers supplied instructions. In this protocol, cells were grown in EGM-2 MV media (Lonza), and it is recommended that all experiments are performed within two weeks of thawing to minimize any variation due to passage number. Our experiments with HMVEC-Lung are performed between passages 4 to 9.
- Day 1: When cells reach 80-90% confluency, trypsinize and resuspend at 120,000 cells per ml. Plate 36,000 cells (0.3 ml) per well into 48-well, polystyrene tissue culture plate(s). Include wells of HMVEC that will not be incubated with neutrophils in order to obtain a background fluorescence reading. Unless using 48-well plates specifically designed for fluorescence assays, alternating rows or columns will minimize any light contamination from neighboring wells. NOTE: Wells can be pre-coated with poly-lysine, collagen or fibronectin.
- Day 2: Add fresh media.
- Day 3: By phase contrast microscopy, confirm the cells are healthy (i.e. "cobblestone" appearance and little cell debris), and they are 100% confluent (Figure 1). If the cells are not 100% confluent, change the media every day until they are ready.
- Once the HMVEC monolayers are ready for treatment, wash the cells once with Hank's balanced salt solution (HBSS) and add 0.3 ml of fresh EGM-2 MV media or media containing inflammatory agonist to the appropriate wells. In this protocol the HMVEC-Lung were treated with TNFα (100 ng/ml) (PeproTech, New Jersey) for 3 hr since this is a well-described activator of ECs 21. NOTE: It is recommended that you time your experiment so that your activated HMVEC cells (Step 4.1) and calcein AM labeled neutrophils (Step 3.5) are ready to use at the same time. It takes us approximately 2.5 hr to isolate and label neutrophils.
2. Neutrophil Isolation using Polymorphprep
- Isolate neutrophils as described previously 22, or with a ready-made density gradient solution to isolate polymorphonuclear granulocytes from whole blood. Employing Polymorphprep following the protocol by Nuzzi, et al. 2007 results in yields of approximately 2-3 x 106 neutrophils per ml of whole blood 23. NOTE: To avoid excessive erythrocyte lysis, a dextran sedimentation to remove red blood cells may be performed prior to density gradient centrifugation 24.
- Bring the Polymorphprep density gradient solution to RT.
- Collect 30 ml of whole blood from a healthy human volunteer in the presence of an anti-coagulant such as heparin, citrate or EDTA. The blood should be used within 2 hr, and kept between 18-22 °C.
- Layer 5 ml of whole blood over 5 ml of the density gradient solution in a 15 ml conical tube (Figure 2A). Avoid altering volumes of blood and the density gradient solution as this may change the subsequent centrifugation conditions.
- Centrifuge at 450 x g for 30 min at 18-22 °C. Make sure to slowly speed up and down the centrifuge (i.e. turn off the centrifuge brake), and balance the tubes well to prevent vibrations to help reduce activation of the neutrophils and prevent the mixing of layers. Longer centrifugation times or higher centrifugal force will result in the lower leukocyte layer, which contains neutrophils, to migrate further down towards to the pelleted red blood cells.
- Aspirate the plasma and upper leukocyte band containing PBMCs to prevent the contamination of the layer containing neutrophils (Figure 2B).
- Use a 1-2 ml serological pipet to remove the lower leukocyte layer containing neutrophils. Combine the neutrophil layers into 30 ml of PBS (without Ca2+ and Mg2+) in a 50 ml conical tube.
- Bring the volume up to 50 ml with PBS (without Ca2+ and Mg2+) and centrifuge at 450 x g for 10 min at 18-22 °C.
Note: Steps 2.9 and 2.10 are optional but highly recommended.
- Aspirate the supernatant. Resuspend the neutrophils in 8 ml of sterile water for 30 sec to lyse red blood cells, immediately add the cell suspension to 2 ml of 5x PBS (without Ca2+ and Mg2+) and gently mix by hand. DO NOT incubate cells in water for longer than 30 sec since significant neutrophil death can occur.
- Centrifuge at 250 x g for 5 min at 18-22 °C.
- Wash the cells once with 10 ml of PBS (without Ca2+ and Mg2+) and centrifuge again at 250 x g for 5 min at 18-22 °C.
