Stabilizing Hepatocellular Phenotype Using Optimized Synthetic Surfaces
Summary
This article will focus on developing polymer coated surfaces for long-term, stable culture of stem cell derived human hepatocytes.
Abstract
Currently, one of the major limitations in cell biology is maintaining differentiated cell phenotype. Biological matrices are commonly used for culturing and maintaining primary and pluripotent stem cell derived hepatocytes. While biological matrices are useful, they permit short term culture of hepatocytes, limiting their widespread application. We have attempted to overcome the limitations using a synthetic polymer coating. Polymers represent one of the broadest classes of biomaterials and possess a wide range of mechanical, physical and chemical properties, which can be fine-tuned for purpose. Importantly, such materials can be scaled to quality assured standards and display batch-to-batch consistency. This is essential if cells are to be expanded for high through-put screening in the pharmaceutical testing industry or for cellular based therapy. Polyurethanes (PUs) are one group of materials that have shown promise in cell culture. Our recent progress in optimizing a polyurethane coated surface, for long-term culture of human hepatocytes displaying stable phenotype, is presented and discussed.
Introduction
Biological materials have been widely used in the maintenance and differentiation of pluripotent stem cells 1. While enabling, these biological substrates often contain a myriad of undefined components. Matrigel is a commonly used substrate for stem cell culture and differentiation. Unfortunately, its variable composition influences cell function and phenotype. Although a variety of alternative, more defined biological matrices have been used 2-7, their animal origin or poor scalability makes them unsuitable candidates for industrial manufacture. Therefore the identification of synthetic alternatives, with defined composition and reliable performance, are key goals in stem cell research.
In an attempt to overcome the limitations of undefined cell culture substrates, interdisciplinary collaborations between chemistry and biology have identified synthetic materials with the capacity to support cell phenotype. Synthetic substrates are scalable, cost effective, and can be manufactured into complex 3D structures, mimicking the in vivo environment. Due to these properties synthetic substrates have been widely used to support and drive differentiation of many cell types 8-10.
Advanced and high throughput assays have facilitated the rapid screening of synthetic materials, from large libraries, and delivered novel materials with flexible properties with wide applications in biomedical research and development 11-13. Utilizing high throughput, polymer micro-array screening technology, we rapidly identified a simple polyurethane (PU134), suitable for maintenance of human stem cell derived hepatocytes. This polymer was found to be superior to animal derived substrates with regard to hepatocyte differentiation and function 14-16. We have subsequently optimized the coating conditions, topography and sterilization process to access effects on polymer performance in stabilizing hepatocyte function and lifespan. This has significant implications with regard to understanding fundamentals of hepatocyte biology for cell based modelling and regenerative medicine applications.
The technology here described represents an example of how the surface of a synthetic polymer can be optimized to preserve cell phenotype. We believe that the combination of this technology with an efficient serum free hepatocyte differentiation protocol has the potential to provide a scalable production of hepatocytes for use in in vitro modelling and regenerative medicine.
Protocol
1. Synthesis of PHNGAD (Poly[1,6-hexanodiol/neopentyl glycol/di(ethylene glycol)-alt-adipic acid]diol)
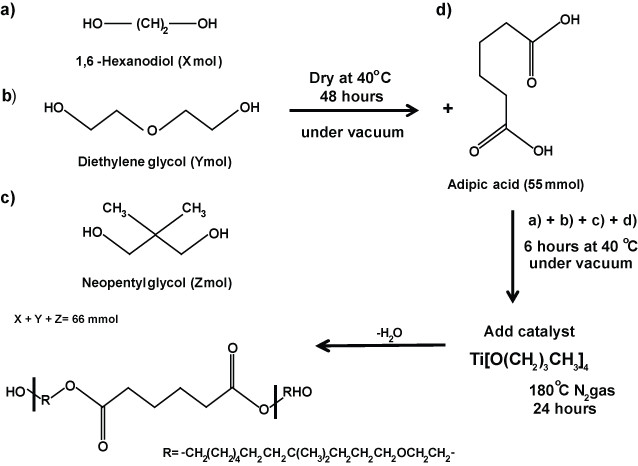
Scheme 1: Synthesis of PHNAGD. Schematic representation of the synthesis of PHNAGD. PHNAGD was prepared by the reaction of 1,6-Hexanodiol, diethylene glycol, neoppentyl glycol and adipic acid. PHNAGD, Poly[1,6-hexanodiol/neopentyl glycol/di(ethylene glycol)-alt-adipic acid]diol.
