Measuring Material Microstructure Under Flow Using 1-2 Plane Flow-Small Angle Neutron Scattering
Summary
A shear cell is developed for small-angle neutron scattering measurements in the velocity-velocity gradient plane of shear and is used to characterize complex fluids. Spatially resolved measurements in the velocity gradient direction are possible for studying shear-banding materials. Applications include investigations of colloidal dispersions, polymer solutions, and self-assembled structures.
Abstract
A new small-angle neutron scattering (SANS) sample environment optimized for studying the microstructure of complex fluids under simple shear flow is presented. The SANS shear cell consists of a concentric cylinder Couette geometry that is sealed and rotating about a horizontal axis so that the vorticity direction of the flow field is aligned with the neutron beam enabling scattering from the 1-2 plane of shear (velocity-velocity gradient, respectively). This approach is an advance over previous shear cell sample environments as there is a strong coupling between the bulk rheology and microstructural features in the 1-2 plane of shear. Flow-instabilities, such as shear banding, can also be studied by spatially resolved measurements. This is accomplished in this sample environment by using a narrow aperture for the neutron beam and scanning along the velocity gradient direction. Time resolved experiments, such as flow start-ups and large amplitude oscillatory shear flow are also possible by synchronization of the shear motion and time-resolved detection of scattered neutrons. Representative results using the methods outlined here demonstrate the useful nature of spatial resolution for measuring the microstructure of a wormlike micelle solution that exhibits shear banding, a phenomenon that can only be investigated by resolving the structure along the velocity gradient direction. Finally, potential improvements to the current design are discussed along with suggestions for supplementary experiments as motivation for future experiments on a broad range of complex fluids in a variety of shear motions.
Introduction
Developing a scientific understanding of a natural phenomenon requires accurate and precise measurements. Metrology is also the basis of successful engineering and design of new processes and materials. Rheology is the science of the deformation and flow of matter. Rheology is central in our ability to process a wide variety of materials and is also used by product formulators to target specific material properties. Typical examples of the former include molding polymers or forming composites, whereas the latter includes the development of everyday consumer products such as paints, shampoos, and foods. Whether the viscosity of a molten polymer is controlled so that it can be effectively injection molded or the viscoelasticity of a shampoo is changed so it has the correct consistency for the consumer, the rheological properties are controlled by changing the formulation of the material1. The rheology of materials and products also depends on the structure in the fluid state and this structure ranges from the microscale to the nanoscale. Furthermore, this structure changes with the processing parameters, such as flow rate and time of flow, which challenges rheologists to measure the structure during flow. It is this challenge that is met, in part, by the novel instrumentation described in this article.
Novel techniques capable of probing the microstructure of soft materials under shear flow can benefit soft material product engineering and processing condition optimization. Many intriguing and long-standing challenges for the application of soft materials in a variety of industries and in fundamental science involve unusual flow behavior, such as shear thickening in colloidal suspensions2, shear and vorticity banding in wormlike micelles3, and heterogeneities inherent in the flow of colloidal gels4-6. Rheologists are constantly challenged to elucidate the microstructural origins of the nonlinearities in the rheological responses and sometimes even in the velocity field of shearing viscoelastic materials. This challenge requires simultaneous acquisition of the microstructure as a function of both the spatial location in the flow field and the time dependent behaviors, which has proven a formidable task for experimentalists.
Small angle neutron scattering (SANS) is particularly well suited for measuring the structure of complex fluids as it can probe materials that are opaque to light. Also selective deuteration can be used to provide contrast between components that may appear similar under X-ray scattering7. Furthermore, neutrons have an advantage over X-rays as there is no radiation damage of biological or other soft-matter samples. In the experiments illustrated here, cold neutrons generated by a reactor or a spallation source are collimated and illuminated upon a sample. The scattering intensity yields information about the structure of the material on length scales from the atomic to hundreds of nanometers (and with ultra-small angle neutron scattering up to tens of microns), but in the form of a Fourier transform of the real space structure. Therefore, interpretation of the data can be challenging and involves an inverse transform or comparison to microstructural models or simulations. More about SANS instrumentation, experiments, and contrast matching can be found on the tutorials posted on the web site of the Center for Neutron Science, www.cns.che.udel.edu.
Here we describe a shear cell designed to extend the SANS method to examine materials under flow. A recent overview of the general methodology and instrumentation, as well as a substantial literature review of recent applications can be found in reference8 and the cited references therein. A convenient and nearly ideal environment to probe fluid structure under shear flow with SANS is a narrow gap Couette geometry, also known as concentric cylinders9. This geometry applies a simple (i.e. laminar) shear flow to the sample while maintaining a sufficient unobstructed volume for the incident neutron beam. The application of flow breaks the symmetry of the microstructure; as such a complete characterization of the material microstructure under simple shear flow requires microstructural measurements in all three planes of shear. Two planes of shear may be investigated using the standard Couette geometry configuration (Figure 1a): the neutron beam is configured to travel along the velocity gradient direction and probe the velocity-vorticity (1-3) plane of shear (“radial” configuration); alternatively, the beam is collimated by a thin slit and aligned parallel to the flow direction, thereby probing the velocity gradient-vorticity (2-3) plane (“tangential” configuration). This instrument is available commercially and has been recently documented for examining complex fluids under shear10. The aforementioned review describes its use and that of related devices for structure-property determination across a broad range of materials and applications8. Time-resolved experiments, such as for oscillatory shear flows have also been reported11,12.