- Resuspend the neutrophils in 10 ml of RPMI-1640 (without phenol red) and count the live cells using the trypan blue dye exclusion method. Avoid prolonged periods of cell densities greater than 5 x 106 per ml since this may result in activation of the neutrophils.
3. Neutrophil Labeling with Calcein AM
- 6 x 105 neutrophils are used per well of a 48-well plate.
- After counting, transfer the resuspended cells to a 50 ml conical tube and dilute the neutrophils to 2-4 x 106 cells per ml with phenol red-free RPMI-1640.
- Add calcein AM stock solution to 3 μM (i.e. 2.98 μl of calcein AM stock (1 mg/ml) per ml of media) and mix well by gently flicking the tube. Greater than 5 μM calcein AM will result in leakage from the neutrophils. Note: Non-fluorescent calcein AM is cleaved within the cells by endogenous esterases to produce the highly fluorescent molecule calcein (i.e. dead cells will not fluoresce).
- Cover the tube with aluminum foil and incubate for 30 min at 37 °C in the dark. Note: Longer incubation times may result in more calcein being liberated from the neutrophils over the course of the adhesion assay.
- Centrifuge at 450 x g for 5 min at 18-22 °C, and wash the neutrophils 2x with 10 ml of RPMI-1640 (without phenol red; pre-warmed to 37 °C). After resuspending the cells for the second wash, first pass the neutrophils though a 40 μM sterile filter to remove clumps and then centrifuge once more at 450 x g for 5 min at 18-22 °C.
- Resuspend the neutrophils in RPMI 1640 (without phenol red; pre-warmed to 37 °C) at 2 x 106 neutrophils per ml.
4. Neutrophil Binding Assay
- Wash the HMVEC monolayers 3x with 0.5 ml of filter-sterilized (0.22 μm) RPMI 1640 (without phenol red) containing 3% BSA per well.
- Aspirate the media and add 6 x 105 calcein-labeled neutrophils (0.3 ml of cell suspension), or 0.3 ml of RPMI 1640 (without phenol red) without neutrophils, to the appropriate wells of the 48-well plate. Note: This is the equivalent of 8 x 105 neutrophils per cm2.
- Incubate for 20 min in a 37 °C, 5% CO2 incubator.
- Total Neutrophil Fluorescence (PRE wash): Measure the fluorescence intensity of each well with an excitation wavelength of 485 nm and an emission wavelength of 520 nm (fluorescein filter set) in a FLUOstar, or equivalent, spectrophotometer. Perform this step just prior to the incubation end point. Note: In this protocol, calcein excitation and detection of the emitted light were performed from the top of the uncovered wells; however, both can also be performed from the bottom of the wells.
- Wash the wells containing HMVEC monolayers and neutrophils 5x with 0.5 ml per well of PBS (w/ Ca2+ and Mg2+). Remove the media and washes by inverting the plate, and patting gently onto paper towels to remove the remaining liquid.
- Add back 0.3 ml of RPMI 1640 (without phenol red) per well.
- Adherent Neutrophil Fluorescence (POST wash): Measure the fluorescence intensity of each well as in Step 4.4.
- Determine the percent adherence and relative adherence per well:
Note: At this stage, images of the calcein-labeled neutrophils can be obtained by using a fluorescence capable microscope equipped with standard fluorescein filters. The images in Figure 4 were obtained on an Olympus IX51 inverted microscope equipped with a Retiga 2000R camera (Q Imaging) using Q-Capture Pro7 software (Q Imaging).
Representative Results
In order to obtain reliable, reproducible results using a neutrophil binding assay, it is essential that the health and the confluency of the microvascular endothelial cells are optimal on the day of the assay, as illustrated with HMVEC-Lung in Figure 1. In addition, it is imperative that low passage number microvascular ECs are used (i.e. less than 9 passages), and accordingly, we recommend performing all experiments within two weeks of thawing. The health of the neutrophils is also of utmost importance, especially since calcein AM will not be metabolized to the fluorescent calcein molecule if the cells are comprised in some manner. We use the trypan blue exclusion method, and do not proceed with the assay if a significant proportion of the cells are dead (i.e. stained).