- Apply heat treatment to the monomers 1,6-hexanediol, di(ethylene glycol) and neopentyl glycol at 40 °C for 48 hr in a vacuum oven to remove any residual water. Allow to cold down to RT under vacuum.
- Add 22 mmol of each monomer and adipic acid (55 mmol) to a two-necked round bottom flask equipped with a stirred bar and connected to a Dean-Stark apparatus.
- Place the whole assembly under a vacuum and gently heat the glassware at 40 °C for 6 hr, in order to avoid any moisture absorption during the addition of the chemical into the flask.
- Add via syringe, drop by drop, 0.0055 mol of the catalyst titanium (IV) butoxide.
- Stir the reaction mixture at 180 °C, under an N2 atmosphere for 24 hr, and collect residual water in the Dean-Stark trap. Allow the product to cool to RT.
2. Synthesis of PU134
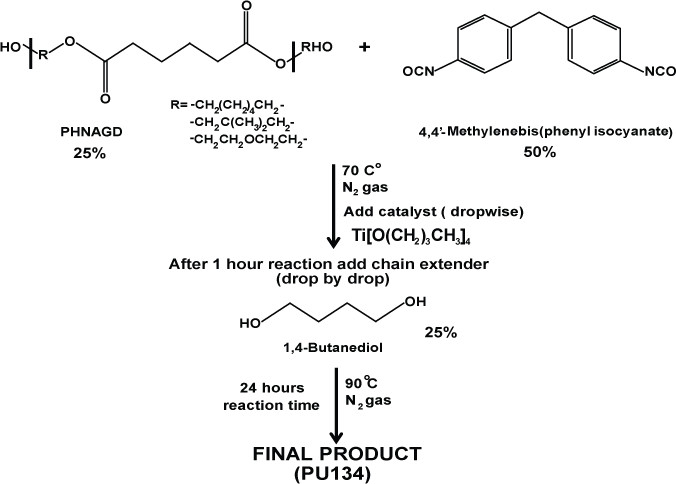
Scheme 2: Synthesis of PU134. Schematic representation of the synthesis of polyurethane 134. PU134 was prepared by the reaction of 1.0 equiv of a PHNGAD with 2.0 equiv of a 4,4′-Methylenebis(phenyl isocyanate), followed by the addition of 1.0 equiv of a 1,4-butanediol chain extender.
- Mix one equivalent of the polyol PHNGAD (Mn ~1,800 Da, 3.2 mmol) with two equivalents of 4’4-Methylenebis(phenyl isocyanate) (6.4 mmol) in anhydrous N,N-Dimethylformamide (12 ml).
- Stir the reaction mixture at 70 °C, under an N2 atmosphere.
- Add via syringe, drop by drop the catalyst titanium (IV) butoxide (0.8% wt).
- After 1 hr, add one equivalent of the chain extender 1,4-butanediol (3.2 mmol). Increase the temperature at 90 °C and stir for 24 hr under an N2 atmosphere.
- Following the reaction, collect the polyurethane by precipitation by adding diethyl ether (hexane or water could also be used) drop wise into the reaction solution until the precipitation occurs.
- Centrifuge the solution at 5,300 x g for 5 min.
- Decant the supernatant and dry off at 40 °C in a vacuum oven until the solvent evaporates.
Note: In order to ensure that the final product of the reaction possess the correct parameters regarding to the molecular weight distribution, the functional groups of the polymer, and the melting and glass transition temperatures, various analytical techniques and methods can be used, such as gel permeation chromatography or FITR spectroscopy.
