Design and Implementation of an fMRI Study Examining Thought Suppression in Young Women with, and At-risk, for Depression
Summary
We aim to identify the neural correlates underlying sustained and transient thought suppression, and thought re-emergence in controls, at-risk and depressed individuals. Activation was greatest for controls compared to the at-risk and the depressed group in the dorsolateral prefrontal cortex during thought suppression and anterior cingulate cortex during thought re-emergence.
Abstract
Ruminative brooding is associated with increased vulnerability to major depression. Individuals who regularly ruminate will often try to reduce the frequency of their negative thoughts by actively suppressing them. We aim to identify the neural correlates underlying thought suppression in at-risk and depressed individuals. Three groups of women were studied; a major depressive disorder group, an at-risk group (having a first degree relative with depression) and controls. Participants performed a mixed block-event fMRI paradigm involving thought suppression, free thought and motor control periods. Participants identified the re-emergence of “to-be-suppressed” thoughts (“popping” back into conscious awareness) with a button press. During thought suppression the control group showed the greatest activation of the dorsolateral prefrontal cortex, followed by the at-risk, then depressed group. During the re-emergence of intrusive thoughts compared to successful re-suppression of those thoughts, the control group showed the greatest activation of the anterior cingulate cortices, followed by the at-risk, then depressed group. At-risk participants displayed anomalies in the neural regulation of thought suppression resembling the dysregulation found in depressed individuals. The predictive value of these changes in the onset of depression remains to be determined.
Introduction
A common trait in individuals with major depressive disorder (MDD) is the tendency to engage in ruminative thought1. This coping mechanism is considered maladaptive as it involves passive fixation on negative thoughts and events with no attempt at resolution2-5. Rumination is associated with increased risk of developing depression1,6-9 and increased length and severity of depressive episodes10.
Individuals who regularly ruminate will often try to reduce the frequency of these negative thoughts by actively suppressing them11. However, engaging in thought suppression can make such thoughts more accessible and likely to quickly re-emerge in the individual’s thoughts12. This may be seen more often in depressed individuals as their ability to actively suppress thoughts may be compromised. Additionally, thought suppression has been shown to increase the likelihood of other negative thoughts in dysphoric individuals13. Therefore, for depressed individuals the suppression of ruminative thoughts may lead to an exacerbation of symptoms; a product of increased cycling of ruminative intrusions and heightened negative thinking.
Neuropathological models of depression posit a dysregulation of the limbic, striatal, thalamic, and cortical brain circuits14. Resting disruptions of regional metabolism and blood flow are consistently reported in MDD, with heightened basal levels observed in the amygdala, orbital frontal cortex, ventral medial prefrontal cortex and medial thalamus. In addition, reduced levels are found in the dorsolateral prefrontal cortex, and subgenual and dorsal anterior cingulate cortex compared to healthy controls15,16. These observations have led to the notion that MDD involves a reduction in the activity of dorsal regions and heightened emotional limbic activity in more ventral brain regions.
Cognitive theories regarding the regulation of thought have identified a role for two separate mechanisms in thought suppression. It is suggested that the first mechanism of control is constantly engaged in order to maintain a baseline level of thought suppression and the second mechanism is transiently activated to re-suppress any unwanted thoughts that manage to intrude above this baseline17. Functional MRI data implicate a number of brain regions in these processes including the dorsolateral and ventrolateral prefrontal cortices18,19, the insula19,20, anterior cingulate cortex20, and dorsomedial prefrontal cortex19,21 during maintenance of thought suppression. Additionally, the re-emergence of a suppressed thought has been specifically associated with engagement of the anterior cingulate cortex18. Thus, there appears to be considerable overlap between the brain regions shown to be dysregulated in depression including the dorsolateral prefrontal cortex, insula, anterior cingulate cortex, dorsomedial prefrontal cortex22 and those involved in thought suppression. This suggests that a neurophysiological, and not just a behavioral link, between thought suppression and depression exists.
Young women who engage in ruminative thought are at greater risk for developing depression23. Risk for depression is also conferred genetically; individuals with a parent or sibling with depression are much more likely to develop depression than individuals with no family history of the disorder24. This study was carried out to explore the neural systems involved in thought suppression in a group of young women with a familial risk for depression, a group of young women currently experiencing depression, and a group of healthy controls. We developed a novel ruminative thought suppression paradigm to examine the changes in neural activity associated with sustained and transient thought suppression of both neutral and personally relevant thoughts. This design allowed us to investigate whether there were differences in neural activity for the suppression of personally relevant thoughts relative to neutral thoughts. Moreover, testing the at-risk group provided an opportunity to explore potential vulnerability markers of depression by determining whether risk for depression is associated with the magnitude of the blood oxygen level dependant (BOLD) signal in regions implicated in depression.
Based on the literature surrounding neural activity in depression15,16, and the studies on rumination and thought suppression25,26 it was predicted that the suppression of thoughts would be associated with reduced engagement of the dorsolateral prefrontal cortex in participants with MDD compared to controls. It was expected that the greater vulnerability to depression in the at-risk group would be reflected in levels of dorsolateral cortical activity that fall between that of the control and depressed groups. Furthermore, it was expected that the re-emergence of suppressed thoughts would be associated with activation of the anterior cingulate cortex, and that this activation would be greater in controls than in the at-risk group. Additionally, it was expected to observe significantly less anterior cingulate cortex activation in depressed participants as compared to both the control and at-risk participants during re-emergence of suppressed thoughts.
Protocol
All participants were briefed about the procedures and signed a consent form prior to study initiation. The McMaster University Health Sciences and St. Joseph’s Healthcare Research Ethics Boards approved all procedures.