There are several ways to isolate neutrophils, including density gradient and antigen-based column separation methods, and any of these should suffice for this procedure. We chose to use the Polymorphprep ready-made density gradient solution from Axis-Shield. As can be seen in Figure 2, the PBMCs are present in the top layer, while the neutrophils are within the lower layer after centrifugation. The PBMC layer is aspirated in order to avoid contamination of the granulocytes upon their removal. It is important to note that step 2.9, in which water is added to lyse any remaining red blood cells, is optional. However, while it is ideal to remove all contaminating erythrocytes, it is critical that the neutrophils do not sit in pure water for more than the 30 sec after which considerable death can occur.
Finally, the neutrophils are incubated with calcein AM for 30 min, washed and resuspended in pre-warmed (37 °C) RPMI-1640 media (without phenol red). It is important to use media without phenol red, which can interfere with the fluorescence reading. The number of neutrophils added to the wells is rather arbitrary, provided that the fluorescence readings pre- and post-wash are within the linear range of the spectrophotometer, and the differences between treatments that either promote or prevent neutrophil adherence can be reproducibly ascertained (see below). We find that the fluorescence OD520nm is within the linear range when 10,000 to 1,000,000 labeled neutrophils are analyzed (Figure 3A). Interestingly, we also find that the percent adherence increases as more neutrophils are added to HMVEC-Lung monolayers treated with TNFa (100 ng/ml) for 3 hr (Figure 3B). We chose to incubate the HMVEC-Lung and calcein-labeled neutrophils together for 20 min, since we find that the percentage of adhesion does not significantly increase after this time point (data not shown). Of note, in this assay we used standard, clear polystyrene 48-well plates and found that the level of fluorescence cross-over readings between the wells did not amount to more than 0.5% of the total fluorescence input (data not shown). Nonetheless, we recommend using 48-well plates purposely made for fluorescence spectrophotometer readings for more sensitive experiments, if available, or at the very least, designing your experiment to minimize cross-over readings (see Step 1.2).
To demonstrate that the number of neutrophils added to the wells is rather arbitrary so long as the number added is within the linear range of the spectrophotometer, we performed a neutrophil adhesion assay with TNFα-treated HMVEC-Lung in the absence or presence of the p38-MAPK inhibitor BIRB7906, using varying input amounts of neutrophils (i.e. 40,000/well, 200,000/well or 1,000,000/well) 25. p38-MAPK has previously been shown to be necessary for E-selectin expression in TNFα-treated human ECs, and its inhibition should therefore reduce neutrophil binding to TNFα-activated HMVEC-Lung 26. In fact, this is what we observe (Figure 3C). Although we find that the percent adherence increases as more neutrophils are added, and the percent adherence may differ between experiments, due to various factors such as inter-donor variations, the quality of the neutrophils and the density of HMVEC at confluence, the relative adherence between conditions remains relatively constant in each independent experiment regardless of the number of neutrophils added (Figure 3C and data not shown). These data also exemplify why it is better to display the adhesion data as relative to a positive control for inter-experimental consistency.
In order to fully illustrate how this assay works, we performed a neutrophil adhesion assay with TNFα-treated HMVEC-Lung pre-incubated with either control antibody or an E-selectin blocking antibody. E-selectin is expressed on the EC surface after treatment with TNFa and captures neutrophils from the vasculature via electrostatic interactions with glycomolecules present on the neutrophil surface 1,27 . As shown numerically in Figure 4A, graphically in Figure 4B and visually in Figure 4C, the TNFα-treated HMVEC-Lung pre-incubated with the E-selectin antibody bound ~75% less neutrophils than the TNFα-treated HMVEC-Lung pre-incubated with control IgG. This suggests E-selectin plays an important role in tethering neutrophils to HMVEC-Lung in our static adhesion assay.
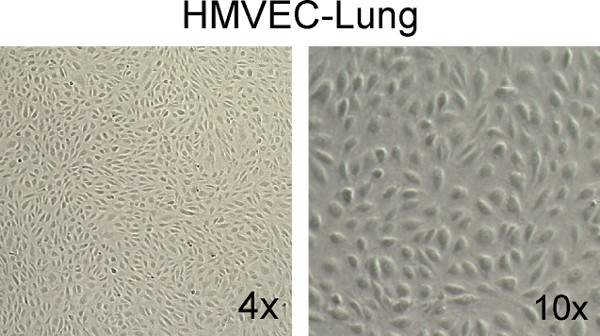
Figure 1. Example of healthy, confluent HMVEC-Lung monolayers. Note the "cobblestone" appearance and total confluency of the monolayers. Images were taken at 4X and 10X magnification on a Fisher Scientific Micromaster inverted digital microscope.