3. Preparation of PU134 Solutions
- Weigh 200 mg of PU134 into a glass bottle.
- Dilute PU134 to a final concentration of 2% in a number of solvents: chloroform, a combination of chloroform and toluene in 1:1 ratio, tetrahydrofuran, and combination of tetrahydrofuran and dicloromethane in 1:1 ratio.
Note: The election of the right solvent varies depending on the polymer to use. Different solvents possess different boiling points that can affect the solubilization of the polymer. - Shake the solution vigorously for 20 min at RT using a shaker at a speed of 200 mot/min, until the solution becomes homogeneous and no precipitate is observed.
4. Coating of Glass Slides with PU134
- Place a round 15 mm2 coverslip on the spin coater.
- Apply 50 μl of the PU134 solution to each using a pipette. Adjust the volume of the PU134 solution accordingly for the required coverslip size keeping the volume to surface ratio proportional.
- Spin each coverslip for 7 sec at 23 x g.
- Air dry coverslip at RT for at least 24 hr before sterilization.
5. Irradiation of Coverslip
- Gamma-irradiate polymer coated coverslip by applying a dose of 10 Grays using a laboratory irradiator for 12 min.
- UV- irradiate polymer coated coverslip using a 30 W, UV bulb for 16 min each side.
- Place the polymer coated coverslip in a suitable tissue culture plate according to the slide size.
6. Scanning Electron Microscopy
- Gold coat polymer coverslip by sputtering for 200 sec in an atmosphere of 5 x 10-1 millibars of pressure.
- Capture the micrographs of the polymer coated coverslip using a scanning electron microscope at an accelerating voltage of 20 kV in secondary electron imaging mode.
7. Atomic Force Microscopy Observations
- Scan an area of 20 x 20 μm of the polymer surface.
- Set up a scan rate from 1.32 Hz to 1.60 Hz.
- Set up a resolution of 512 x 512 pixels in the scanned region.
- Calculate the root mean square (RMS or Rq) of the coating by using the average of height deviations taken from the mean image data plane, expressed as:

Where Zi is the current Z value, and N is the number of points within the given area. - 7.5 Calculate the deviation or mean surface roughness (Ra) of the image using,
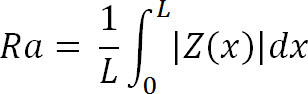
Where Z (x) is the function that describes the surface profile analyzed in terms of height (Z) and position (x) of the sample over the evaluation length “L”. Ra represents the mean value of the surface relative to the center plane.
8. Cell Culture and Differentiation
- Culture and differentiate the human embryonic stem cell line (hESC) H9 as described in Hay 17.
- Detach cells using a dissociation reagent and replate them onto PU134 coated slides at Day 9 of the differentiation process, in the presence of a serum free medium as describe in Szkolnicka 18,19.
Note: The use of an enzymatic cell dissociation is preferred to physical cell detachment.
9. Cytochrome p450 Functional Assay
- Measure CYP3A activity following the manufacturer’s instructions.
- Incubate hESC derived hepatocytes at Day 13 or Day 19, and media without cells – as a negative control, with the appropriate substrate for 5 hr at 37 °C.
- Collect supernatants from cells and media, carry out the assay as per manufacturer’s instructions.
- Measure the level of CYP activity and normalized relative to the surface area (cm2).
10. Immunostaining
- Wash hESC derived hepatocytes with PBS, for 1 min, repeat two times.
- Add ice cold 100% methanol to fix cells, place in -20 °C for 10 min.
- Wash cells with PBS for 5 min and repeat twice.
- Incubate cells with PBS/T (0.1% tween)/10% BSA for 1 hr at RT.
- Aspirate the PBST solution and add the appropriate primary antibody diluted in PBS/T (0.1% tween)/1% BSA, incubate at 4 °C with gentle agitation O/N.
- Wash cells with PBS/T (0.1% tween)/1% BSA for 5 min and repeat three times.