Note: In this protocol, 47 right-handed females between the ages of 16 and 24 years are used. Of which, 15 participants suffer from MDD (physician-confirmed diagnosis) and are experiencing a depressive episode at the time of the study. This group of individuals is denoted as the “MDD group”. The at-risk group in this protocol consists of 16 participants who have a first degree relative (parent or sibling) with a diagnosis of MDD, but diagnosed with a psychiatric disorder and are not currently depressed. The control group in this protocol consists of 16 participants who do not have first-degree relatives with depression, have no lifetime diagnosis of a psychiatric disorder and are not currently depressed.
1. Participants Selection
- Recruit healthy control and at-risk female participants through the internet and print advertisements in the local community, and through the Department of Psychology, Neuroscience and Behavior at the local university. Recruit depressed participants through the Mood Disorders Clinic at the local hospital.
NOTE: Qualifying patients are eligible to participate if they are female and have been diagnosed with a primary mood disorder according to DSM-IV criteria27, and have clinically significant standardized cut-off scores on the Beck Depression Inventory-Version II (BDI-II) and Hamilton Depression Rating Scale (HAM-D) questionnaires, as determined by completion of these measures upon arrival. - Conduct initial phone or in-person interviews with interested participants to establish eligibility. Exclude participants that do not meet scanner safety requirements, or eligibility criteria for the MDD, at-risk or control groups, and all participants with a current or past history of psychosis, mania or generalized anxiety disorder (GAD). Additionally, exclude participants who have experienced a head injury leading to unconsciousness, or have had previous treatment with electroconvulsive therapy or transcranial magnetic stimulation.
- Invite the selected group of individuals for extensive testing, and a functional magnetic resonance imaging (fMRI) scan using the following parameters [3T, T1-weighted SPGR axial acquisition with: 132 – 160 slices (1 mm thick). fMRI scan, 8-channel head coil, 31 axial slices (4 mm thick, no gap), TR/TE = 2,500/35 msec, FOV = 24 cm, matrix = 64 x 64, flip angle 90°].
NOTE: Exclude participants with GAD as it is anticipated that they might experience higher levels of anxiety during the MRI scanning portion of the study, interfering with concentration and task compliance. - Scan the participants during the first 12 days of their menstrual cycles (follicular phase) to reduce the potential influences of hormonal fluctuations.
2. Build Thought Suppression Task
- Build a modified version of the suppression paradigm described by Mitchell and colleagues18, that will be viewed by the participants in the scanner. The paradigm consists of 4 blocks, each marked by a newly presented screen.
- Use psychology paradigm building software to program the paradigm. Using the software, assemble text and image slides to be presented serially, with responses collected with millisecond timing. The first block presents a target statement on the screen for 12.5 sec. Format the paradigm so that a 3-color traffic signal is present on the left hand side of all subsequent screens.
- Configure the paradigm to present a red traffic light signal for 30 sec on the following screen. Program the paradigm to present a green traffic signal for 30 sec on the next screen presented.
- Configure the final screen to present a flashing yellow light. Flash the light four times at pseudorandom intervals between 1,500 – 2,500 msec apart. Repeat this series of screens 12 times.
- Insert the target statements into the paradigm during the participants visit, and therefore construct the paradigm in a way that makes it easily modifiable. During the participants visit, instruct the participants to suppress the target thought when the red light is presented, to free think when the green light is presented, and to press the response button each time the yellow light flashes. Completing this task in the MRI scanner will allow the assessment of neural activity difference during each instructed task.
3. Participant Visit
- Upon arrival, assess depression severity and psychiatric status of all participants with the BDI-II, HAM-D and Mini International Neuropsychiatric Inventory (MINI) questionnaires. Collect information regarding medication status and educational background.
NOTE: These questionnaires were completed as part of a larger investigation including other measures not listed here. - Ask participants to list troubling thoughts or concerns that they have been repeatedly revisiting over the past few weeks and that they have been unable to shake. Record these oral statements while discussing them with the participant. Working with the participant, paraphrase the statement, shortening it to 7 – 10 words in length and identify a key word that is emotionally significant and explicitly conveys the meaning of the target sentence to the participant.
- Prior to the scan, instruct the participant to observe the traffic signal on the screen throughout the paradigm. Instruct the participant to suppress the presented target statement when the red light is presented. Ask the participant to think freely about anything and let their minds wander when the green light is presented.
- Instruct the participants to press the button on the scanner response box each time the target thought re-emerges during both the thought suppression and free thought periods. Finally, ask the participants to press the response box button each time a flashing yellow light is presented.
NOTE: The order of blocks in each functional run is as follows: a) target statement presentation period b) personal or distracter thought suppression period, c) free thought period, and d) motor response period to control for activation of motor areas induced during button presses in the thought suppression and free thought blocks. This pattern repeats 12 times, four times in each of the 3 runs. - Insert personally relative and distractor target statements and key words into the paradigm. Within each run, ensure the first block consists of two personal thought suppression blocks and two distracter thought suppression blocks (See Figure 1).
- Present the shortened statements and key words in a subsequent order and for 1TR each, followed by the traffic light signal for the rest of the target statement presentation period. Counterbalance the order of personal and distracter thought periods within each of the 3 fMRI scans and across participants.
Note: Target statements and key words will consist of both personally relevant negative ruminative thoughts provided by the participant (for example: “Think about not getting into university”), and neutral distractor statements taken from a battery prepared by Nolen-Hoeksema (for example: “Think about a row of shampoo bottles on display” 2).
- Present the shortened statements and key words in a subsequent order and for 1TR each, followed by the traffic light signal for the rest of the target statement presentation period. Counterbalance the order of personal and distracter thought periods within each of the 3 fMRI scans and across participants.