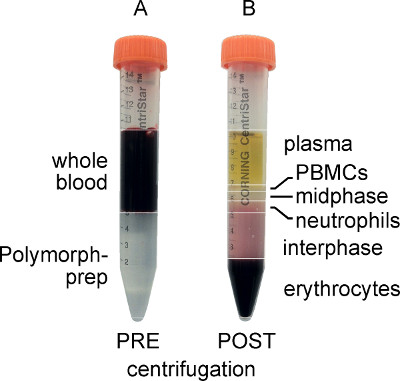
Figure 2. Whole blood and Polymorphprep layered prior to centrifugation (A) and separated after centrifugation (B). Note that after centrifugation, the PBMCs form a distinct upper band, while the polymorphonuclear granulocytes (neutrophils) form a distinct lower band.
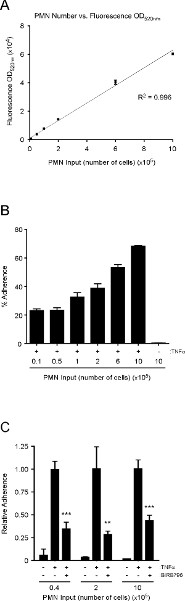
Figure 3. The relationship between neutrophil number and fluorescence OD520nm is linear; the percent adhesion of neutrophils to TNFα-treated HMVEC-Lung increases as more neutrophils are added; p38-MAPK promotes neutrophil adherence to TNFα-activated HMVEC-Lung monolayers. (A) Graph depicting the linear relationship between fluorescence OD520nm and neutrophil numbers over two orders of magnitude. An R2 value of 0.996 indicates a linear relationship between neutrophil number and fluorescence OD520nm when 10,000 to 1,000,000 neutrophils are analyzed. (B) Graphical representation of the percent adherence when varying numbers of calcein-labeled neutrophils were added to untreated or TNFα-treated (100 ng/ml; 3 hr) HMVEC-Lung monolayers in 48-well plates. (C) HMVEC-Lung monolayers were pre-incubated with the p38-MAPK inhibitor BIRB796 (10 mM) (Axon Medchem) or DMSO (control) for 1 hr prior to TNFa (100 ng/ml) treatment for 3 hr while in the continuous presence of BIRB796 (10 mM). The relative adherence was calculated as in step 4.8. Data are expressed as the mean ± SD. GraphPad Prism unpaired t-test was used for statistical analysis (n = 3) (TNFα-treated vs. TNFα-treated plus BIRB796). p-value ** < 0.01; *** < 0.001. Click here to view larger figure.
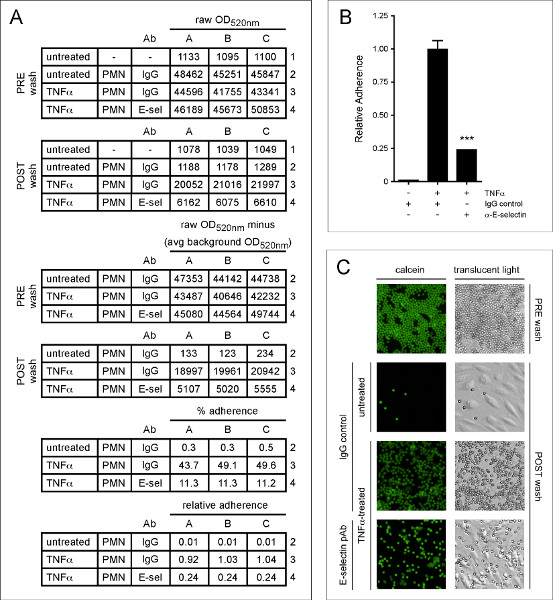
Figure 4. E-selectin promotes the adherence of neutrophils to TNFa-treated HMVEC-Lung. HMVEC-Lung were treated with TNFα (100 ng/ml) for 3 hr. After washing the HMVEC-Lung with RPMI 1640 containing 3% BSA (step 4.1), either sheep IgG (50 μg/ml) (5-001-A; R&D Systems) or E-selectin polyclonal antibody (pAb) (50 mg/ml) (AF724; R&D Systems) were added to the appropriate wells for 20 min. The HMVEC-Lung monolayers were then washed once more with RPMI-1640 before 6 x 105 neutrophils were added per well for an additional 20 min. (A) The calculations to determine the percent and relative adherence are shown. Calcein light emission is measured at OD520 nm. Background OD520 nm average is derived from HMVEC-Lung in the absence of TNFα treatment and neutrophils. (B) Graphical representation of relative percent adherence of calcein-labeled neutrophils to untreated or TNFα-treated HMVEC-Lung monolayers after pre-incubation with either control IgG or E-selectin blocking pAb. Data are expressed as the mean ± SD. GraphPad Prism unpaired t-test was used for statistical analysis (n = 3). Calculated p-value was < 0.001. (C) Images of calcein-labeled neutrophils bound to untreated and TNFα-treated HMVEC-Lung monolayers pre-incubated with either control IgG or E-selectin pAb. An example well containing 6 x 105 neutrophils prior to washing with PBS is shown (PRE wash). Translucent light microscopy images are shown in the right-hand column, while fluorescence images of the same field of reference, using a fluorescein filter set, are shown in the left-hand column. Images were taken at 10X magnification on an Olympus IX51 inverted microscope equipped with a Retiga 2000R camera (Q Imaging). PRE wash = total neutrophil fluorescence OD520nm (Step 4.4); POST wash = adherent neutrophil fluorescence OD520nm (Step 4.7); PMN – polymorphonuclear granulocytes; avg – average; IgG – sheep IgG control; E-sel/α-E-selectin – E-selectin blocking pAb. Click here to view larger figure.