Often the most interesting and most important plane of flow is the velocity-velocity gradient (1-2) plane (Figure 1b) but it is also the most difficult to investigate as it requires special instrumentation. A custom shear cell has been designed to enable direct investigation of the velocity-velocity gradient (1-2) plane by SANS such that the neutron beam travels parallel to the vorticity axis of shear13-16. Measurements in the 1-2 plane of flow are critical to gaining a quantitative understanding for the shear viscosity because they elucidate the orientation of the structure relative to the flow direction15,17,18. This is important for materials such as polymers, self-assembled surfactants, colloids, and other complex fluids. In addition, it is possible to investigate the materials’ microstructure as a function of position across the gap in the gradient direction of shear flow. With the addition of spatial resolution, the method provides a means for studying materials that exhibit microstructural changes along the gradient direction of shear. An example for which investigating changes in microstructure and composition along the gradient direction of flow is shear-banding. Shear banding is a phenomenon caused by a coupling between the microstructure and flow direction that results in an inhomogeneous flow field13. In this article, we describe the instrument, its assembly and the flow-SANS measurement technique as implemented at the NIST Center for Neutron Research (NCNR) at the National Institute of Standards and Technology (NIST) in Gaithersburg, MD. This sample environment is the result of a collaboration between the University of Delaware, NIST and the Institut Laue-Langevin (ILL), and has been successfully implemented at both ILL and NIST. For purposes of this article, where the SANS specific portions of the protocol are concerned, the technique is described as implemented at NIST. However, modifying those instrument specific details should be straightforward and the overall technique can be implemented on any SANS instrument for steady flow (section 5.1). In addition, instruments equipped with time-resolved SANS capabilities may also perform oscillatory shear flow-SANS experiments (section 5.2). Technical drawings of the shear cell components are provided as Figures 12-23.
Protocol
Figure 2 shows an assembled shear cell attached to the baseplate, which is mounted to the breadboard on the sample environment stage and aligned in the neutron beam for a SANS experiment. The stepper motor, gear box and belt drive, slit motor stage, shear cell and direction of the neutron beam are labeled in Figure 2. The present protocol provides directions for assembling the shear cell (section 1), mounting the shear cell onto the sample environment stage (section 2), calibrating the geometry for a SANS experiment (section 3), loading a sample (section 4), running an experiment and data collection (section 5) and ending an experiment (section 6). For reference, Figure 3 is a schematic of the assembled cell and Figure 4 shows the disassembled shear cell parts laid out from front plate to back plate, left to right, and the necessary tools for assembly (1/16 in and 3/16 in Allen wrenches and a 3/8 in open end wrench). From left to right in Figure 4 are the front plate, bearing, spring-loaded bushing, O-rings, quartz window, middle plate with O-rings, sample access ports and syringe connectors, set-screws, mandrel, and the parts for the back plate (quartz window, O-rings, spring-loaded bushing, bearing), back plate, four socket head cap screws and quick connect cooling hose with quick-connectors attached.
1. Assemble the Shear Cell (Inset to the Right in Figure 2)
- Prepare the middle plate for assembly.
- Clean the middle plate including sample and set-screw pathways and identify the top of the plate by the score mark.
- Seal the sample loading pathways using the three set-screws. Wrap each set-screw in thread seal tape and use a 1/16 in Allen wrench to insert each screw into each hole at the “bottom” (2) and one set-screw into the hole on the “side”.
- Place the round white O-rings (2-14 in ID, Viton standard AS568 size 035) in the grooves on both sides of the middle plate.
- Prepare the back and front plates for assembly (Figure 5).
- Press fit the bearing into each of the front and back plates.
- Insert the spring-loaded bushing (which is a seal) with the spring-side open toward the sample into the front and back plates.
- Place the small (1-5/8 in ID) and large (2-1/4 in ID) Buna-N square double seal O-rings into the grooves in each of the front and back plates.
- Place the quartz windows on top of the square O-rings in each plate.
- Assemble the front and middle plates together.
- Place the front plate on a flat surface; align the score on the top of the middle and front plates and place the middle plate on the front plate. If necessary, apply a small amount of appropriate grease to the rounded O-rings in the middle plate to hold them in place during assembly.
- Assemble the mandrel and back plate together.
- Insert the short end of the mandrel shaft into the back plate. Use evenly applied force and the mandrel will “click” into place. Take note that the mandrel is now holding the quartz window and square O-rings in place on the back plate.
- Assemble the front plate, middle plate, mandrel and back plate together.
- Place the front and middle plate assembly on a raised platform with the middle plate facing up. This raised platform is to allow space for the mandrel shaft to extend below the assembly without hitting the table.
- Align the score on the top of the front plate assembly with the score on the back plate assembly.
- Insert the long part of the mandrel shaft into the front plate assembly. Make sure the rounded O-rings on the middle plate remain properly seated during assembly. The cell will slide together and again, “click” when properly assembled.
- Screw the assembly together using the four socket head cap screws and a 3/16 in Allen wrench. Tighten the screws in a cross-pattern so the cell maintains concentricity.