- Dilute the appropriate antibody in PBS/T (0.1% tween)/1% BSA, add to the cells and incubate in the dark at RT for 1 hr with gentle agitation.
- Wash cells with PBS for 5 min and repeat three times.
- Add MOWIOL 488 (containing DAPI 1:1,000) to each well, add a coverslip gently to reduce number of air bubbles. Store fixed cells at 4 °C in the dark. Observe staining using a microscope with appropriate filter and fluorescent lamp. The optimized primary and secondary antibodies are listed in Table 1.
Representative Results
Polymer solvent influences the topography of the polymer coated surface
Polyurethane 134 was solubilized in chloroform, either alone or in combination with toluene or tetrahydrofuran or dichloromethane and the glass slides spin-coated with the different formulations. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were used to characterize the physical properties of the polymer coatings (Figure 1). The coating obtained using toluene or chloroform was not homogeneous, demonstrating an uneven coating with PU134 precipitation (Figure 1A). In contrast, tetrahydrofuran was a suitable solvent allowing the spin coating of a much more uniform surface (Figure 1A). Surface topography was measured by AFM using the root mean square (RMS) and the mean roughness (Ra). PU134 dissolved tetrahydrofuran exhibited a 40% reduction in the roughness in comparison to the other solvents (Figure 1B and 1C). These data demonstrate that the use of tetrahydrofuran as a solvent provides a more uniform coating of PU134 for biological application.
Polymer topography has a significant impact on hepatocyte function
hESC can be efficiently differentiated to hepatic endoderm in vitro, exhibiting many of the functions found in the adult liver 17-20. Hepatic endoderm differentiation was induced and at Day 9 hepatoblast-like cells were apparent, with the majority of cells expressing cytokeratin 19 (99% ± 1.2), α-fetoprotein (99% ± 0.6), and hepatic nuclear factor 4α (97% ± 2.6); and low levels of albumin (20% ± 1.7) (Figure 2). At this point the cells were detached using TrypLE express and replated onto the different polymer coated surfaces. As a measure of metabolic function, cytochrome P450 function was assessed 4 days post-replating. We observed a 2-fold increase in metabolic activity in cells replated on tetrahydrofuran/PU134 coated surfaces (Figure 3). These results demonstrate that hESC derived hepatocyte metabolic activity is improved with a more uniform coating of PU134, this is consistent with previous approaches 21.
Surface sterilization impacts on the bioactive properties of the polymer
The effect of sterilization on PU134 performance was also investigated. Polymer coated glass slides were exposed to UV or gamma irradiation. Phase contrast imaging of PU134 coated glass slides showed no gross differences post UV or gamma irradiation (Figure 4A). These observations were further confirmed by SEM analysis (Figure 4B). The biological performance of PU134 was examined using hESC derived hepatocytes at 10 days post-replating. hESC derived hepatocytes replated on γ-radiated PU134 coated glass slides displayed a 3-fold increase in CYP3A activity over cells replated on UV-radiated PU134 coated glass slides (Figure 4C). These observations demonstrated that gamma-irradiation was the optimal sterilization technique for our purposes.

Figure 1. Optimization of the polyurethane 134 coated surface. (A) Glass slides were coated with a 2% solution of PU134 dissolved in different solvents. Scanning Electron Microscopy (SEM) images of the different coated glass slides show the presence of a homogeneous coated surface and the absence of polymer precipitates when tetrahydrofuran was used compared with toluene, chloroform or mixtures of the solvents (black and white arrows). The images were taken at x1664 magnification and scales bars are shown below the images. (B-C) Atomic Force Microscopy (AFM) was employed to analyze the roughness of the PU134 coated surfaces on selected solvents. Analysis of the roughness parameters RMS (the root mean square) (B) and Ra (the mean roughness) (C) of the different PU134 coated surfaces show that the coating obtained by using tetrahydrofuran as a solvent had the smoothest surface when compared with the rest of the conditions (n=7). Data are expressed as mean ± s.d., p<0.05 is denoted *, p<0.01 is denoted **, and p <0.001 is denoted ***, measured by students t-test in comparison to slides coated with PU134 solubilized in tetrahydrofuran. Error bars represent 1 standard deviation. Please click here to view a larger version of this figure.