- Scan each participant with three functional MRI scans.
4. Functional Magnetic Resonance Imaging Data Acquisition and Analysis
- Conduct imaging on a 3T whole body short bore scanner with an 8-channel parallel receiver head coil. Perform a T1 weighted three-dimensional SPGR axial anatomical scan with 132 – 160 slices (1 mm thick). Acquire three functional MRI runs using a gradient-echo EPI sequence consisting of 31 axial slices (4 mm thick, no gap) beginning at the cerebral vertex and encompassing the entire cerebrum (TR/TE = 2,500/35 msec, FOV = 24 cm, matrix = 64 x 64, flip angle 90°).
- Transfer the acquired images to a work station.
- Transform anatomical MRI data sets into Talairach space28 to perform co-registration on functional data sets and average to generate a composite image.
- Temporally correct the functional data sets29. 3D motion correct the functional data sets29. Realign the functional data sets to the 5th frame of each run29. Smooth using a 6 mm Gaussian kernel and normalize to Talairach space29.
- Use an event-related analysis method when analyzing the data. Build an analysis protocol that extracts intervals of time associated with thought suppression, thought re-emergence and successful re-suppression, as outlined below.
- Define a re-emergence event as the interval beginning 500 msec before the button press (re-emergence of thought prior to button press) and continuing for 2,000 msec after the button press, during the thought suppression or free thinking block. The time spanning the thought suppression block with re-emergence events excluded is to define thought suppression in the analysis protocol.
NOTE: Define successful re-suppression in the analysis protocol as maintained suppression, without an intrusion event, within one TR following the re-suppression event. Define motor control in the protocol as event times when the yellow light flashed during the motor response period. - Using the neuroimaging software, contrast activation maps using a general linear model to identify clusters of activity associated with contrasts between and within groups. Conduct a random effects analyses (3 x 2) with a priori hypothesis testing. Conduct between group contrasts for thought suppression vs. motor control and thought re-emergence vs. re-suppression for control/at-risk vs. depressed, control vs. depressed, control vs. at-risk, and at-risk vs. depressed groups.
- Correct contrasts for multiple comparisons using the false discovery rate (FDR, set at p = 0.05) methodology implemented in the neuroimaging software.
Representative Results
Block Condition Analyses: Thought Suppression versus Motor Control
ANOVA analyses were used to determine the brain activation associated with block periods of thought suppression (with intrusions removed) relative to a motor control. Contrast results for control and at-risk versus MDD, control versus MDD, control versus at-risk, and at-risk versus MDD are detailed in Table 1. There were no between or within group differences in activity associated with suppression of personally relevant thoughts with distractor thoughts. As a result, all further analyses collapsed the personal and distractor thought suppression conditions in each group. Examining group differences between the control and MDD groups revealed greater activation in the dorsolateral prefrontal cortices (DLPFC) (BA 8), dorsal anterior cingulate, medial prefrontal and superior parietal regions for controls during suppression relative to MDD participants (Figure 2A). Comparisons between the at-risk and MDD groups similarly revealed greater activation of the dorsolateral prefrontal cortices as well as greater inferior prefrontal activation in at-risk participants during suppression as compared to MDD participants (Figure 2B). Collapsing the control and at-risk groups, and contrasting this larger group of healthy young women with MDD patients revealed greater activation in many of the same regions as the separate analyses of controls vs. MDD, and at-risk vs. MDD detailed above. Greater activation was identified in the dorsolateral prefrontal (BA 8), inferior frontal, and anterior insula cortices for the control/at-risk group during suppression as compared to the MDD group. In contrast, greater activation of more dorsal aspects of the insula, inferior parietal cortices and the cuneus were elicited during thought suppression in the MDD group relative to the control & at-risk groups. Finally, group contrasts exploring differences between the control and at-risk participants revealed greater activation for controls in the dorsolateral and dorsomedial prefrontal cortices during suppression relative to the at-risk (Figure 2C).
Event-Related Analyses: Thought Re-emergence versus Re-suppression
An ANOVA was used to examine the brain activation associated with transient re-emergence of target thoughts compared to thought re-suppression. The ANOVA analysis compared control and at-risk versus MDD, control versus MDD participants, control versus at-risk participants, and at-risk versus MDD participants. The results of these analyses are detailed in Table 2. Between group contrasts (thought re-emergence – thought re-suppression) revealed significant clusters of activation in the anterior cingulate cortices (ACC) for the control & at-risk group compared to MDD group. These group differences were attributable to greater activation in the ACC for control and at-risk groups compared to MDD group. The group contrast exploring differences between the control and MDD groups identified greater activation for controls in the anterior cingulate / medial prefrontal cortices, inferior and middle frontal cortices and superior temporal cortices (Figure 3A). The group contrast exploring differences between the control and at-risk participants revealed greater activation for controls in the anterior cingulate, inferior frontal and dorsomedial prefrontal cortices (Figure 3B). Finally, comparisons between the at-risk and MDD groups revealed greater activation of the anterior cingulate, inferior frontal cortices, dorsomedial prefrontal, insula and the uncus in at-risk participants than MDD participants (Figure 3C). These results indicate that the re-emergence and subsequent re-suppression of intrusive thoughts produced a continuum of group activation differences across a consistent set of regions that included the anterior cingulate cortex. Control participants showed the greatest difference in activation between the re-emergence and re-suppression periods followed by at-risk, and then MDD participants displaying the activation changes in these regions (Figure 4).
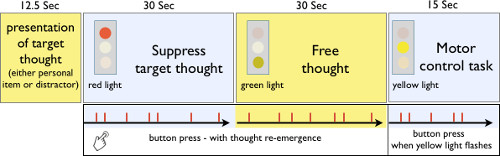
Figure 1: Thought suppression MRI paradigm. Pictorial of the thought suppression paradigm, which included the presentation of the target thought, thought suppression period, free thought period and motor control task. Reprinted with permission30.