Discussion
The most critical steps for a successful neutrophil / microvascular endothelial cell adhesion assay are: 1) Use of low passage number (<9), healthy endothelial cells; 2) Maintaining the isolated neutrophils at a low density (i.e. <5 x 106 cells / ml) and using them within two hours of isolation; and 3) Fastidious washing of the calcein AM-labeled neutrophils and use of the calcein AM-labeled neutrophils in a timely fashion to minimize EC contamination and loss of calcein from the neutrophils, respectively.
We add 8 x 105 neutrophils per cm2 of tissue culture well surface area (i.e. 600,000 neutrophils per well of a 48-well plate). We find that an input of 600,000 neutrophils is within the linear range of our spectrophotometer, and reliably reproduces data when relative adherences are measured. However, a very similar decrease in neutrophil binding is observed in the p38-MAPK inhibition assay (Figure 3C) when 40,000, 200,000 or 1,000,000 neutrophils are added per well, suggesting that less than 600,000 neutrophils can be used.
The protocol described herein only assesses the extent of endothelial activation since the neutrophils themselves are not pre-activated, or treated in any way, which is of course an option if your particular assay requires it. We show that the expression of E-selectin on TNFa-treated HMVEC-Lung is important for the attachment of the neutrophils in this assay, consistent with previous observations 27. The electrostatic binding of E-selectin to glycomolecules present on the neutrophil surface are relatively weak, but under flow conditions, these interactions are thought to induce the high-affinity integrin-mediated attachments between neutrophils and ECs 1,7-10. Therefore, this assay may be more indicative of the initial steps in inflammation that are necessary to capture neutrophils from the vasculature. Nonetheless, we have verified that our results obtained with TNFα in this report were highly similar, if not identical, to ones obtained using a flow module (data not shown), indicating the results of this static assay are physiologically relevant and suggests that this assay is particularly useful for researchers that do not have access to a flow system.
Variations of the described assay, using calcein as the detection fluorophore, were first described in 1993 28,29. Importantly, these initial reports found that calcein did not affect cell viability and had the least effect on cell function in adhesion assays when compared to other fluorescein-derived dyes 29,30. It was also reported that cell number is linear with respect to fluorescent intensity, consistent with what we observe (Figure 3A) 28.Initial reports also highlighted that a major advantage of fluorometric assays is that they do not require radiolabeling (e.g. 111In or 51Cr) of the purified leukocytes 31-33. The advantages and consistencies of using calcein AM-labeled cells over radiolabeled cells have been thoroughly discussed previously 28,29,34. Although we use calcein AM to label the neutrophils in our assays, several other cell permeant dyes that are esterase substrates are available. For example, Life Technologies sells CellTrace calcein red-orange (C34852), calcein violet (C34858) and calcein blue (C34853) that excite/emit at various wavelengths. The different dyes each have their advantages and disadvantages. For instance, the calcein red-orange AM dye has some intrinsic fluorescence prior to hydrolysis 18.