Figure 2. Characterisation of the hESC derived hepatoblasts. Immunocytochemistry showing expression of hepatic markers AFP, CK19, HNF4α and Albumin in hESC (H9) derived hepatic endoderm. Negative controls were performed with corresponding immunoglobulin G (IgG) and representative images are shown. The percentage of positive cells and standard deviation were estimated from at least five random fields of view containing at least 300 cells per view. The images were taken at 20X magnification. Data is expressed as mean ± s.d. Error bars represent 1 standard deviation. HNF4α, Hepatocyte nuclear factor 4α; AFP, α-fetoprotein; ALB, Albumin; CK19, Cytokeratin 19, IgG goat, Immunoglobulin G anti-goat, IgG mouse, Immunoglobulin G anti-mouse. Please click here to view a larger version of this figure.
Figure 3. Functional implications of the topography of the PU134 coated surface on the function of hESC derived hepatocytes. Measurement of the Cytochrome P450 3A activity on hESC-derived hepatocytes maintained for 96 hr on glass slides coated with PU134 dissolved in different solvents. An improvement in the in the cytochrome activity on cells maintained on PU134 coated glass slides solubilized with tetrahydrofuran (n=6). Data are expressed as mean ± s.d., p<0.05 is denoted *, p<0.01 is denoted **, and p <0.001 is denoted ***, measured by students t-test in comparison to hESC derived hepatocytes maintained on slides coated with PU134 solubilized on tetrahydrofuran. Error bars represent 1 standard deviation.

Figure 4. Optimizing the sterilization of the coated surfaces. Phase contrast images (A) or SEM images (B) show no gross differences in the PU134 coated surface between γ-radiation or UV radiation (UV) treatments. Phase contrast images were taken at 40X magnification and SEM images at 1,664X magnification. (C) Analysis of the cytochrome P450 3A activity on hESC derived hepatocytes maintained for 10 days on PU134 coated glass slides. An increase in the cytochrome P450 3A activity in cells replated on γ-radiated PU134 coated glass slides when compared with cells replated on UV radiated PU134 coated glass slides. Units of activity are expressed as relative light units (RLU) ml−1 cm−2 of culture surface (n=3). Data are expressed as mean ± s.d., p<0.001 is denoted *** measured by students t-test in comparison to hESC derived hepatocytes maintained on PU134 coated glass coverslips sterilized by γ-radiation. Error bars represent 1 standard deviation. Please click here to view a larger version of this figure.
| Primary antibodies | |||
| Antigen | Type | Company | Dilution |
| HNF4α | Rabbit polyclonal | Santa Cruz | 1/100 |
| Albumin | Mouse monoclonal | Sigma | 1/500 |
| AFP | Mouse monoclonal | Abcam | 1/500 |
| Cytokeratin 19 | Mouse monoclonal | Dako | 1/50 |
| IgG | Mouse monoclonal | Dako | 1/500 |
| Secondary antibodies | |||
| Anti-rabbit 568 Alexa Flour | Goat | Invitrogen | 1/400 |
| Anti-mouse 488 Alexa Flour | Rabbit | Invitrogen | 1/500 |
Table 1. List of optimized primary and secondary antibodies and concentrations used in this study. HNF4α, Hepatocyte nuclear factor 4α; AFP, α-fetoprotein; IgG, Immunoglobulin G.
Discussion
Many of the current methods used to generate hepatocytes from stem cells rely on undefined matrices of animal origin. These substrates can be costly and highly variable, affecting cell function and stability, representing a significant barrier to application. Therefore, we performed a screen for synthetic materials which support the culture of stem cell derived hepatocytes. We have identified, a simple polyurethane (PU134), formed by polymerizing PHNGAD, MDI and an extender, that in combination with a robust hepatocyte differentiation approach, stabilizes the hepatocyte phenotype and improves cell function when compared with matrigel 15.
