Enrichment and Purging of Human Embryonic Stem Cells by Detection of Cell Surface Antigens Using the Monoclonal Antibodies TG30 and GCTM-2
Summary
We describe the use of the monoclonal antibodies TG30 (CD9) and GCTM-2 for the combined detection of cell surface antigens via fluorescence activated cell sorting (FACS) for the identification and enrichment of live human embryonic stem cells (hESC) using positive selection and also the use of negative selection to purge hESCs from a mixed cell population.
Abstract
Human embryonic stem cells (hESC) can self-renew indefinitely in vitro, and with the appropriate cues can be induced to differentiate into potentially all somatic cell lineages. Differentiated hESC derivatives can potentially be used in transplantation therapies to treat a variety of cell-degenerative diseases. However, hESC differentiation protocols usually yield a mixture of differentiated target and off-target cell types as well as residual undifferentiated cells. For the translation of differentiated hESC-derivatives from the laboratory to the clinic, it is important to be able to discriminate between undifferentiated (pluripotent) and differentiated cells, and generate methods to separate these populations. Safe application of hESC-derived somatic cell types can only be accomplished with pluripotent stem cell-free populations, as residual hESCs could induce tumors known as teratomas following transplantation. Towards this end, here we describe a methodology to detect pluripotency associated cell surface antigens with the monoclonal antibodies TG30 (CD9) and GCTM-2 via fluorescence activated cell sorting (FACS) for the identification of pluripotent TG30Hi-GCTM-2Hi hESCs using positive selection. Using negative selection with our TG30/GCTM-2 FACS methodology, we were able to detect and purge undifferentiated hESCs in populations undergoing very early-stage differentiation (TG30Neg-GCTM-2Neg). In a further study, pluripotent stem cell-free samples of differentiated TG30Neg-GCTM-2Neg cells selected using our TG30/GCTM-2 FACS protocol did not form teratomas once transplanted into immune-compromised mice, supporting the robustness of our protocol. On the other hand, TG30/GCTM-2 FACS-mediated consecutive passaging of enriched pluripotent TG30Hi-GCTM-2Hi hESCs did not affect their ability to self-renew in vitro or their intrinsic pluripotency. Therefore, the characteristics of our TG30/GCTM-2 FACS methodology provide a sensitive assay to obtain highly enriched populations of hPSC as inputs for differentiation assays and to rid potentially tumorigenic (or residual) hESC from derivative cell populations.
Introduction
HPSC (hPSC) include human embryonic stem cells (hESC) and human induced pluripotent stem cells (hIPSC). HPSC can self-renew indefinitely in appropriate culture conditions without losing their ability known as "pluripotency". Pluripotency is defined as the potential for a cell to differentiate into essentially any somatic cell lineage including cells representative of each of the three embryonic germ cell layers. The remarkable potential of hPSC provides a means for a large variety of cell-based applications including therapeutic options. For instance, there are disorders involving cell death and degeneration, where normal functionality of cells in the human body is compromised, as in heart failure, spinal injuries, diabetes, Parkinson's disease, some cancers, and other clinical pathologies. Patients suffering these conditions could potentially be treated by transplanting healthy and functional somatic cells that have been derived from hPSC in the laboratory. However, the current hPSC differentiation protocols are not 100% efficient and yield a mixture of differentiated target and off-target cell types, as well as residual hPSCs that have not undergone differentiation, instead continuing to self-renew1-3. The presence of even a small number of hPSC in a sample of hPSC-derived somatic cells intended for transplantation into patients constitutes a serious clinical risk as these cells by their inherent nature to form tissues of all three embryonic germ layers, could potentially form in vivo a type of tumor known as a teratoma. Therefore, only target cell populations determined to be free of pluripotent stem cells can be considered safe for transplantation into patients. There are several approaches reported to potentially accomplish the purging of residual hPSCs following differentiation, (for review see Polanco and Laslett4). We have previously reported the use of the monoclonal antibodies TG30 (CD9) and GCTM-2 coupled to fluorescence activated cell sorting (FACS) to identify pluripotent stem cells and discriminate them from cells undergoing early stage of differentiation in cultures of hESC lines5-7.
One advantage of using antibodies to detect cell surface antigens is that the target cells are usually viable after antibody binding and/or labeling. Therefore, target cells can be collected after antibody labeling and recultured for expansion and further applications before transplantation. One caveat for cell surface antigens expressed on hPSC is that they are not exclusive to the pluripotent stage and are in many cases re-expressed temporally during development and will therefore be detected in some differentiated cell types. Therefore, if the aim is to use antibodies to detect human pluripotent cells and purge them from a sample of hPSC-derived cells, selected antibodies should not also react with antigens on the specific differentiated cell types intended for transplantation. Unfortunately, there are limited numbers of antibodies that detect cell surface markers on live hPSCs 4, making limited the options for selection. In addition, a few studies have pointed out that detection of one single cell-surface marker is not sufficient to eliminate all hPSC, suggesting that any attempt to eliminate all hPSC pluripotent subpopulations should rely on methods that use two or more antibodies detecting different epitopes expressed by hPSCs 9-10. As mentioned above, only hPSC-derived cells that could be determined as pluripotent stem cell-free cell populations are appropriate for human transplantation. Reaching this level of stringency may not be achieved with a single pass through an antibody-mediated cell sorting technology. Reculture of the enriched population of differentiated target cells and subsequent rounds of cell sorting may be required to definitively obtain pluripotent stem cell-free samples.