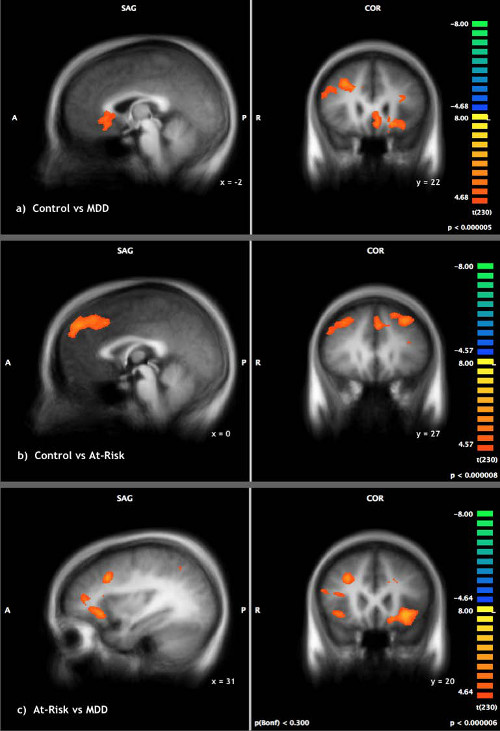
Figure 2: Thought Suppression. Thought suppression compared to motor control in (A) control versus MDD individuals (B) at-risk versus MDD individuals (C) control versus at-risk individuals. Reprinted with permission30. Please click here to view a larger version of this figure.
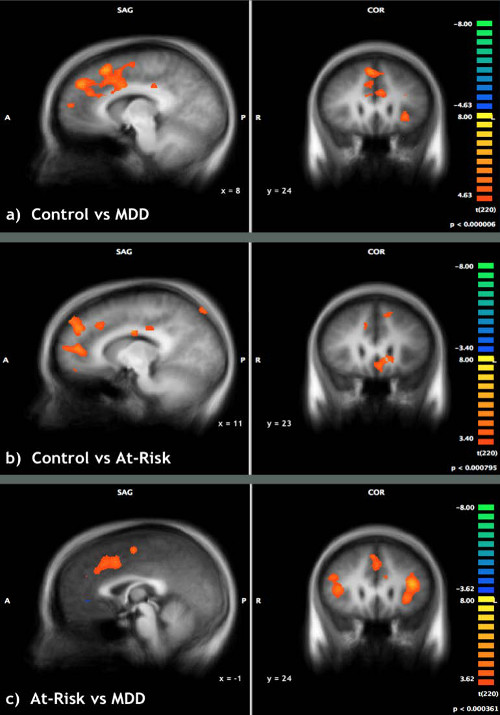
Figure 3: Though re-emergence vs. thought suppression. Re-emergence of target thoughts compared to successful thought suppression in (A) control versus MDD individuals (B) control versus at-risk individuals (C) at-risk versus MDD individuals. Reprinted with permission30.
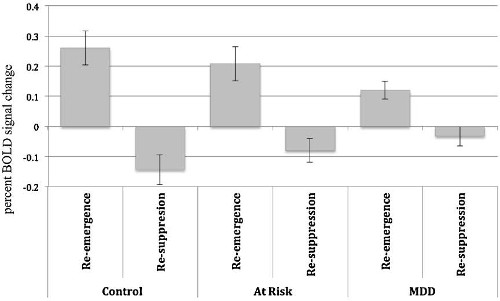
Figure 4: Anterior Cingulate Cortex activity during thought suppression and re-emergence. BOLD signal change in the anterior cingulate cortex for re-emergence and re-suppression of thought conditions. Greatest BOLD differences between the two conditions were elicited by the controls, then at-risk and finally MDD participants. Reprinted with permission30.
| Suppression vs Motor task | |||||||
| Region | BA | x | y | z | t- value | p-value | No. Voxels |
| a) Control & At-Risk vs. MDD | |||||||
| Control & At-Risk > MDD | |||||||
| Inferior Frontal Gyrus | 9 | 53.85 | 17.34 | 22.29 | 7.408 | <0.001 | 473 |
| Inferior Frontal Gyrus | 47 | 42.19 | 25.85 | -3.22 | 9.044 | <0.001 | 4554 |
| Dorsolateral Prefrontal Cortex | 8 | 26.16 | 21.28 | 34.45 | 7.461 | <0.001 | 1011 |
| Cingulate Gyrus | 32 | 11.46 | 23.68 | 29.97 | 7.223 | <0.001 | 783 |
| Superior Frontal Gyrus | 6 | -8.23 | 13.27 | 47.31 | 8.456 | <0.001 | 11795 |
| Precuneus | 7 | -2.48 | -69.17 | 39.1 | 7.417 | <0.001 | 1534 |
| Insula | 13 | -36.11 | 22.58 | 4.08 | 10.770 | <0.001 | 30885 |
| Middle Temporal Gyrus | 21 | -55.72 | -32.24 | -7.34 | 9.660 | <0.001 | 4080 |
| MDD > Control/At-Risk | |||||||
| Cuneus | 18 | -23.73 | -91.4 | -1.15 | -6.653 | <0.001 | 392 |
| Insula | 13 | -40.53 | -11 | 8.2 | -7.457 | <0.001 | 977 |
| Inferior Parietal Lobule | 40 | -51.62 | -31.52 | 23.08 | -8.290 | <0.001 | 1776 |
| b) Control vs. MDD | |||||||
| Control > MDD | |||||||
| Fusiform Gyrus | 37 | 54.57 | -60.2 | -12.05 | 5.753 | <0.001 | 366 |
| Middle Frontal Gyrus | 9 | 47.84 | 20.07 | 28.47 | 5.563 | <0.001 | 624 |
| Fusiform Gyrus | 20 | 38.21 | -24.52 | -26.78 | 5.990 | <0.001 | 461 |
| Superior Parietal Lobule | 7 | 28.48 | -59.26 | 44.5 | 7.155 | <0.001 | 1617 |
| Middle Frontal Gyrus | 6 | 24.66 | -1.78 | 53.11 | 5.688 | <0.001 | 500 |
| Dorsolateral Prefrontal Cortex | 8 | 28.62 | 21.46 | 35.58 | 6.205 | <0.001 | 1112 |
| Ventromedial Frontal Gyrus | 10 | 16.92 | 32.46 | -8.48 | 5.845 | <0.001 | 973 |