Handling of existing biological matrices, commonly used in culturing and differentiating stem cells such as vitronectin 22, laminin 521 23 or matrigel, is effective for short term maintenance. In contrast, PU134 coated surfaces provide a superior substrate for hepatocyte culture. Moreover, the optimization of surface topography and polymer sterilization have led to further improvements in cell performance, a key factor in delivering human liver models at scale with dependable performance.
The nature of the chain extender and phenyl isocyanate represents one of the critical stages of the process; as elimination or replacement of either component would significantly impair the polymer surface and therefore the cellular attachment and functional activity of stem cell derived hepatocytes. The composition of the polymer affects physical parameters such as elasticity and wettability 24, which has impacts the capacity of the polymer to absorb key extracellular matrix proteins involved in hepatocyte function. In addition, variations in the catalyst, reaction time and temperature affect the molecular weight distribution of the polymer.
The selection of the solvent to solubilize the polymer represents another critical point in the obtaining of an optimized polymer coated surface. We have observed that the nature of the solvent affects the topography of the coated surface, which has direct implications in the function of the cells 25-31. In addition, the spinning time and speed are also crucial in obtaining this uniform and homogeneous coating. Another step to take in consideration is the sterilization procedure applied on the polymer coated surface, as the activity of the cells can be influenced by the sterilization treatment used. This may be due to the presence of sensitive area(s) within the structure of the polymer to different sterilization procedures 32-34.
Current pluripotent stem cell based cell models possess phenotypic and functional limitations. We believe that this optimized polymer coated surface represents an advance in the field, circumventing some of the limitations associated with the use of biological substrates. Furthermore, the possibility of using the polymer within 3D matrices to support the attachment of different cellular types found in the tissue of interest, may further improve cell fidelity.
In conclusion, the use of synthetic materials is becoming increasingly important in the delivery of human soma from pluripotent stem cells. We have developed an optimized surface and coating procedure that is robust and reliably delivers functional human hepatocytes with significant implications for cell based modeling and regenerative medicine.
Divulgations
The authors have nothing to disclose.
Acknowledgements
D.C.H., M.B. and F.K. were supported by an EPSRC Follow on Fund. B.L-V and D.S. were each supported by MRC PhD studentships. K.C. was supported by funding from the UK Regenerative Medicine Platform.
Materials
| Synthesis, preparation, coating and characterization of polymer PU134 coated coverslips | |||
| Shaker | Edmun Bühler | KS-15 | |
| Irradiator | CIS Biointernational | IBL 637 | |
| Spin coater | Specialty Coating System | P-6708 | |
| Scanning Electron Microscope | Philips | XL30CPSEM | |
| Atomic Force Microscope | DimensionV Nanoscope, VEECO | ||
| p4-GLO CYP3A4 | Promega | V8902 | |
| UV bulb | ESCO | ||
| NanoScope analysis software | VEECO | version 1.20 | |
| Fluorescence microscope | Olympus | TH45200 | Use Volocity 4 Software |
| Tissue culture plates | Corning, UK | 3527 | |
| glass slides | Scientific Laboratory Supplies | MIC3308 | |
| Diethylene glycol | Sigma–Aldrich | 93171 | |
| 1,6-hexanediol | Sigma–Aldrich | 240117 | |
| Neopentyl glycol | Sigma–Aldrich | 408255 | |
| Adipic acid | Sigma–Aldrich | 9582 | |
| anhydrous N,N-Dimethylformamide | Sigma–Aldrich | 227056 | |