In our laboratory, we have extensively characterized two hES cell-surface antibodies, TG30 (CD9) and GCTM-2, for the detection of live pluripotent cells. Our studies have shown that combined detection of both TG30 and GCTM-2 strongly correlates with the expression of canonical pluripotency-associated genes in hESC lines 5-7. TG30/GCTM-2 FACS immunoprofiling has consistently shown that hESC cultures constitute a quantitative continuous gradient of TG30/GCTM-2 expression 5-7. We have arbitrarily established four populations (P) of cells within this TG30/GCTM-2 gradient: P4 (TG30Neg-GCTM-2Neg), P5 (TG30Low-GCTM-2Low), P6 (TG30Mid-GCTM-2Mid) and P7 (TG30Hi-GCTM-2Hi) 5-7. Our characterization of these P4, P5, P6 and P7 cell populations has shown that the P6 and P7 subfractions express a large number of pluripotency-associated genes and efficiently form stem-like colonies when recultured post-FACS 2-3. On the other hand, P4 (TG30Neg-GCTM-2Neg) cells express a large number of differentiation markers and constitute the spontaneously differentiated cell types that typically occur in expanding cultures of hESC lines 5-6. We decided to test the potentiality of our TG30/GCTM-2 FACS for the selective elimination of residual hPSCs following early stage differentiation, and also for the enrichment of pluripotent stem cell populations. The protocol described below shows how to collect and reculture differentiated P4 (TG30Neg-GCTM-2Neg) cells post-FACS to accomplish purging of pluripotent P7 (TG30Hi-GCTM-2Hi). Furthermore, we also explain the collection and reculture of pluripotent P7 (TG30Hi-GCTM-2Hi) cells to obtain an enriched culture of pluripotent cells, which could subsequently be used as a defined input population to potentially increase the efficiency and consistency of differentiation assays.
Protocol
The following protocol was performed using hESC-MEL111 standard bulk cultures provided by the StemCore facility at Monash University (Melbourne). This cell line is routinely cultured on a layer of mitotically inactivated mouse embryonic fibroblasts (MEFs) in bFGF supplemented hESC/KOSR media7 and is maintained with enzymatic dissociation (Collagenase) each 5-7 days8. hESC cultures grown to ~80% confluency in 75 cm2 (T75) flasks are used as input populations for this protocol. All the cell manipulation procedures described below should be performed under aseptic conditions in a HEPA-filtered class II bio-safety cabinet.
Two days prior to performing a TG30/GCTM-2 FACS assay, prepare the T75 culture plates (described in section 1) that are to be used for the reculture of cells recovered post-FACS (described in section 4).
1. Seeding of MEFs and Preparation of MEF-conditioned Medium
1.1 DAY -2: Plating of MEFs into T75 flasks
- Pipette 10 ml of 0.1% w/v gelatin into each T75 flask and tilt to coat surface evenly.
- Incubate for 30 min at room temperature in a biosafety cabinet.
- Aspirate gelatin solution.
- Add 20 ml MEF-medium to the flask and pre-equilibrate to 37 °C/5% CO2 for 30 min in a cell culture incubator.
- Plate mitotically inactivated MEFs onto prepared flask(s) at the following densities. For 1/3 MEF density seed 1.4 x 104 cells/cm2 (T75= 1 x 106 MEFs). For full density MEF seed 5.3 x 104 cells/cm2 (T75 = 4 x 106 MEFs).
Note: We routinely use MEFs that were mitotically inactivated by γ-irradiation. A protocol for preparation of γ-irradiated MEFs can be found in Michalska12.
- Incubate irradiated MEF cultures at 37 °C/5% CO2 overnight. The 1/3 flasks can be incubated for 1-4 days without MEF-medium change. These flasks are used for replating of post-FACS cells (as in protocol 4). Full MEF flasks are incubated overnight to generate CM as per below in step 1.2.
1.2 DAY -1: Preparation of MEF-conditioned medium (CM)
- Aspirate MEF-medium from full-MEF T75 flasks.
- Add pre-equilibrated 25 ml hESC/KOSR medium supplemented with 5 ng/ml human FGF-2 per T75 flask. Incubate at 37 °C/5% CO2 for 24 hr.
- Collect MEF-conditioned medium (CM) into a sterile tissue culture tube, freshly supplement CM with 10 ng/ml human FGF-2 and filter using a 0.22 μm polyethersulfone filter.
- To produce additional CM, repeat steps 1.2.2 and 1.2.3 daily on the same MEF culture for one week.