| Anterior Cingulate | 32 | 10.72 | 33.08 | 25.96 | 5.731 | <0.001 | 561 |
| Caudate | -15.22 | 23.75 | -6.17 | 7.428 | <0.001 | 6062 | |
| Superior Parietal Lobule | 7 | -25 | -54.08 | 39.14 | 5.915 | <0.001 | 1183 |
| Middle Frontal Gyrus | 46 | -35.33 | 29.34 | 19.61 | 6.629 | <0.001 | 1693 |
| Inferior Temporal Gyrus | 20 | -54.85 | -34.52 | -12.19 | 7.253 | <0.001 | 2062 |
| c) Control vs. At-Risk | |||||||
| Control > At-Risk | |||||||
| Dorsolateral Prefrontal Cortex | 8 | 35.55 | 28.27 | 38.61 | 5.949 | <0.001 | 5053 |
| Precuneus | 7 | 21.29 | -69.02 | 35.03 | 5.312 | <0.001 | 391 |
| Dorsomedial Frontal Gyrus | 8 | -10.18 | 38.86 | 39.05 | 6.524 | <0.001 | 22307 |
| Cuneus | 19 | 4.55 | -87.54 | 39.61 | 5.127 | <0.001 | 1086 |
| Lingual Gyrus | 18 | 2.46 | -89.17 | -18.33 | 5.475 | <0.001 | 1886 |
| Cingulate Gyrus | 31 | -2.7 | -44.13 | 40.29 | 4.933 | <0.001 | 803 |
| Posterior Cingulate | 29 | -3.85 | -44.61 | 7.25 | 4.966 | <0.001 | 641 |
| Middle Frontal Gyrus | 6 | -27.97 | -0.74 | 56.75 | 5.694 | <0.001 | 5025 |
| Fusiform Gyrus | 20 | -44.03 | -4.97 | -24.44 | 4.710 | <0.001 | 324 |
| Dorsolateral Prefrontal Cortex | 9 | -46.06 | 20.83 | 32.63 | 5.336 | <0.001 | 569 |
| Middle Temporal Gyrus | 22 | -54.22 | -47.41 | 1.45 | 5.166 | <0.001 | 1710 |
| d) At-Risk vs. MDD | |||||||
| At- Risk > MDD | |||||||
| Middle Frontal Gyrus | 46 | 45 | 18.86 | 18.56 | 5.393 | <0.001 | 555 |
| Inferior Frontal Gyrus | 47 | 37.93 | 31.63 | -0.06 | 6.854 | <0.001 | 5826 |
| Dorsolateral Prefrontal Cortex | 8 | 28.78 | 18.53 | 34.15 | 6.718 | <0.001 | 889 |
| Inferior Frontal Gyrus | 47 | -27.97 | 24.94 | -5.92 | 8.273 | <0.001 | 8208 |
| Inferior Temporal Gyrus | 20 | -48.48 | -34.62 | -11.66 | 6.695 | <0.001 | 2349 |
| Inferior Frontal Gyrus | 9 | -43.33 | 5.5 | 31.04 | 5.926 | <0.001 | 880 |
| MDD > At-Risk | |||||||
| Precentral Gyrus | 4 | -58.64 | -4.45 | 22.75 | -6.031 | <0.001 | 500 |
| Insula | 13 | -45.77 | -34.9 | 22.43 | -6.123 | <0.001 | 1062 |
Table 1: Thought Suppression. (a) Control & At-Risk: Suppression of personal and neutral statements – Motor condition, MDD: Motor condition – Suppression of personal and neutral statements. (b) Control: Suppression of personal and neutral statements – Motor condition, MDD: Motor condition – Suppression of personal and neutral statements. (c) Control: Suppression of personal and neutral statements – Motor condition, At-Risk: Motor Condition – Suppression of personal and neutral statements. (d) At-Risk: Suppression of personal and neutral statements- Motor Condition, MDD: Motor Condition – Suppression of personal and neutral statements. Reprinted with permission30.