- Wrap thread seal tape around the two access ports and screw them into the top of the middle plate. Tighten with a 3/8 in open-end wrench.
- Place the cadmium mask (Figure 6) into the receiving slot machined into the front of the front plate. Apply tape or tack to hold the mask in place if needed.
- Use the quick-connectors to cross-connect the coolant hose between the top ports on the front and back plates.
2. Mount the Shear Cell into the Beamline
- Cover the SANS detector window with the safety shield.
- Ask the responsible facility instrument scientist to align the sample environment stage with the neutron beam.
- Mount the breadboard to the sample environment stage using four ¼ in x 20 socket head Allen bolts and a 3/16 in Allen wrench.
- Attach the shear cell assembly to the cell-mounting bracket located on the baseplate (already attached to the breadboard (Figure 7)).
- Identify the cell-mounting bracket and shaft coupler attached to the baseplate (Figure 8). Make sure the set-screws for the shaft coupler are loosened.
- Align the shaft coupler and the mandrel shaft such that the set-screws on the coupler will screw into the flat part of the mandrel shaft.
- Horizontally slide the shear cell into the cell-mounting bracket so that the assembly looks like that shown in Figure 8. This step should be performed with care as it is important not to bend the mandrel shaft or the shaft coupler.
- Attach the shear cell assembly to the cell-mounting bracket with two socket head cap-screws using a 3/16 in Allen wrench. Tighten securely always making sure the shear cell is flush against the cell-mounting bracket.
- Tighten the two set-screws on the shaft connector using a 1/16 in Allen wrench to connect the shear cell mandrel shaft to the drive assembly.
- Align the shear cell geometry with the neutron beam.
- Use the laser to adjust the SANS sample environment stage such that the height of the mandrel shaft is the same as the neutron beam. Align the center of the gap in the shear cell to the center of the neutron beamline path.
- Insert the appropriate cadmium slit into the slit motor stage assembly that is mounted on the breadboard (Figure 8). Secure the slit with tack if necessary.
Note: The slit should be flush with the front plate and approximately placed within the gap of the shear cell. Choose the slit accordingly for the desired experiment. For gap resolution experiments 0.1 mm and 0.2 mm curved slits are available. Whereas for measurements that do not require spatial resolution a 0.8 mm rectangular slit is advisable. - Move the motor position using the crank to adjust the tension of the drive belt so that there is approximately ¼ in deflection in the belt. When tensioned properly, lock the motor location by tightening the set-screw located below the wheel using a 7/64 in Allen wrench.
Note: An optional gear reducer may be added to the motor assembly. This option may be necessary based upon the required shear rates required for a specific experiment. - Connect the two coolant bath hoses to the shear cell using the quick-connectors.
- Adjust any observation cameras or other auxiliary equipment specific to observing the experiment.
- Remove the safety shield protecting the SANS detector window.
3. SANS Setup and Calibration
- Attach a 0.5 in aperture to the end of the snout on the incident neutron beam.
- Set the desired SANS detector position (q-range), neutron wavelength, and wavelength spread following standard SANS protocols and optimized for the experimental conditions.
Note: The calculation for the sample-to-detector distance is based on the sample environment stage located on the “Huber table”. - Align the slit position with the gap of the shear cell.
- Use the slit motor stage (Figure 8) to align the slit position with the gap of the shear cell. Use a laser to emulate the neutron beam and a mirror to detect the laser once it passes through the quartz windows within the gap of the shear cell assembly.
- Fine-tune the position of the slit using SANS transmission measurements. Systematically vary the slit motor position from the inner wall of the shear cell gap to the outer wall of the shear cell gap using 0.1 mm motor slit translation steps. Observe the transmission (typically 2 sec) using SANS and record the slit motor position for each transmission measurement (Figure 9).
Note: If spatial resolution is desired, identify the motor positions necessary for the SANS experiments. If spatial resolution is not necessary identify the single motor position that aligns the slit with the middle of the shear cell gap. Aligning the slit with the gap in the shear cell is crucial for completing a good experiment. It is also possible (and recommended) to use water to align the position of the slit using SANS transmission measurements. Using water reduces the transmission and provides contrast with the shear cell housing (Figure 9).
Note: Load the water into the cell by following the sample loading protocol (section 4). Using water will generally require the shear cell to be removed from the baseplate, disassembled, dried, reassembled and remounted to the baseplate prior to loading the sample for the experiment. As long as the baseplate IS NOT removed from the sample environment stage this should not be a problem, but it is always important to verify the slit alignment with the gap.
- Calibrate the sample geometry
- Perform a blocked beam dark count and an empty cell measurement according to standardized SANS procedures. Note that the empty cell measurements should be performed at each spatial location as determined by the slit calibration performed in section 3.3.
4. Sample Loading Protocol
- Place the safety shield on the SANS detector window.
- Mount the two syringe connectors (Nylon) and threaded syringe fixtures (blue and yellow) to the steel pipes at the top of the sample cell. Make sure the stopcocks are in the closed position.
- Preload the sample into a 10 ml threaded syringe (minimum sample volume is 6 ml). Make sure the sample is free of bubbles.
- Eliminate bubbles by either centrifuging lightly or heating the sample to reduce the viscosity of the sample while loading the syringe. If the sample is heated, it is strongly recommended that the temperature of the shear cell is also increased to aid in loading the sample.