On the day of performing a TG30/GCTM-2 assay, replace MEF-medium in 1/3 MEF T75 flasks with CM and pre-equilibrate for at least 30 min prior to seeding any hESCs or hESC-derivative cells recovered from the procedure as described below. CM is either used fresh or stored at 4 °C for up to 2 weeks. CM is used in this protocol as in our hands it increases the viability of the cells post-FACS compared to nonconditioned media (results not shown).
2. Performing a TG30/GCTM-2 FACS Assay: Dissociation of hESC Bulk Cultures into Single Cells
Note: Prechill 20% FBS/DMEM-F12 medium at 4 °C.
- Aspirate the hESC/KOSR media from two T75 flasks with a cultured hESC line.
- Rinse the cells in each T75 with 10 ml DPBS and aspirate to remove.
- Add 3 ml TrypLE Express dissociation solution into each T75 flask. Incubate at 37 °C/5% CO2 for 5 min.
- Bump the side of the flasks to obtain complete dislodgement of the cells. Check under microscope. If cells do not dislodge easily, incubate for a further 2-3 min at 37 °C/5% CO2.
- Add 10 ml 20% FBS/DMEM-F12 (kept at 4 °C) to each T75 flask. Using a sterile 10 ml pipette gently triturate the dissociated cells several times against the flask wall to achieve a predominantly single cell suspension.
- Place a 70 μm cell strainer on a 50 ml sterile polypropylene tube. Use a sterile 10 ml pipette to force the hESC single cell suspensions pooled from the two T75 flasks through the strainer.
- Discard cell strainer, replace the tube lid and centrifuge the pooled hESC suspension at 1,000 x g for 2 min.
- Discard supernatant and wash the cells by gently resuspending the pellet in 10 ml 20% FBS/DMEM-F12 (prechilled at 4 °C).
- Perform a second centrifugation at 1,000 x g for 2 min.
- Discard supernatant, add 10 ml 20% FBS/DMEM-F12 (prechilled at 4 °C) and gently triturate to resuspend cells. Place the 50 ml tube on ice. This tube will be used for the Sort Sample immunostaining procedure described below.
- Take 20 μl of the hESC single cell suspension (step 2.10) for cell number counting using a hemotocytometer. You should expect a yield of 10-15 million cells per T75 flask.
Note: FBS is used in this part of the protocol to increase cell viability post-FACS.
3. Immunofluorescent Staining of Cell Surface Antigens TG30 and GCTM-2
- Aliquot 150 μl (~100,000 cells) of a hESC single cell suspension (from step 2.11) into each of six microcentrifuge tubes of 1.5 ml. These six aliquots will be used for the staining controls required to calibrate the assay on the FACS machine. The remaining ~9 ml cell suspension will be your Sort Sample in which the dissociated culture will be costained for detection of TG30 and GCTM-2, according to Tables 1 and 2.
- Perform binding reactions of primary antibodies in 20% FBS/DMEM-F12. Final reaction volumes should be ~9 ml for the Sort Sample and 200 μl for the controls. Include isotype controls as primary antibodies. Place tubes horizontally on ice and mix gently for 30 min on a rocking platform.
- Centrifuge primary antibody reactions at 1,000 x g for 2 min.
- Discard supernatant and wash the cells by resuspending the pellet in 20 ml 20% FBS/DMEM-F12 (prechilled at 4 °C) for the Sort Sample and 200 μl for the controls.
- Perform a second spin at 1,000 x g for 2 min.
- Perform binding reactions of secondary antibodies in 20% FBS/DMEM-F12. Final reaction volumes should be 2.5 ml for the Sort Sample and 200 μl for the controls. Prepare the PE-anti-mouse CD90.2 reaction tube at this stage. Place tubes horizontally on ice and mix gently for 30 min on a rocking platform.
Note: During this incubation time, it is convenient to collect CM and prepare 1/3 MEF T75 flasks with CM as in section 1.2.
- Centrifuge secondary antibody reactions at 1,000 x g for 2 min.
- Discard supernatant and wash the cells TWICE by resuspending the pellet in 20 ml 20% FBS/DMEM-F12 (prechilled at 4 °C) for the Sort Sample and 200 μl for the controls.
- Perform a spin at 1,000 x g for 2 min following each wash and place tubes on ice.
- Discard supernatants and resuspend cell pellets in 20% FBS/DMEM-F12 supplemented with propidium iodide (PI) at 0.3 μg/ml. Use 2-3 ml for the Sort Sample and 300 μl for the controls.
Note: DO NOT add PI to the control tube with unstained cells.
- Use a P1000 micropipette to force each individual cell suspension through a 35 μm lid cell strainer, coupled to 5 ml tube polystyrene round bottom test tubes.
- Centrifuge at 50 g/min to force through residual cell suspension on the strainer lids.
- Resuspend each single cell suspension by tapping the sides of the tubes, and place on ice. Your cells are now ready for FACS. Please proceed straightaway.