| Re-emergence vs Re-suppresion | |||||||
| Region | BA | x | y | z | t- value | p-value | No. Voxels |
| a) Control & At-Risk vs. MDD | |||||||
| Control & At-Risk > MDD | |||||||
| Superior Temporal Gyrus | 13 | 47.55 | -48.85 | 15.16 | 6.935 | <0.001 | 18665 |
| Inferior Temporal Gyrus | 20 | 50.5 | -11.15 | -34.26 | 6.002 | <0.001 | 804 |
| Fusiform Gyrus | 37 | 38.04 | -46.75 | -17.86 | 5.106 | <0.001 | 531 |
| Anterior Cingulate | 32 | 3.15 | 14.93 | 41.26 | 6.755 | <0.001 | 20349 |
| Cingulate Gyrus | 23 | 7.05 | -18.18 | 25.01 | 6.334 | <0.001 | 568 |
| Insula | 13 | -39.51 | 16.12 | 11.4 | 7.167 | <0.001 | 24746 |
| Supramarginal Gyrus | 40 | -46.36 | -43.59 | 31.1 | 7.248 | <0.001 | 14751 |
| b) Control vs. MDD | |||||||
| Control > MDD | |||||||
| Superior Temporal Gyrus | 22 | 50.2 | -48.65 | 10.5 | 6.480 | <0.001 | 8042 |
| Inferior Frontal Gyrus | 9 | 45.18 | 8.16 | 23.21 | 6.390 | <0.001 | 5739 |
| Insula | 36.95 | -0.51 | -2.36 | 6.222 | <0.001 | 2542 | |
| Dorsomedial Frontal Gyrus/ Anterior Cingulate | 32 | 4.8 | 22.94 | 39.24 | 6.758 | <0.001 | 10780 |
| Cingulate Gyrus | 23 | 7.67 | -14.61 | 26.13 | 7.135 | <0.001 | 406 |
| Anterior Cingulate | 24 | -6.41 | 22.89 | 25.5 | 5.876 | <0.001 | 670 |
| Cingulate Gyrus | 24 | -10 | 2.38 | 35.19 | 5.888 | <0.001 | 380 |
| Middle Frontal Gyrus | 10 | -32.54 | 34.35 | 21.99 | 5.870 | <0.001 | 2918 |
| Insula | 13 | -39.56 | 4.6 | 2.22 | 6.740 | <0.001 | 8142 |
| Inferior Parietal Lobule | 40 | -41.12 | -30.05 | 35.15 | 6.189 | <0.001 | 936 |
| Middle Temporal Gyrus | 21 | -48.87 | -32.53 | -5.01 | 5.960 | <0.001 | 591 |
| Superior Temporal Gyrus | 39 | -53.69 | -53.65 | 23.97 | 5.547 | <0.001 | 1144 |
| c) Control vs. At-Risk | |||||||
| Control > At-Risk | |||||||
| Insula | 13 | 38.49 | -10.73 | -2.5 | 6.743 | <0.001 | 7591 |
| Inferior Frontal Gyrus | 47 | 24.74 | 33.12 | -3.47 | 5.159 | <0.001 | 1135 |
| Hippocampus | 27.45 | -40.77 | 1.97 | 4.207 | <0.001 | 630 | |
| Dorsomedial Frontal Gyrus | 9 | 0.45 | 46.97 | 29.98 | 6.248 | <0.001 | 13057 |
| Cingulate Gyrus | 31 | 10.4 | -25.45 | 34.63 | 4.061 | <0.001 | 439 |
| Anterior Cingulate | 32 | -2.93 | 38.46 | -6.89 | 5.453 | <0.001 | 4329 |
| Cuneus | 18 | 3.55 | -94.91 | 24.44 | 4.708 | <0.001 | 339 |
| Cingulate Gyrus | 31 | -10.53 | -36.01 | 34.5 | 4.541 | <0.001 | 453 |
| Caudate | -17.99 | -31.31 | 16.85 | 4.488 | <0.001 | 489 | |
| Superior Temporal Gyrus | -47.13 | -24.52 | 3.47 | 5.639 | <0.001 | 8162 | |
| d) At-Risk vs. MDD | |||||||
| At-Risk > MDD | |||||||
| Superior Temporal Gyrus | 39 | 47.02 | -48.19 | 14.17 | 6.649 | <0.001 | 15860 |
| Inferior Frontal Gyrus | 9 | 43.17 | 9.48 | 21 | 6.122 | <0.001 | 16140 |
| Inferior Temporal Gyrus | 20 | 52.27 | -10.57 | -33.72 | 4.815 | <0.001 | 584 |
| Dorsomedial Frontal Gyrus | 32 | 4.87 | 7.97 | 46.92 | 5.688 | <0.001 | 8580 |
| Anterior Cingulate | 32 | -9.46 | 18.24 | 24.38 | 5.869 | <0.001 | 494 |
| Uncus | 36 | -24.63 | -3.52 | -27.89 | 5.165 | <0.001 | 827 |
| Insula | 13 | -40.1 | 15.42 | 16.26 | 7.314 | <0.001 | 21421 |
| Superior Parietal Lobule | 7 | -30.73 | -54.74 | 40 | 6.175 | <0.001 | 3551 |
| Inferior Parietal Lobule | 40 | -46.34 | -36.74 | 33.83 | 6.364 | <0.001 | 4717 |
| Middle Temporal Gyrus | 37 | -52.46 | -54.47 | 1.43 | 5.899 | <0.001 | 1484 |
Table 2: Though re-emergence vs. thought suppression. (a) Control & At-Risk: Re-emergence – Suppression of personal and neutral statements, MDD: Suppression – re-emergence of personal and neutral statements. (b) Control: Re-emergence – Suppression of personal and neutral statements, MDD: Suppression – re-emergence of personal and neutral statements. (c) Control: Re-emergence – Suppression of personal and neutral statements, At-Risk: Suppression – re-emergence of personal and neutral statements. (d) At-Risk: Re-emergence – Suppression of personal and neutral statements, MDD: Suppression – re-emergence of personal and neutral statements. Reprinted with permission30.
Discussion
Elements of the neural circuitry disrupted in depression15,16,25 are also associated with the regulation of conscious thought17,18. By examining suppression-related neural processing in at-risk and depressed participants we were able to examine whether there are alterations in brain activation patterns that are common in both individuals with a genetic predisposition to depression and a current depressive episode.