- Place an empty syringe without the plunger on the connector in the middle of the shear cell to receive excess sample (Figure 8).
- Place the sample syringe on the other connector (Figure 8).
- Open both stopcocks.
- Inject the sample slowly until the sample begins to enter into the empty syringe.
- Remove any air bubbles from the gap of the shear cell.
- Turn the motor control off to release the motor and allow the belt to be manually moved.
- Shear the sample by hand to help move the bubbles to the top of the shear cell, whereby additional sample injection will typically push the bubble into the outlet and out of the shear cell gap.
- Close the stopcocks to lock the sample in the cell.
- Change the temperature of the water bath to the required experimental temperature, and precondition the sample’s shear history as appropriate.
- Check for any bubbles (and do so regularly during the course of the experiment). If bubbles are observed; open the stopcocks, use rotation to move the bubbles to the top of the shear zone, and inject additional sample to push the bubbles out of the shear zone of the cell.
- Remove the safety shield and any extraneous tools and supplies from the beam area.
5. Running the Shear Experiment and Collecting SANS Data
- For simple steady shear experiments:
- Set the shear rate in the steady shear control file associated with the motor control software (see associated documentation for motor control software operation).
- Identify the shear direction of the sample during the experiment.
- Set up the desired SANS experiments according to the standardized SANS procedures.
- Start the shear cell motor.
- Start the SANS experiment. Check the detector counts and observe the SANS 2D pattern to insure SANS results are being properly recorded during shearing. An example of a typical pattern observed for the surfactant solutions discussed in the section on representative results is shown in Figure 10.
- Repeat the procedure (section 5.1) for each desired shear rate.
- For time-resolved oscillatory shear experiments:
- Verify the trigger position for the oscillatory shear experiment. For oscillatory shear, this is at the point of maximum strain and minimum (zero) strain rate.
- Set the oscillation frequency and strain amplitude in the time-resolved control file associated with the motor control software (see associated documentation for motor control software operation). Note that the strain amplitude is defined according to the amplitude of the applied strain centered at zero and is the rheologically defined strain amplitude.
- Start the shear cell motor for the oscillatory shear experiment.
- Start the SANS experiment. Check detector counts and observe the 2D pattern to insure SANS is properly being recorded during oscillatory shearing.
- Copy the time-stamped neutron detector log file from NISTO to Charlotte and preprocess the data using software provided by the NCNR.
- Reduce the preprocessed data set with the reduction software package in IGOR.
- Repeat the procedure (section 5.2) for each, desired oscillation frequency and strain amplitude condition.
6. End of Experiment
- Turn off the neutron beam and motor control.
- Place the safety shield on the SANS detector window.
- Let the sample and apparatus stand in the closed beam for 5 min. Perform a standard radiation check before removing the shear cell from the baseplate.
- Open the stopcocks on the sample ports and withdraw or push out the sample using the sample syringes. Recover the sample, close the stopcocks, and remove the syringes.
- Turn off the temperature bath. Uncouple the fluid bath cooling hoses from the shear cell quick connect ports.
- Loosen the Allen screws on the shaft coupler between the mandrel and the drive shaft using a 1/16 in Allen wrench. Use a 3/16 in Allen wrench to unscrew the two socket head cap screws that attach the shear cell to the cell-mounting bracket. Slide the shear cell out of the cell mounting bracket.
- Disassemble the shear cell by reversing the assembly protocol (section 1 of the protocol).
- Clean the shear cell using soapy water. Rinse and dry thoroughly.
Representative Results
Representative results of a successful flow-SANS experiment are given in Figures 9, 10, and 11. These examples are from investigations made on a wormlike micelle solution (WLM) (Table 1) known to exhibit shear banding during certain conditions of shear. A complete discussion of the scientific findings can be found in references15-17.
Figure 10 represents results of a scattering pattern obtained under shear flow using the shear cell. The sample studied is a viscoelastic wormlike micelle (WLM) solution comprised of long, entangled threadlike self-assembled micelles amphiphilic molecules13-15. The composition of the solution studied is given in Table 1. Upon shearing these systems the WLM solution demonstrates shear thinning behavior as a consequence of a complex combination of micelle flow alignment, disentanglement, and possibly micelle breakage (Vazquez-Cook-McKinley (VCM) model19). A particularly interesting complexity in these systems is the onset of shear banding. Shear banding was originally observed visually as birefringent bands near the rotating wall of a Couette geometry20. During shear banding the flow field segregates into two or more regions, or “bands”, each with a different characteristic shear rate as illustrated in Figure 9. For the WLM studied here, two bands form at sufficiently high shear rates – one with a shear rate higher than the expected, average value, and one at a lower shear rate. These bands coincide with the stress plateau observed in steady state shear rheometry measurements (Figure 11).