4. Post-FACS Culture of TG30/GCTM-2 Subfraction Cells in 75 cm2 (T75) Flasks
- We have previously reported how to set up a FACS machine for TG30/GCTM-2 immunoprofiling7. The procedure allows the recovery of viable single hESCs, free of MEFs, and within the gates of P4 (TG30Neg-GCTM-2Neg), P5 (TG30Low-GCTM-2Low), P6 (TG30Mid-GCTM-2Mid) and P7 (TG30Hi-GCTM-2Hi) – see Figure 1E. Set up the FACS machine for TG30/GCTM-2 FACS as in our previous report to recover the cell subfractions to be used next.
- Add 1 ml CM, prepared as in step 1.2, to each of two 5 ml polystyrene round bottom test tubes prior to collecting cells from the FACS machine. Place on ice.
- Recover 400,000 cells each from differentiated P4 (TG30Neg-GCTM-2Neg) and pluripotent P7 (TG30Hi-GCTM-2Hi) cell subfractions using TG30/GCTM-2 FACS.
- Centrifuge the collection tubes at 1,000 x g for 5 min.
- Aspirate the supernatants. Add 1 ml prequilibrated 37 °C/5% CO2 CM to resuspend each cell pellet using a P1000 micropipettor. Seed the cells for each fraction onto a pre-equilibrated 1/3 MEF T75 flask with CM (prepared as per step 1.2)
- Culture at 37 °C/5% CO2 for 24 hr in a cell culture incubator.
- Aspirate CM daily and replace with freshly prepared CM (as in step 1.2) for approximately two weeks. Cultures of pluripotent P7 (TG30Hi-GCTM-2Hi) cells show human stem-like colonies after one week and reach ~80% confluency at 9-11 days. Cultures of P4 (TG30Neg-GCTM-2Neg) cells mostly grow as a lawn of fibroblast-like cells that reaches confluency at 11-13 days. At this stage, if required, P4 and P7 cell cultures can be used for TG30/GCTM-2 FACS mediated consecutive reculture of either P4 or P7 subfractions (See Figures 2 and 3).
Representative Results
hESC-derivatives that are pluripotent stem cell-free can be obtained by consecutive passaging of cells from the P4 (TG30Neg-GCTM-2Neg) subfraction.
Previous reports have established that in hESC standard cultures a mixture of subpopulations coexist exhibiting an expression gradient of genes associated with pluripotency5,6,13. Combined detection of cell surface antigens like TG30 (CD9) and GCTM-2 allows for the discrimination of a number of subsets of cells at the pluripotent stage and at various stages of early differentiation, as indicated by their expression of stem cell markers and lineage specific transcription factors5,6,13. In agreement with previous reports, we were able to discriminate P4 (TG30Neg-GCTM-2Neg), P5 (TG30Low-GCTM-2Low), P6 (TG30Mid-GCTM-2Mid), and P7 (TG30Hi-GCTM-2Hi) cells in the hESC-MEL1 line used for this study (Figure 1). With this result, we proceeded to collect differentiated P4 (TG30Neg-GCTM-2Neg) cells and pluripotent P7 (TG30Hi-GCTM-2Hi) cells for further culture in studies described below.
We investigated whether using a negative selection approach we could purge undifferentiated hESCs from cell populations undergoing very early-stage differentiation (TG30Neg-GCTM-2Neg). We used a defined number of P4 (TG30Neg-GCTM-2Neg) cells that were consecutively cultured post-FACS (Figure 2A) for approximately two weeks, and reanalyzed using TG30/GCTM-2 FACS prior to replating at each passage. Results showed that the P4-subfraction enriched for TG30Neg-GCTM-2Neg differentiated cell types after consecutive passaging, and a minor presence of P7 (TG30Hi-GCTM-2Hi) cells at the initial passage (Figure 2A, Passage 1) eventually disappeared after further passaging, becoming pluripotent stem cell-free samples (Figure 2A, Passage 3).
Enrichment of pluripotent hESC cells can be achieved by collection of cells TG30Hi-GCTM-2Hi cells.
Based on previous observations using this assay, we would expect an enrichment of pluripotent hESCs by collecting P7 (TG30Hi-GCTM-2Hi) cells that should form hESC colonies. Therefore, we also investigated TG30/GCTM-2 FACS-mediated consecutive passaging of pluripotent P7 (TG30Hi-GCTM-2Hi) cells. In these P7 cultures, TG30/GCTM-2 FACS immunoprofiling was performed prior to replating cells at each passage and only P7 (TG30Hi-GCTM-2Hi) cells were recultured. In this way, we observed that the majority of cells were located in the subfractions associated with pluripotency (P6 and P7), indicating an enrichment of pluripotent cells that also maintained a capacity to differentiate and generate a small percentage of P4 (TG30Neg-GCTM-2Neg) cells (Figure 3A). Furthermore, cultures of P7 cells showed a large number of hESC-like colonies during passaging (Figure 3B), also indicating maintenance of the pluripotency state.