In keeping with our hypotheses and the existing literature examining thought suppression in healthy controls, engagement of the dorsolateral prefrontal cortices in response to demands to suppress thoughts over an extended period of time was identified18,20. Activation of this area was evident across all three subject groups. However, we also identified group differences in the activation of the DLPFC during sustained thought suppression (compared to motor control), not only distinguishing between MDD patients and healthy controls, and between at-risk and MDD patients, but also between the control and at-risk groups. Thus differential engagement of the DLPFC during sustained thought suppression followed a progression with the greatest activation seen in controls, followed by at-risk and then MDD. Group differences in the activation of superior parietal cortices in the contrast between control and MDD groups further suggests that there is diminished engagement of dorsal control systems in MDD during thought suppression. Controls also engaged an area of the ventromedial prefrontal/subgenual cingulate cortices more greatly than MDD patients. The ventromedial prefrontal cortices have been implicated in previous research on thought suppression. Moreover, previous fMRI work has associated activation of the ventromedial prefrontal cortices with self-reflection and emotional processing28 and this region has been shown to be up-regulated during self-reflection31. Thus heightened engagement of the ventromedial prefrontal cortex by controls during thought suppression may be related to the processing of emotionally relevant thoughts during this condition. However, it should be noted, our analyses included both personally relevant and neutral distractor statements, and therefore, these findings may reflect elements of cognitive control and internal monitoring.
To further explore the brain regions involved in active suppression of intrusive thought, the event-related changes in activation associated with the re-emergence of target thoughts and their subsequent re-suppression was examined. In keeping with the extant literature it was found that the anterior cingulate cortex was transiently activated when intruding thoughts were returned to their suppressed state17,18. Moreover, a continuum of ACC engagement with controls showing the greatest activation of this area was identified, followed by the at-risk group and then finally the depressed group. While a limited amount of previous research has been conducted examining transient cognitive processes in the context of thought suppression, our task likely draws on a similar neural network identified in a recent study involving memory suppression. Anderson et al.32, found memory suppression to be associated with significant activity in the dorsolateral and ventrolateral prefrontal cortices and the anterior cingulate cortices. Their findings suggested the anterior cingulate cortex might play an integral role in suppression, signaling the dorsolateral prefrontal cortex to engage during the intrusion of suppressed memories. That said, such conclusions should be considered with caution as previous research has implicated ACC activation with a number of roles including conflict monitoring, error detection and inhibition. Further research is required to distinguish the role of the ACC in active thought suppression.
Research on MDD has identified hypoactivation of the anterior cingulate cortex and dorsolateral prefrontal cortex during a motor response inhibition task in a study of medication naïve adolescents experiencing a first episode of depression. It was suggested that these findings signaled a dysregulation of these neural regions that occurred early in the course of depression33. Our results build on this observation with young adults with MDD showing hypoactivation of the DLPFC during sustained thought suppression and reduced ACC engagement during transient thought suppression. Moreover, the present findings extend the observation of dysregulation of the DLPFC and ACC to at-risk individuals. In this regard, research has found that compared to controls, young adults at familial risk for depression showed reduced activation of the anterior cingulate cortex during an emotional Stroop task34. Thus, it may be suggested that hypoactivation of the ACC and DLPFC during thought suppression may be both an early marker of neural dysregulation in MDD and confer vulnerability to depression in those at risk. This suggestion is supported by work from Koenigs and colleagues35 who found that naturally occurring lesions in the dorsolateral prefrontal cortex made individuals much more likely to develop depression.
The decreased activation of the dorsolateral prefrontal cortex and anterior cingulate cortex in at-risk and depressed participants indicates alteration in brain activity that may impair transient thought regulation in individuals with and at-risk for depression. Controls showed robust activation of the anterior cingulate cortex and dorsolateral prefrontal cortex during intrusions of target thoughts, providing a neural mechanism for monitoring lapses in thought regulation and quickly reinitiating suppression. Without this monitoring system individuals who are exposed to a negative stressor may be more prone to ruminate about an event, which may facilitate an onset or worsening of depressive symptoms in at-risk and depressed individuals.
This study explored changes in regional patterns of brain activation associated with thought suppression in healthy controls as well as individuals with and at-risk for depression. The findings provide important evidence of neural dysregulation present in patients with a major depression as well as in individuals with a familial risk for depression. While it is by no means certain that these at-risk individuals will go on to develop depression, it may be that these changes in thought regulation circuitry confer vulnerability to increased intrusive or ruminative thoughts, thereby increasing the risk of eventually developing depressive symptoms. Additionally, these changes may confer vulnerability for the worsening of depressive symptoms in already depressed individuals. Future research is needed to examine the trajectory of these neural changes over time, and their usefulness in predicting the eventual onset and progression of depression.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank Richie Davidson, Waisman Center, University of Wisconsin-Madison, for his guidance and support.
Materials
| Magnetic Resonance Imaging Scanner | General Electric | 3T, whole body, short bore scanner, Milwaukee, WI | |
| Brain Voyageur, QX, V2.1 | Brain Innovation (B.V.) | Maastricht, The Netherlands | |
| E-prime | Psychology Software Tools | Pennsylvania, USA | |
| Hamilton Depression Rating Scale (HAM-D) | Hamilton M (1967) Development of a rating scale for primary depressive illness. The British journal of social and clinical psychology 6: 278–296 | ||
| Rosenberg Self-Esteem Questionnaire (RSE) | Rosenberg M (1965). Society and the Adolescent Self-Image. Princeton University Press : Princeton, NJ. | ||
| Childhood Trauma Questionnaire (CTQ) | Bernstein DP, Stein JA, Newcomb M, et al. (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect 27: 169–190. | ||
| NEO-FFI Neuroticism questionnaire | Costa, P. T., & McCrae, R. R. (1992). Revised NEO Personality Inventory (NEO‐PI‐R) and NEO Five‐Factor Inventory (NEO‐FFI) professional manual. Odessa, FL: Psychological Assessment Resources. | ||
| Mini International Neuropsychiatric Inventory (MINI) | Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state.” Journal of Psychiatric Research, 12(3), 189–198. | ||
| Beck Depression Inventory-Version II (BDI-II) | Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression Archives of General Psychiatry 4:561 – 571 | ||
References
- Gotlib, I. H., Joormann, J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 6, 285-332 (2010).