| Diethyl ether | Sigma–Aldrich | 676845 | |
| titanium (IV) butoxide | Sigma–Aldrich | 244112 | |
| 1,4-butanediol | Sigma–Aldrich | 493732 | |
| Vacuum oven | Thermoscientific | ||
| 4,4’-Methylenebis(phenyl isocyanate) | Sigma–Aldrich | 101688 | |
| Tetrahydrofurane | Sigma–Aldrich | 401757 | |
| Sputter coater | Bal-Tec SCD 050 | ||
| Inmunostaining | |||
| Phosphate buffer saline (-MgCl2, -CaCl2) | Gibco | 10010031 | Store at room temperature |
| PBST, PBS made up with 0.1% TWEEN 20 | Scientific Laboratory Supplies Ltd | EC607 | |
| Methanol | Scientific Laboratory Supplies Ltd | CHE5010 | |
| Bovine Serum Albumin | Sigma-Aldrich, UK | A7906 | |
| MOWIOL 488 DAPI | Calbiochem | 475904 | Made up in Tris HCL and glycerol as per manufacturers instructions |
| Cell culture and Functional assay | |||
| CYP3A activity pGLO kit | Promega | V8902 | |
| Hepatozyme | Gibco | 17705021 | |
| TryLE express | Life Technologies | 12604013 |
References
- Zhou, W., et al. SUMOylation of HNF4α regulates protein stability and hepatocyte function. J Cell Sci. 125 (15), 3630-3635 (2012).
- Banerjee, A., et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 30 (27), 4695-4699 (2009).
- Shanbhag, M. S., et al. Neural Progenitor Cells Grown on Hydrogel Surfaces Respond to the Product of the Transgene of Encapsulated Genetically Engineered Fibroblasts. Biomacromolecules. 11 (11), 2936-2943 (2010).
- Battista, S., et al. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 26 (31), 6194-6207 (2005).
- Tian, W. M., et al. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: in vitro. Journal of Controlled Release. 102 (1), 13-22 (2005).
- Keshaw, H., Forbes, A., Day, R. M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials. 26 (19), 4171-4179 (2005).
- Baharvand, H., Hashemi, S. M., Kazemi Ashtiani, S., Farrokhi, A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. The International Journal of Developmental Biology. 50 (7), 645-652 (2006).
- Cameron, K., Travers, P., Chander, C., Buckland, T., Campion, C., Noble, B. Directed osteogenic differentiation of human mesenchymal stem/precursor cells on silicate substituted calcium phosphate. Journal of Biomedical Materials Research Part A. 101 (1), 13-22 (2013).
- Pernagallo, S., Unciti-Broceta, A., Diaz-Mochon, J. J., Bradley, M. Deciphering cellular morphology and biocompatibility using polymer microarrays. Biomedical Materials. 3 (3), 034112 (2008).
- Li, Z., Guo, X., Matsushita, S., Guan, J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials. 32 (12), 3220-3232 (2011).
- Tare, R. S., Khan, F., Tourniaire, G., Morgan, S. M., Bradley, M., Oreffo, R. O. C. A microarray approach to the identification of polyurethanes for the isolation of human skeletal progenitor cells and augmentation of skeletal cell growth. Biomaterials. 30 (6), 1045-1055 (2009).
- Khan, F., Tare, R. S., Kanczler, J. M., Oreffo, R. O. C., Bradley, M. Strategies for cell manipulation and skeletal tissue engineering using high-throughput polymer blend formulation and microarray techniques. Biomaterials. 31 (8), 2216-2228 (2010).
- Zhang, R., et al. A thermoresponsive and chemically defined hydrogel for long-term culture of human embryonic stem cells. Nature Communications. 4 (1335), (2013).
- Medine, C. N., et al. Developing high-fidelity hepatotoxicity models from pluripotent stem cells. Stem Cells Translational Medicine. 2 (7), 505-509 (2013).
- Hay, D. C., et al. Unbiased screening of polymer libraries to define novel substrates for functional hepatocytes with inducible drug metabolism. Stem Cell Research. 6 (2), 92-102 (2011).
- Lucendo-Villarin, B., Khan, F., Pernagallo, S., Bradley, M., Iredale, J. P., Hay, D. C. Maintaining hepatic stem cell gene expression on biological and synthetic substrata. BioResearch Open Access. 1 (1), 50-53 (2012).