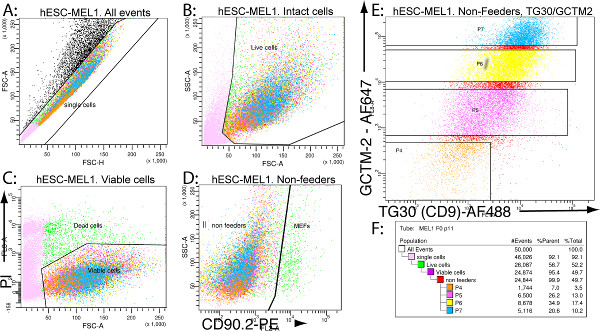
Figure 1. Representative sorting strategy to obtain TG30/GCTM-2 FACS subfractions. (A): Dissociated hES cell suspensions are sorted as single cells and potential clumps or doublets are gated out with the forward scatter for height and area (FSC-H and FSC-A). (B): Potential debris is removed with FSC-A and SSC-A, and only intact cells are further sorted. (C): Nonviable cells are excluded based on propidium iodide (PI) fluorescence (FSC-A and FL3-A). (D): MEF feeder cells were gated out by negative selection based on expression of mouse CD90.2-PE (FL2-A and SSC-A). (E): The isolated viable hESC fraction, free of MEF feeders, is finally fractionated into P4 (TG30Neg-GCTM-2Neg), P5 (TG30Low-GCTM-2Low), P6 (TG30Mid-GCTM-2Mid), and P7 (TG30Hi-GCTM-2Hi) subpopulations. The negative gate (TG30Neg-GCTM-2Neg, named P4) was set according to background autofluorescence for the unstained cells and also to isotype controls. (F): Percentages of the different gated subpopulations typical of a TG30/GCTM-2 flow cytometry analysis. Click here to view larger figure.
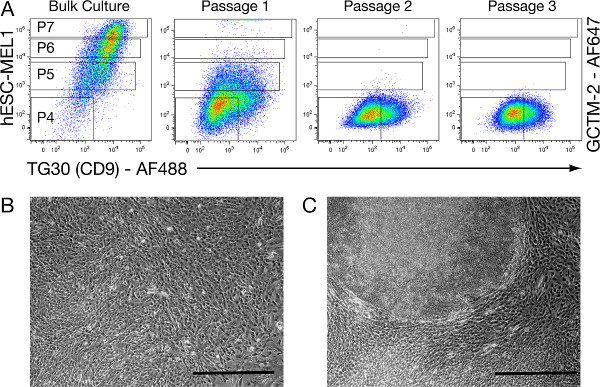
Figure 2. Representative consecutive cultures of spontaneously differentiated P4 (TG30Neg-GCTM-2Neg) cells from the hESC-MEL1 line. (A): Representative TG30/GCTM-2 FACS plots showing gated subsets of cells (P4, P5, P6 and P7) discriminated by the level of detected expression of TG30 and GCTM-2 cell surface markers. An initial population of 400,000 hESC-derivatives exhibiting P4 (TG30Neg-GCTM-2Neg) immunoprofiling is collected from bulk cultures of hESC-MEL1 line. These P4 (TG30Neg-GCTM-2Neg) cells are consecutively passaged post-FACS in conditions supportive for hESC renewal in T75 flasks, until reaching confluency (11-13 days). Prior to each passage, TG30/GCTM-2 FACS is performed to collect and reculture only the P4 (TG30Neg-GCTM-2Neg) differentiated cell types. (B): Typical morphology of the lawn of differentiated P4 (TG30Neg-GCTM-2Neg) cells after 14 days. (C): Sporadic hESC-like colonies seen after 14 days intermingled with the lawn of P4 (TG30Neg-GCTM-2Neg) cells at passage 1 only, are most likely generated by contaminating P7 (TG30Hi-GCTM-2Hi) cells. Scale bar = 200 μm. Click here to view larger figure.
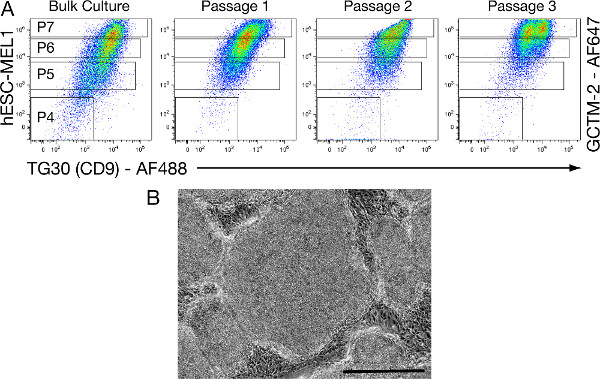
Figure 3. P7 (TG30Hi-GCTM-2Hi) cells derived from hESC lines can be consecutively passaged post-FACS without losing their intrinsic pluripotent properties. Representative TG30/GCTM-2 FACS immunoprofiling of the different consecutive passages, showing the discriminated subsets of cells (P4, P5, P6, and P7) according to the level of expression of TG30 and GCTM-2. (A): A starting population of 400,000 cells showing P7 (TG30Hi-GCTM-2Hi) characteristics is retrieved from bulk cultures of the hESC-MEL1 line. Pluripotent P7 (TG30Hi-GCTM-2Hi) cells are consecutively passaged post-FACS in conditions supportive for hESC renewal in T75 flasks, until reaching ~80% confluency (9-11 days). TG30/GCTM-2 FACS immunoprofiling is performed prior to replating cells at each passage and only theP7 (TG30Hi-GCTM-2Hi) cells are recultured. Results show that P7 (TG30Hi-GCTM-2Hi) cells conserve their pluripotent characteristics with subsequent passaging, including conservation of (TG30Hi-GCTM-2Hi) expression, forming hESC-like colonies (shown in B) and maintaining the ability to differentiate as can be inferred from the recapitulation of a small fraction of differentiated P4 (TG30Neg-GCTM-2Neg) in each of the FACS gradients. Scale bar = 200 μm. Click here to view larger figure.