A primary question concerning shear banding is the microstructural state of the surfactant at shear rates where shear banding is observed. It was unknown as to how the surfactant organized in the high shear band relative to the low shear band. The new shear cell SANS instrument with spatial resolution across the gap is uniquely suited to study this problem. Through independent rheometry and flow-velocimetry measurements in a comparable Couette cell, the location of the shear bands are defined across the gap in the Couette cell. Using a narrow slit aperture (0.1 mm) SANS data is collected at different positions across the gap in the velocity-velocity gradient (1-2) plane of shear during steady shear flow. Here we report results for a WLM comprised of cationic surfactant cetyltrimethylammonium bromide (CTAB) in deuterated water (D2O) at 0.49 mol/L (490 mM) and 32 °C 6. Flow-SANS measurements are performed at eight positions across the 1.0 mm Couette gap by systematically translating the 0.1 mm slit aperture across the window in the shear cell. Figure 11 displays a visual summary of the results, where the intensity ring is a correlation peak due to segment-segment interactions. Anisotropy in this ring indicates segmental flow alignment, with high alignment typical for a nematic phase. A significant difference in scattering anisotropy is observed between positions in the low- shear and high-shear bands. A detailed explanation of the significance of these measurements in realizing the goal of explaining the mechanism of shear banding as observed in the rheology and flow-velocimetry results can be found in references13-15. These measurements have recently been successfully extended to time-dependent deformations by time resolved neutron scattering methods as described in section 5.2 of this work and these results have been submitted for publication21.
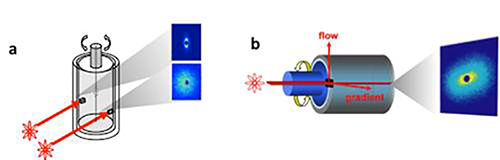
Figure 1. a) Geometry for Time-resolved Oscillatory Rheo-SANS (tOr-SANS) experiments in the 1,3 and 2,3 planes of flow. b) New geometry that probes the velocity-velocity gradient (1-2) plane of shear (adapted from M. E. Helgeson, N. J. Wagner, & L. Porcar, “Neutron transmission measurements of concentration profiles in nonhomogeneous shear flows”, 2010 Annual Report, NIST Center for Neutron Research, Gaithersburg, MD. p. 38-39, 2010).
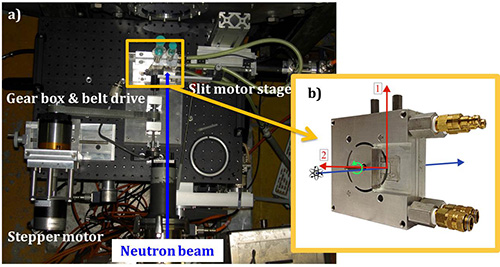
Figure 2. The basic 1-2 shear cell instrumentation in the D22 SANS beamline at the Institute Laue-Langevin, Grenoble, France. a) top view of the instrumentation with gear box and belt drive, slit motor stage, stepper motor and neutron beam highlighted for clarity; b) side view of the shear cell with sample access ports attached.
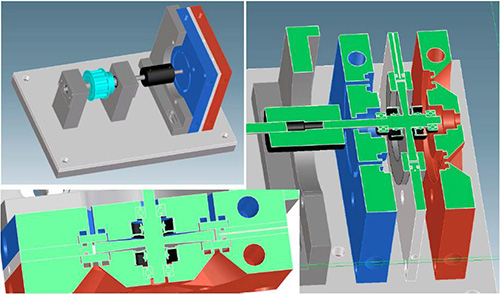
Figure 3. Schematic drawing of the shear cell with back plate (red), spacer plate (white) and front plate (blue) comprise the housing for the rotating mandrel.
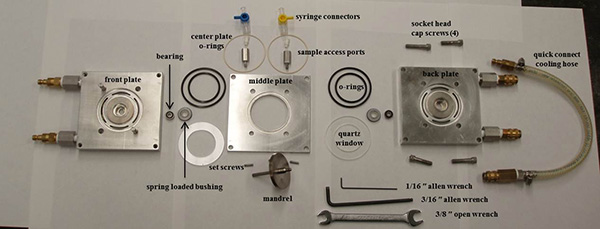
Figure 4. Disassembled view of all of the parts and tools required to assemble the 1-2 plane shear cell instrument.

Figure 5. Back plate assembly showing bearing, spring-loaded bushing, two O-rings and the quartz window properly assembled.
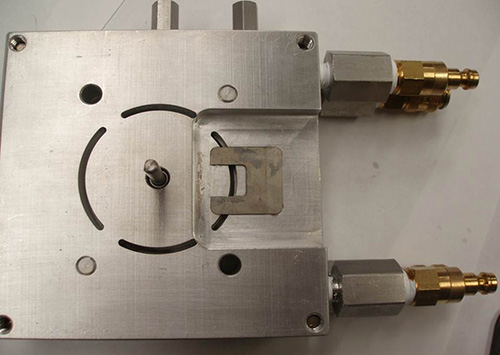
Figure 6. Assembled shear cell featuring the front plate to demonstrate proper placement of the cadmium aperture.
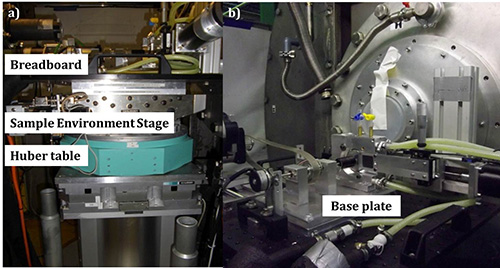
Figure 7. Side view of the sample environment at the D22 beamline, at the Institute Laue-Langevin, Grenoble, France. a) From bottom to top: Huber table, sample environment stage and breadboard; b) safety cover in place and cell mounted onto the base plate, which is attached to the breadboard.