- Nolen-Hoeksema, S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 100, 569-582 (1991).
- McBride, C., Bagby, R. M. Rumination and interpersonal dependency: Explaining women’s vulnerability to depression. Canadian Psychology. 47, 184-194 (2006).
- Thomsen, D. K. The association between rumination and negative affect: A review. Cognition and Emotion. 20 (8), 1216-1235 (2006).
- Moulds, M. L., Kandris, E., Williams, A. D. The impact of rumination on memory for self-referent material. Memory. 15, 814-821 (2007).
- Nolen-Hoeksema, S., Parker, L. E., Larson, J. Ruminative coping with depressed mood following loss. J Pers Soc Psychol. 67, 92-104 (1994).
- Just, N., Alloy, L. B. The response styles theory of depression: Tests and an extension of the theory. J Abnorm Psychol. 106, 221-229 (1997).
- Broderick, P. C., Korteland, C. A prospective study of rumination and depression in early adolescence. Clinical Child Psychology and Psychiatry. 9, 383-394 (2004).
- Kuhn, S., Vanderhasselt, M., De Raedt, R., Gallinat, J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders. 141 (2-3), 352-360 (2012).
- Kuyken, W., Watkins, E., Holden, E., Cook, W. Rumination in adolescents at risk for depression. J Affect Disord. 96, 39-47 (2006).
- Williams, A. D., Moulds, M. L. Cognitive avoidance of intrusive memories: Recall vantage perspective and associations with depression. Behav Res Ther. 45, 1141-1153 (2007).
- Wegner, D. M., Schneider, D. J., Carter, S. R. White TL. Paradoxical effects of thought suppression. J Pers Soc Psychol. 53, 5-13 (1987).
- Dalgleish, T., Yiend, J. The effects of suppressing a negative autobiographical memory on concurrent intrusions and subsequent autobiographical recall in dysphoria. J Abnorm Psychol. 115, 467-473 (2006).
- Price, J. L., Drevets, W. C. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences. 16, 61-71 (2012).
- Drevets, W. C., Price, J. L., Furey, M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 213, 93-118 (2008).
- Kupfer, D. J., Frank, E., Phillips, M. L. Major depressive disorder: new clinical, neurobiological and treatment perspectives. Lancet. 379, 1045-1055 (2012).
- Matsumoto, K., Tanaka, K. Conflict and cognitive control. Science. 303, 969-970 (2004).
- Mitchell, J. P., Heatherton, T. F., Kelley, W. M., Wyland, C. L., Wegner, D. M., Neil Macrae, C. Separating sustained from transient aspects of cognitive control during thought suppression. Psychol Sci. 18, 292-297 (2007).
- Goldin, P. R., McRae, K., Ramel, W., Gross, J. J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 63, 577-586 (2008).
- Wyland, C. L., Kelley, W. M., Macrae, C. N., Gordon, H. L., Heatherton, T. F. Neural correlates of thought suppression. Neuropsychologia. 41, 1863-1867 (2003).
- Fossati, P., et al. In search of the emotional self: An fMRI study using positive and negative emotional words. Am J Psychiatry. 160, 1938-1945 (2003).
- Disner, S. G., Beevers, C. G., Haigh, E. A. P., Beck, A. T. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 12, 467-477 (2011).
- Nolen-Hoeksema, S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 109, 504-511 (2000).
- Sullivan, P. F., Neale, M. C., Kendler, K. S. Genetic epidemiology of major depression: Review and meta-analysis. Am J Psychiatry. 157, 1552-1562 (2000).
- Drevets, W. C. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 126, 413-431 (2000).
- Ray, R. D., Ochsner, K. N., Cooper, J. C., Robertson, E. R., Gabrieli, J. D. E., Gross, J. J. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 5, 156-168 (2005).
- . . Diagnostic and Statistical Manual of Mental Disorders. , (2000).
- Phan, K. Neural correlates of individual ratings of emotional salience: a trial related fMRI study. NeuroImage. 21, 768-780 (2004).
- Carew, C., Milne, A. M., Tatham, E. L., MacQueen, G. M., Hall, G. B. C. Neural Systems underlying thought suppression in young women with, and at-risk, for depression. Behavioural Brain Research. 257, 13-24 (2013).
- Jenkins, A. C., Macrae, C. N., Mitchell, J. P. Repetition suppression of ventromedial prefrontal activity during judgment of self and others. PNAS. 105, 4507-4512 (2008).
- Anderson, M. C., et al. Neural systems underlying the suppression of unwanted memories. Science. 303, 232-235 (2004).
- Halari, R., et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naive adolescents with depression compared to controls. J Child Psychol Psychiatry. 50, 307-316 (2009).
- Mannie, Z. N., Norbury, R., Murphy, S. E., Inkster, B., Harmer, C. J., Cowen, P. J. Affective modulation of anterior cingulate cortex in young people at increased familial risk of depression. Br J Psychiatry. 192, 356-361 (2008).
- Koenigs, M., Huey, E. D., Calamia, M., Raymont, V., Tranel, D., Grafman, J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 28, 12341-12348 (2008).