We use this method to study inflammatory activation of the endothelium in response to bacterial and endogenous factors (such as TNFa), and thus use HMVECs collected from cadavers, which can be expanded in sufficient quantity to do the assay with proper controls and enough replicates to perform statistical analyses. Conceivably, this assay could also be used as a tool to study endothelial-based disease processes that involve leukocyte binding to the microvascular endothelium. Examples of disorders that might be studied using HMVECs from patients include chronic obstructive pulmonary disease (COPD), sepsis, inflammatory bowel disease, vasculitides, such as the anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides, and brain vascular malformations 35-40. For instance, HMVECs could be collected from humans with vasculitides or sepsis post-mortem, or potentially from patients with endothelial based disease processes that have undergone a biopsy or surgical resection of an organ, and their binding to neutrophils assessed under different conditions. However, this assay requires sufficient quantities of HMVECs to form confluent monolayers, and thus HMVECs are expanded and used after multiple passages. This process of expansion may lead to phenotypic changes in the ECs, which would need to be taken into account in designing studies using primary ECs from patients with EC-based diseases. Nevertheless, the versatility of this assay allows it to be adapted to many other adherent and leukocyte cell types. In fact, this assay, or variants of it, have been successfully used with other endothelial cells types, and primary cells and cell lines such as monocytes, THP-1, Jurkat, HL-60 and U937 28,31,41. This assay has proven to be rapid, easy to perform, reproducible and highly sensitive, and is therefore a very useful tool to detect endothelial cell activation by inflammatory mediators.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the UCSF Department of Anesthesia and Perioperative Care.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| HMVEC-Lung | Lonza | CC-2527 | |
| EGM-2 MV | Lonza | CC-3202 | |
| HBSS | Life Technologies | 14175-095 | Can be substituted with any vendor |
| 48-well Tissue Culture Plates | BD Falcon | 353078 | |
| PBS (without phenol red) | UCSF Cell Culture Facility | CCFAL001 | Can be substituted with any vendor |
| D-PBS (without Ca2+ and Mg2+ and phenol red) | UCSF Cell Culture Facility | CCFAL003 | Can be substituted with any vendor |
| RPMI-1640 (without phenol red) | Life Technologies | 11835-030 | |
| Polymorphprep | Axis-Shield | 1114683 | |
| calcein AM | Life Technologies | C3099 | (0.995 mM) stock soln |
| Trypan Blue Solution | Sigma-Aldrich | T8154 | |
| 40 μM filters | VWR | 21008-949 | Can be substituted with any vendor |
| FLUOstar OPTIMA Spectrophotometer | BMG LABTECH | – |
References
- Ley, K., Laudanna, C., Cybulsky, M. I., Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678-689 (2007).
- Collins, T., et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 9, 899-909 (1995).
- Medzhitov, R. Origin and physiological roles of inflammation. Nature. 454, 428-435 (2008).
- Chen, G. Y., Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826-837 (2010).
- Middleton, J., et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 91, 385-395 (1997).
- Rot, A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol. Today. 13, 291-294 (1992).
- Detmers, P. A., et al. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 171, 1155-1162 (1990).
- Laudanna, C., Kim, J. Y., Constantin, G., Butcher, E. Rapid leukocyte integrin activation by chemokines. Immunol. Rev. 186, 37-46 (2002).
- Simon, S. I., Hu, Y., Vestweber, D., Smith, C. W. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 164, 4348-4358 (2000).
- Zarbock, A., Ley, K. Mechanisms and consequences of neutrophil interaction with the endothelium. The American Journal of Pathology. 172, 1-7 (2008).
- Muller, W. A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 6, 323-344 (2011).
- Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303, 1532-1535 (2004).
- Kumar, V., Sharma, A. Neutrophils: Cinderella of innate immune system. International Immunopharmacology. 10, 1325-1334 (2010).
- McDonald, B., et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 330, 362-366 (2010).
- Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173-182 (2006).
- Soehnlein, O., Lindbom, L., Weber, C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 114, 4613-4623 (2009).