| Sample (reaction volumes) | Primary Antibody | Secondary Antibody | PE Rat Anti-Mouse CD90.2 b | Propidium Iodide c |
| Sort Sample (~9 ml for primary ABs, 2.5 ml for secondary ABs) | (TG30 + GCTM-2)a | AF 488 goat anti-mouse IgG2a + AF 647 goat anti-mouse IgM | + | + |
| Control-1: TG30 (200 μl) | TG30 | AF 488 goat anti-mouse IgG2a | – | + |
| Control-2: GCTM-2 (200 μl) | GCTM-2 | AF 647 goat anti-mouse IgM | – | + |
| Control-3: Anti-mouse CD90.2 (200 μl) | – | – | + | + |
| Control-4: Phycoerythrin (PE) (200 μl) | Mouse IgG2a isotype | R-phycoerythrin goat anti-mouse IgG2a | – | + |
| Control-5: Mouse Immunoglobulin isotype (200 μl) | Mouse IgG2a isotype + IgM isotype | AF 488 goat anti-mouse IgG2a + AF 647 goat anti-mouse IgM | – | + |
| Control-6: Unstained cells (200 μl) | – | – | – | – |
Table 1. Sort sample and controls for FACS sorting. aSort sample is immunostained simultaneously with TG30 and GCTM-2 antibodies. bPE-anti-mouse CD90.2 is used to recognize mouse cells and remove MEFs from hESCs during FACS14. cNonviable cells are excluded using propidium iodide at a final concentration of 0.3 μg/ml. Reagent added (+), not added (-) to reaction tube.
| Antibody | Host | Isotype | Working dilution |
| TG30 (1.4 mg/ml) | Mouse | IgG2a | 1:1,000 |
| GCTM-2 (hybridoma supernatant) | Mouse | IgM | 1:5 ~ 1:10 a |
| AF 488 goat anti-mouse IgG2a | Goat | Polyclonal | 1:500 |
| AF 647 goat anti-mouse IgM | Goat | Polyclonal | 1:500 |
| R-phycoerythrin (PE) goat anti-mouse IgG2a | Goat | Polyclonal | 1:1,000 |
| Anti-mouse CD90.2 | Rat | IgG2b | 1:100 |
| Purified Mouse IgG2a, κ Isotype Control | Mouse | IgG2a | 1:200 |
| Purified Mouse IgM, κ Isotype Control | Mouse | IgM | 1:200 |
Table 2. Antibody details and dilutions for FACS sorting. a Due to the extremely very high expression of GCTM-2 on the cell surface of hESCs it is not possible to reach saturation with this antibody. Each batch of GCTM-2 hybridoma supernatant must be titrated against a standard control or a previous batch to obtain similar results with hESCs in scale and to avoid over staining.
| Name of medium | Composition |
| hESC/KOSR medium | Dulbecco's Modified Eagle Medium:Nutrient Mixture F-12 (DMEM/F-12) supplemented with 20% Knockout Serum Replacement (KOSR), 2 mM GlutaMAX, 1% MEM Nonessential Amino Acids, 0.1 mM 2-Mercaptoethanol, 10 ng/ml human fibroblast growth factor 2 (FGF-2) and Pen/Strep at 1x. |
| MEF medium | Dulbecco's Modified Eagle Medium, high glucose (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 2 mM GlutaMAX, and Pen/Strep at 1x. |
Table 3. Composition of culture media.
Discussion
In the clinical context, differentiated somatic cell types are the final desired product for transplantation and therapeutic applications, and hPSC are a self-renewing and scalable source to generate those somatic cells or their progenitors in the laboratory. The presence of residual undifferentiated hESCs with an inherent potential for teratoma formation is a safety risk when considering hESC-derived somatic cells for transplantation into patients. For a review of this and other risks associated with cell therapy please see Sharpe et al.16 Therefore, to realize such applications, it is imperative that researchers aim to generate target cell populations that are also pluripotent stem cell-free. Towards this end, we demonstrate here that using our TG30/GCTM-2 FACS methodology it is possible to monitor for the presence of pluripotent cells (TG30Hi-GCTM-2Hi) in a differentiating cell population and obtain viable hESC-differentiated derivatives that can be enriched and recultured post-FACS. Although it is not possible to obtain 100% pluripotent stem cell-free samples with a single pass of our protocol, enriched hESC-derivatives can be recultured and after consecutive passaging 100% somatic-cell purity is achieved. The demonstrated viability of somatic cells for expansion post-FACS also potentially allows for the production of adequate numbers of cells suitable for potential transplantation therapies.