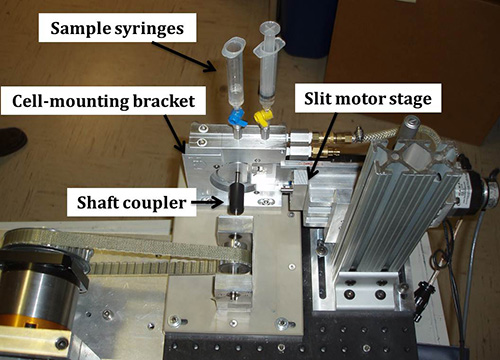
Figure 8. The assembled shear cell complete with sample syringes attached to the cell-mounting bracket and shaft coupler on the baseplate.
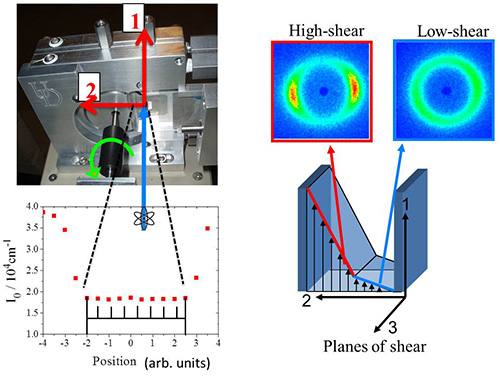
Figure 9. Left) Diagram of the 1-2 plane flow-SANS shear cell showing velocity (1) and velocity gradient directions (2) (red arrows) relative to the incident beam (blue arrow) and direction of mandrel rotation (green arrow). Transmission measurements are made using 0.1 mm slit and are presented as a function of position across the gap of the shear cell geometry. Right) The illustration shows results from SANS experiments performed at two different positions in the gap corresponding to the high and low shear bands.
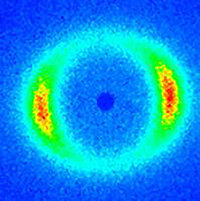
Figure 10. Typical SANS scattering pattern observed in the 1-2 plane for worm-like micelles under shear flow.
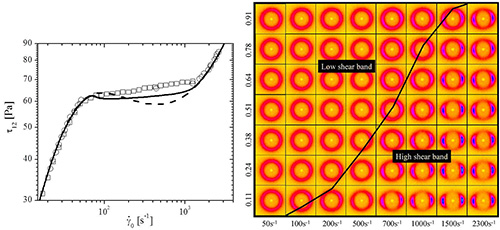
Figure 11. Left) shear stress versus shear rate for CTAB solution. The lines are the Giesekus model fit with (solid) and without (dashed) diffusion as described in reference15. Right) two-dimensional SANS scattering results for nominal applied shear rates and normalized gap positions spanning the shear banding transition for the CTAB sample. The black line indicates the measured location of the interface between the high-shear and low-shear bands. In these figures, the flow direction is vertical downward and the velocity-gradient direction is horizontal to the right. (Reprinted with permission from reference15. Copyright 2009, The Society of Rheology.)
Figure 12. Part drawing: 1-2 shear cell front plate.
Click here to view larger image.
Figure 13. Part drawing: 1-2 shear cell quartz window.
Click here to view larger image.
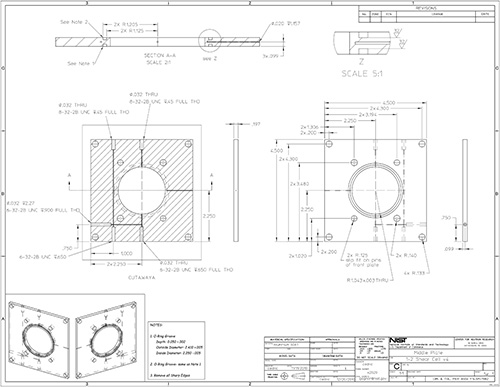
Figure 14. Part drawing: 1-2 shear cell middle plate.
Click here to view larger image.
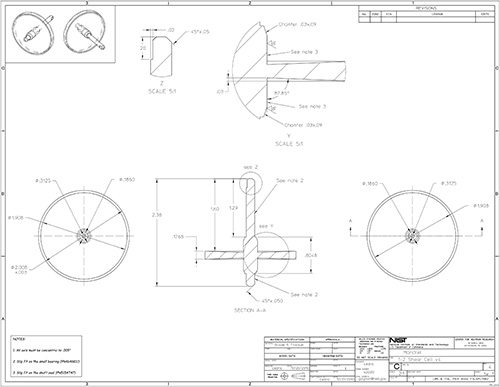
Figure 15. Part drawing: 1-2 shear cell mandrel.
Click here to view larger image.
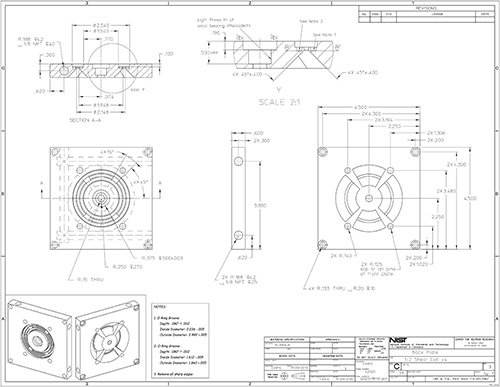
Figure 16. Part drawing: 1-2 shear cell back plate.