- Wilhelmsen, K., Mesa, K. R., Prakash, A., Xu, F., Hellman, J. Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response. Innate Immun. 18, 602-616 (2012).
- Haugland, R. P., Johnson, I. D., Basey, A. . The Handbook: A Guide to Fluorescent Probes and Labelling Technologies. , (2005).
- Shin, H. S., et al. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J. Immunol. 186, 1119-1130 (2011).
- Wilhelmsen, K., Mesa, K. R., Lucero, J., Xu, F., Hellman, J. ERK5 protein promotes, whereas MEK1 protein differentially regulates, the Toll-like receptor 2 protein-dependent activation of human endothelial cells and monocytes. J. Biol. Chem. 287, 26478-26494 (2012).
- Pober, J. S., Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803-815 (2007).
- Oh, H., Siano, B., Diamond, S. Neutrophil isolation protocol. J. Vis. Exp. (17), e745 (2008).
- Nuzzi, P. A., Lokuta, M. A., Huttenlocher, A. Analysis of neutrophil chemotaxis. Methods Mol. Biol. 370, 23-36 (2007).
- Garcia-Garcia, E., Uribe-Querol, E., Rosales, C. A simple and efficient method to detect nuclear factor activation in human neutrophils by flow cytometry. J. Vis. Exp. (74), e50410 (2013).
- Kuma, Y., et al. BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J. Biol. Chem. 280, 19472-19479 (2005).
- Westra, J., Kuldo, J. M., van Rijswijk, M. H., Molema, G., Limburg, P. C. Chemokine production and E-selectin expression in activated endothelial cells are inhibited by p38 MAPK (mitogen activated protein kinase) inhibitor RWJ 67657. International Immunopharmacology. 5, 1259-1269 (2005).
- Leeuwenberg, J. F., Jeunhomme, G. M., Buurman, W. A. Adhesion of polymorphonuclear cells to human endothelial cells. Adhesion-molecule-dependent, and Fc receptor-mediated adhesion-molecule-independent mechanisms. Clinical and Experimental Immunology. 81, 496-500 (1990).
- Akeson, A. L., Woods, C. W. A fluorometric assay for the quantitation of cell adherence to endothelial cells. Journal of Immunological Methods. 163, 181-185 (1993).
- Vaporciyan, A. A., Jones, M. L., Ward, P. A. Rapid analysis of leukocyte-endothelial adhesion. Journal of Immunological Methods. 159, 93-100 (1993).
- De Clerck, L. S., Bridts, C. H., Mertens, A. M., Moens, M. M., Stevens, W. J. Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. Journal of Immunological Methods. 172, 115-124 (1994).
- Bevilacqua, M. P., Pober, J. S., Wheeler, M. E., Cotran, R. S., Gimbrone, M. A. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. The Journal of Clinical Investigation. 76, (1985).
- Schleimer, R. P., Rutledge, B. K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J. Immunol. 136, 649-654 (1986).
- Gamble, J. R., Harlan, J. M., Klebanoff, S. J., Vadas, M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc. Natl. Acad. Sci. U.S.A. 82, 8667-8671 (1985).
- Braut-Boucher, F., et al. A non-isotopic, highly sensitive, fluorimetric, cell-cell adhesion microplate assay using calcein AM-labeled lymphocytes. Journal of Immunological Methods. 178, 41-51 (1995).
- Ait-Oufella, H., Maury, E., Lehoux, S., Guidet, B., Offenstadt, G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Medicine. 36, 1286-1298 (2010).
- Andonegui, G., et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. The Journal of Clinical Investigation. 111, 1011-1020 (2003).
- Sharma, J., et al. Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. American Journal of Physiology. Lung Cellular and Molecular Physiology. 302, L47-L55 (2012).
- Deban, L., Correale, C., Vetrano, S., Malesci, A., Danese, S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. The American Journal of Pathology. 172, 1457-1466 (2008).
- Gross, W. L., Trabandt, A., Csernok, E. Pathogenesis of Wegener's granulomatosis. Annales de Medecine Interne. 149, 280-286 (1998).
- Chen, Y., et al. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery. 62, 1340-1349 (2008).
- Martens, C. L., et al. Peptides which bind to E-selectin and block neutrophil adhesion. J. Biol. Chem. 270, 21129-21136 (1995).