The encouraging results of this pilot study prompted us to perform a more comprehensive investigation using our TG30/GCTM-2 protocol in a comparative study with several human induced pluripotent stem cell (hiPSC) lines15. In that study, we found that pluripotent stem cell-free samples obtained using our TG30/GCTM-2 FACS protocol did not generate teratomas when transplanted into immune-deprived mice. Therefore, we can confidently state that with the use of this in vitro protocol, early stage differentiation cell cultures determined to be free of pluripotent TG30Hi-GCTM-2Hi cells do not develop teratomas in vivo. This strongly supports the robustness of our assay, providing a potential approach to eliminate the risk of teratoma formation prior to use of hPSC-derived cells in clinical applications.
Conversely, we also show that the TG30/GCTM-2 FACS assay can be used to retrieve a pluripotent cell population with high level of expression of these two surface antigens (TG30Hi-GCTM-2Hi). Previous studies have shown that TG30Hi-GCTM-2Hi cells exhibit the highest correlation with OCT4hi expression and form the highest number of hESC-like colonies5,6,13,15. We therefore reason that the pluripotency network is activated at its maximum levels in TG30Hi-GCTM-2Hi expressing cells. We consider that using our TG30/GCTM-2 FACS protocol and retrieving P7 (TG30Hi-GCTM-2Hi) cells could allow for large-scale purification of enriched live hESC cultures, as inputs to differentiation assays.
In conclusion, we demonstrate here the potentiality of our FACS assays based on the combined expression of surface antigens TG30 and GCTM-2 for both the purging and enrichment of hESCs. Future studies with protocols for directed differentiation of hPSCs are likely to also be informative regarding this potentiality. We are aware that in some assays where differentiated hESC-derivatives temporally express TG30 (CD9) or GCTM-2, these two antibodies might not be appropriate or sufficient to purge pluripotent cells from a differentiated population at a particular time point in the assay6. Therefore, there is an ongoing need to find novel antibodies that specifically recognize live hESCs to overcome the possible limitation of cross-reaction with some differentiated cell types.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank the StemCore facility at Monash University for provision of hESC lines and the FlowCore facility at Monash University for FACS services. This work was supported by research grants from the Australian Stem Cell Centre, the New South Wales and Victorian Government Stem Cell Research Grant Program to ALL. ALL is a Partner Investigator of the Australian Research Council (ARC) Special Research Initiative in Stem Cell Science, Stem Cells Australia.
Materials
| Short Name | Company | Catalog Number | Long Name |
| DMEM/F-12 | Life Technologies | 11320082 | Long Name: Dulbecco's Modified Eagle Medium:Nutrient Mixture F-12 |
| KOSR | Life Technologies | 10828028 | Long Name: Knockout Serum Replacement |
| GlutaMAX | Life Technologies | 35050061 | Long Name: GlutaMAX supplement |
| Nonessential Amino Acids | Life Technologies | 11140050 | Long Name: MEM Nonessential Amino Acids Solution |
| Mercaptoethanol | Life Technologies | 21985023 | Long Name: 2-Mercaptoethanol |
| DPBS | Life Technologies | 14190250 | Long Name: Dulbecco's Phosphate-Buffered Saline, no calcium, no magnesium |
| TrypLE Express | Life Technologies | 12604039 | Long Name: TrypLE Express (1x), no Phenol Red |
| AF-488 | Life Technologies | A21131 | Long Name: Alexa Fluor 488 goat anti-mouse IgG2a |
| AF-647 | Life Technologies | A21238 | Long Name: Alexa Fluor 647 goat anti-mouse IgM |
| PE-anti IgG2a | Life Technologies | P21139 | Long Name: R-Phycoerythrin Goat Anti-Mouse IgG2a (γ2a) Conjugate |
| DMEM | Life Technologies | 11965-092 | Long Name: Dulbecco's Modified Eagle Medium, high glucose |
| Pen/Strep | Life Technologies | 15140-122 | Long Name: Penicillin-Streptomycin, Liquid |
| Distilled Water | Life Technologies | 15230-089. | Long Name: Sterile Distilled Water |
| PI | SIGMA | P4864 | Long Name: Propidium iodide solution |
| FBS | SIGMA | 12203C | Long Name: Fetal Bovine Serum |
| Gelatin | SIGMA | G-1890 | Long Name: Gelatin from porcine skin |