Click here to view larger image.
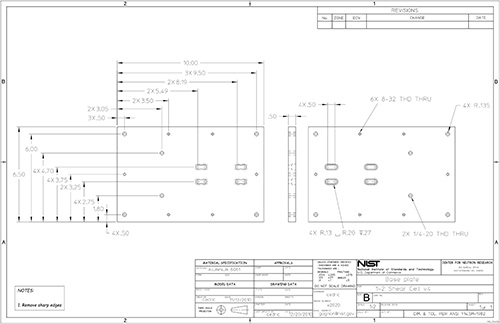
Figure 17. Part drawing: 1-2 shear cell base plate.
Click here to view larger image.
Figure 18. Part drawing: 1-2 shear cell plastic liner.
Click here to view larger image.
Figure 19. Part drawing: 1-2 shear cell front bracket.
Click here to view larger image.
Figure 20. Part drawing: 1-2 shear cell drive shaft support.
Click here to view larger image.
Figure 21. Part drawing: 1-2 shear cell drive shaft.
Click here to view larger image.
Figure 22. Part drawing: 1-2 shear cell retaining plate.
Click here to view larger image.
Figure 23. Part drawing: 1-2 shear cell shaft coupler.Click here to view larger image.
| Deuterated Water (99.9%) | Cambridge Isotopes | 7789-20-0 | 83.3 wt % in formulation D2O |
| CTAB- Cetyltrimethylammonium Bromide | Sigma-Aldrich | 57-09-0 | 16.7 wt % in formulation CH3(CH2)15N(Br)(CH3)3 |
| 1/16 in Allen wrench | |||
| 3/16 in Allen wrench | |||
| 3/8 in open end wrench | |||
| tape | |||
| thread seal tape | |||
| syringes (2) |
Table 1. Composition of Surfactant Solution Studied and tools required for assembly.
Discussion
A new instrument capable of measuring the microstructure of shearing complex fluids in the velocity-velocity gradient plane of shear via small angle neutron scattering is developed and validated. The shear cell design complements other instruments using radiation sources, such as X-ray and light scattering, as well as rheo-SANS instruments capable of characterizing the microstructure in the two other planes of shear (velocity-vorticity and velocity gradient-vorticity)8,10. This instrument functions for both steady shear and time dependent flows, such as oscillatory or start-up shear flows, the latter using a stroboscopic methodology and time-resolved neutron scattering techniques11,12,21. An advantage to using SANS is that contrast matching methods can be employed to explore the individual components in complex mixtures and materials that are opaque, or lack contrast necessary for X-ray scattering. The flow-SANS instrument and methods have been successfully extended to resolve the internal microstructure during shear banding14,15. Further, as SANS is an absolute measurement technique, measurements of the incident beam transmission through the sample can be used to determine absolute chemical composition changes across the Couette gap, recently demonstrated in13. As such, the new flow-SANS technique is a robust and versatile method for obtaining direct microstructural information, from the atomistic to the ~micron length scale, on a broad range of colloidal, self-assembled surfactant, protein, and polymer solutions and their mixtures under nonequilibrium conditions. This instrumentation is currently available for use by proposal submission on both SANS and USANS instruments at the NIST Center for Neutron Research at the National Institute of Standards and Technology in the U.S. and in Europe, on the D22 neutron beam of the Institute Laue-Langevin in Grenoble, France.
The current shear cell geometry design permits the addition of supplementary methods, such as light scattering for collecting simultaneous neutron and optical photon scattering (SNAPS) data, as well as direct microscope imaging. The latter can be used to help resolve the flow-field in situ by particle tracking methods. Future developments include enhanced synchronization for time-dependent flows, which currently is limited to ~10 microseconds in resolution. Of course, there are also limitations to the current mechanical design such as the maximum shear rates achievable are of order 103 sec-1 and strain amplitudes and frequencies for oscillatory flow are limited by the time-resolution as well as motor fidelity. Some of these issues are being resolved using additional gear reducers. Further, the sample viscosity must be such that it can be loaded by syringe. Typically, when possible, samples are heated to facilitate loading and enable removing any air bubbles trapped during loading. Care must be taken to consider possible flow-instabilities and wall slip, which are typical concerns addressed in making the complementary rheological measurements. There is also a trade-off between the accuracy of the applied flow field and the thickness of the sample (currently 5-7 mm) this can limit some applications due to concerns about multiple scattering and adsorption. The geometry requires a sample volume of order 6 ml, which can be a challenge for studying rare materials. As with any good design, there is room for improvement on the shear cell detailed here. In fact, the current instrument is a flow-SANS method in that SANS measurements are made while a simultaneous shear flow is applied, however with the current design no rheometry measurements are possible. Impending developments will enable simultaneous SANS and torque measurements. A true rheo-SANS instrument for investigating the velocity-velocity gradient plane of shear will be possible given that the shear stress will be resolved from the torque and hence, simultaneous rheometry and SANS measurements will be achieved. Engineering new shear cells that are mechanically sealed and magnetically driven is a welcome challenge and currently, designs and construction of the next generation shear cell are underway to address some of these issues.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We acknowledge Master Machinist Al Lance of the University of Delaware for machining the shear cell and Mr. Cedric Gagnon for design and drafting. This manuscript was prepared under cooperative agreement 70NANB7H6178 from NIST, U.S. Department of Commerce. This work utilized facilities supported in part by the National Science Foundation under Agreement No. DMR-0944772. The statements, findings, conclusions and recommendations are those of the author(s) and do not necessarily reflect the view of NIST or the U.S. Department of Commerce.