- Hay, D. C., et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proceedings of the National Academy of Sciences. 105 (34), 12301-12306 (2008).
- Szkolnicka, D., Zhou, W., Lucendo-Villarin, B., Hay, D. C. Pluripotent Stem Cell–Derived Hepatocytes: Potential and Challenges in Pharmacology. Annu Rev Pharmecol Toxicol. 53, 147-149 (2013).
- Szkolnicka, D., et al. Accurate prediction of drug-induced liver injury using stem cell-derived populations. Stem Cells Translational Medicine. 3 (2), 141-148 (2014).
- Medine, C. N., Lucendo-Villarin, B., Zhou, W., West, C. C., Hay, D. C. Robust Generation of Hepatocyte-like Cells from Human Embryonic Stem Cell Populations. Journal of Visualized Experiments. (56), (2011).
- Freed, L. E., Vunjak-Novakovic, G. Culture of organized cell communities. Advanced Drug Delivery Reviews. 33 (1-2), 15-30 (1998).
- Braam, S. R., et al. Recombinant Vitronectin Is a Functionally Defined Substrate That Supports Human Embryonic Stem Cell Self-Renewal via αVβ5 Integrin. Stem Cells. 26 (9), 2257-2265 (2008).
- Rodin, S., et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nature Communications. 5 (3195), (2014).
- Thaburet, J. -. F. O., Mizomoto, H., Bradley, M. High-Throughput Evaluation of the Wettability of Polymer Libraries. Macromolecular Rapid Communication. 25 (1), 336-370 (2003).
- Lim, J. Y., Donahue, H. J. Cell Sensing and Response to Micro- and Nanostructured Surfaces Produced by Chemical and Topographic Patterning. Tissue Engineering. 13 (8), 1879-1891 (2007).
- Teixeira, A. I., Abrams, G. A., Bertics, P. J., Murphy, C. J., Nealey, P. F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. Journal of Cell Science. 116 (10), 1881-1892 (2003).
- Biggs, M. J. P., Richards, R. G., Wilkinson, C. D. W., Dalby, M. J. Focal adhesion interactions with topographical structures: a novel method for immuno-SEM labelling of focal adhesions in S-phase cells. Journal of Microscopy. 231 (1), 28-37 (2008).
- Karuri, N. W., Porri, T. J., Albrecht, R. M., Murphy, C. J., Nealey, P. F. Nano- and microscale holes modulate cell-substrate adhesion, cytoskeletal organization, and -beta1 integrin localization in SV40 human corneal epithelial cells. IEEE Transactions on Nanobioscience. 5 (4), 273-280 (2006).
- Hamilton, D. W., Brunette, D. M. The effect of substratum topography on osteoblast adhesion mediated signal transduction and phosphorylation. Biomaterials. 28 (1), 1806-1819 (2007).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell. 126 (4), 677-689 (2006).
- Dang, J. M., Leong, K. W. Myogenic Induction of Aligned Mesenchymal Stem Cell Sheets by Culture on Thermally Responsive Electrospun Nanofibers. Advanced Materials. 19 (19), 2775-2779 (2007).
- Azevedo, E. C., Nascimento, E. M., Chierice, G. O. UV and gamma irradiation effects on surface properties of polyurethane derivate from castor oil. Polímeros. 23 (3), 305-311 (2013).
- Rosu, L., Cascaval, C. N., Ciobanu, C., Rosu, D. Effect of UV radiation on the semi-interpenetrating polymer networks based on polyurethane and epoxy maleate of bisphenol A. Journal of Photochemistry and Photobilogy A: Chemistry. 169 (2), 177-185 (2005).
- Yang, X. F., Tallman, D. E., Bierwagen, G. P., Croll, S. G. Blistering and degradation of polyurethane coatings under different accelerated weathering tests. Polymer Degradation and Stability. 77 (1), 103-109 (2002).