| Human FGF-2 | Millipore | GF003AF-100UG | Long Name: Fibroblast Growth Factor basic, human recombinant, animal-free |
| Filter 0.22 μm | Millipore | SCGPU02RE | Long Name: Stericup-GP, 0.22 μm, polyethersulfone, 250 ml, radio-sterilized |
| 70 μm Cell Strainer | Becton Dickinson | 352350 | Long Name: Cell strainer with 70 μm Nylon mesh |
| 35 μm Lid cell strainer, 5 ml tube | Becton Dickinson | 352235 | Long Name: 5 ml polystyrene round bottom test tube, with a cell strainer cap (35 μm) |
| 5 ml Polystyrene round bottom test tube | Becton Dickinson | 352054 | Long Name: 5 ml polystyrene round bottom test tube, sterile |
| IgG2a Isotype Control | Becton Dickinson | 554126 | Long Name: Purified Mouse IgG2a, κ Isotype Control |
| IgM Isotype Control | Becton Dickinson | 553472 | Long Name: Purified Mouse IgM, κ Isotype Control |
| PE-CD90.2 | Becton Dickinson | 553014 | Long Name: PE Rat Anti-Mouse CD90.2 |
| 8-peaks calibration | Becton Dickinson | 559123 | Long Name: Rainbow Calibration Particles (8 peaks) |
| 50 ml sterile Polypropylene tube | Greiner Bio-One | 227261 | Long Name: 50 ml Polypropylene tube with conical bottom, Sterile |
| T75 flask | Greiner Bio-One | 658175 | Long Name: CELLSTAR Filter Cap Cell Culture 75 cm2 Flasks |
| TG30 | Merck Millipore | MAB4427 | Long Name: TG30 Antibody, clone TG30 |
| GCTM-2 | Merck Millipore | MAB4346 | Long Name: GCTM-2 is not commercially available but TG343 and equivalent monoclonal antibody is |
| Table 4. List of materials and reagent | |||
| [header] | |||
| FACS machine | Becton Dickinson | FACSVantage SE with FACSDiva option | |
| Inverted microscope | Olympus | IX71 | |
| Table 5. List of equipment. |
References
- Pera, M. F., Tam, P. P. Extrinsic regulation of pluripotent stem cells. Nature. 10 (7299), 713-720 (2010).
- Pistollato, F., Bremer-Hoffmann, S., Healy, L., Young, L., Stacey, G. Standardization of pluripotent stem cell cultures for toxicity testing. Expert Opin. Drug Metab. Toxicol. 8 (2), 239-257 (2012).
- Miura, K., Okada, Y., Aoi, T., Okada, A., Takahashi, K., Okita, K., Nakagawa, M., Koyanagi, M., Tanabe, K., Ohnuki, M., et al. Variation in the safety of induced pluripotent stem cell lines. Nat. Biotechnol. 27 (8), 743-745 (2009).
- Polanco, J. C., Laslett, A. L., Lenka, N. Safety Assessment of Reprogrammed Cells Prior to Clinical Applications: Potential Approaches to Eliminate Teratoma Formation. Pluripotent Stem Cells. , (2013).
- Laslett, A. L., Grimmond, S., et al. Transcriptional analysis of early lineage commitment in human embryonic stem cells. BMC Dev. Biol. 7, 12 (2007).
- Kolle, G., Ho, M., et al. Identification of human embryonic stem cell surface markers by combined membrane-polysome translation state array analysis and immunotranscriptional profiling. Stem Cells. 27, 2446-2456 (2009).
- Zhou, Q., Chy, H., Laslett, A. L. Preparation of defined human embryonic stem cell populations for transcriptional profiling. Current protocols in stem cell biology. Chapter 1, Unit1B 7 (2010).
- Fong, C. Y., Peh, G. S., Gauthaman, K. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Stem Cell Rev. 5, 72-80 (2009).
- Schriebl, K., Satianegara, G., et al. Selective removal of undifferentiated human embryonic stem cells using magnetic activated cell sorting followed by a cytotoxic antibody. Tissue Eng. Part A. 18, 899-909 (2012).
- Adewumi, O., Aflatoonian, B., et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 25, 803-816 (2007).
- Michalska, A. E. Isolation and propagation of mouse embryonic fibroblasts and preparation of mouse embryonic feeder layer cells. Current protocols in stem cell biology. Chapter 1, Unit1C 3 (2007).
- Hough, S. R., Laslett, A. L., Grimmond, S. B., Kolle, G., Pera, M. F. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS One. 4, e7708 (2009).
- Filipczyk, A. A., Laslett, A. L., Mummery, C., Pera, M. F. Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem Cell Res. 1, 45-60 (2007).
- Polanco, J. C., Ho, M. S. H., Wang, B., Zhou, Q., Wolvetang, E., Mason, E., Wells, C., Kolle, G., Grimmond, S. M., Bertoncello, I., O’Brien, C., Laslett, A. L. Identification of unsafe human IPSC lines using a robust surrogate assay for pluripotency. Stem Cells. 31 (8), 1498-1510 (2013).
- Sharpe, M. E., Morton, D., Rossi, A. Nonclinical safety strategies for stem cell therapies. Toxicol. Appl. Pharmacol. 262 (3), 223-231 (2012).