Materials
| Deuterated Water (99.9%) | Cambridge Isotopes | 7789-20-0 | 83.3 wt % in formulation D2O |
| CTAB- Cetyl Trimethyl Ammonium Bromide | Sigma-Aldrich | 57-09-0 | 16.7 wt % in formulation CH3(CH2)15N(Br)(CH3)3 |
| 1/16" Allen wrench | |||
| 3/16" Allen wrench | |||
| 3/8" open end wrench | |||
| tape | |||
| thread seal tape | |||
| syringes (2) |
References
- Larson, R. G. . The Structure and Rheology of Complex Fluids. , (1999).
- Wagner, N. J., Brady, J. F. Shear thickening in colloidal dispersions. Phys.Today. 62, 27-32 (2009).
- Fardin, M. A., et al. Potential "ways of thinking" about the shear-banding phenomenon. Soft Matter. 8, 910-922 (2012).
- Eberle, A. P. R., et al. Shear-induced anisotropy in nanoparticle gels with short-ranged interactions. Phys. Rev. Lett. , (2013).
- Zaccarelli, E. Colloidal gels: equilibrium and non-equilibrium routes. J. Phys. Cond. Matter. 19, (2007).
- Hsiao, L. C., Newman, R. S., Glotzer, S. C., Solomon, M. J. Role of isostaticity and load-bearing microstructure in the elasticity of yielded colloidal gels. Proc. Natl. Acad. Sci. U.S.A. 109, 16029-16034 (2012).
- Zemb, T., Linder, P. Neutron, X-rays, and Light. Scattering Methods Applied to Soft Condensed Matter. Elsevier Science. 552, (2002).
- Eberle, A. P. R., Porcar, L. Flow-SANS and Rheo-SANS applied to soft matter. Curr. Opin. Coll. Inter. Sci. 17, 33-43 (2012).
- Liberatore, M. W., Nettesheim, F., Wagner, N. J., Porcar, L. Spatially resolved small-angle neutron scattering in the 1-2 plane: A study of shear-induced phase-separating wormlike micelles. Phys. Rev. E. 73, (2006).
- Porcar, L., Pozzo, D., Langenbucher, G., Moyer, J., Butler, P. D. Rheo-small-angle neutron scattering at the National Institute of Standards and Technology Center for Neutron Research. Rev. Sci. Instr. 82, (2011).
- Lopez-Barron, C. R., Porcar, L., Eberle, A. P. R., Wagner, N. J. Dynamics of Melting and Recrystallization in a Polymeric Micellar Crystal Subjected to Large Amplitude Oscillatory Shear Flow. Phys. Rev. Lett. 108, 258301-2510 (2012).
- Rogers, S., Kohlbrecher, J., Lettinga, M. P. The molecular origin of stress generation in worm-like micelles, using a rheo-SANS LAOS approach. Soft Matter. 8, 3831-3839 (2012).
- Helgeson, M. E., Porcar, L., Lopez-Barron, C., Wagner, N. J. Direct Observation of Flow-Concentration Coupling in a Shear-Banding Fluid. Phys. Rev. Lett. 105, (2010).
- Helgeson, M. E., Reichert, M. D., Hu, Y. T., Wagner, N. J. Relating shear banding, structure, and phase behavior in wormlike micellar solutions. Soft Matter. 5, 3858-3869 (2009).
- Helgeson, M. E., Vasquez, P. A., Kaler, E. W., Wagner, N. J. Rheology and spatially resolved structure of cetyltrimethylammonium bromide wormlike micelles through the shear banding transition. J. Rheol. 53, 727-756 (2009).
- Liberatore, M. W., et al. Microstructure and shear rheology of entangled wormlike micelles in solution. J. Rheol. 53, 441-458 (2009).
- Maranzano, B. J., Wagner, N. J. Flow-small angle neutron scattering measurements of colloidal dispersion microstructure evolution through the shear thickening transition. J. Chem. Phys. 117, 10291-10302 (2002).
- Wagner, N. J., Ackerson, B. J. Analysis of nonequilibrium structures of shearing colloidal suspensions. J. Chem. Phys. 97, 1473-1483 (1992).
- Zhou, L., Vasquez, P. A., Cook, L. P., McKinley, G. H. Modeling the inhomogeneous response and formation of shear bands in steady and transient flows of entangled liquids. J. Rheol. 52, 591-623 (2008).
- Spenley, N. A., Cates, M. E., McLeish, T. C. B. Nonlinear rheology of wormlike micelles Phys. Rev. Lett. 71, 939-942 (1993).
- Lopez-Barron, C., Gurnon, A. K., Porcar, L., Wagner, N. J. Structural Evolution of a Model, Shear-Bading Wormlike Micellar Soution during Shear Start Up and Cessation. Phys. Rev. Lett.. , (2013).

