Organotypic Slice Cultures for Studies of Postnatal Neurogenesis
Summary
Here we describe a technique for studying hippocampal postnatal neurogenesis using the organotypic slice culture technique. This method allows for in vitro manipulation of adult neurogenesis and allows for the direct application of pharmacological agents to the cultured hippocampus.
Abstract
Here we describe a technique for studying hippocampal postnatal neurogenesis in the rodent brain using the organotypic slice culture technique. This method maintains the characteristic topographical morphology of the hippocampus while allowing direct application of pharmacological agents to the developing hippocampal dentate gyrus. Additionally, slice cultures can be maintained for up to 4 weeks and thus, allow one to study the maturation process of newborn granule neurons. Slice cultures allow for efficient pharmacological manipulation of hippocampal slices while excluding complex variables such as uncertainties related to the deep anatomic location of the hippocampus as well as the blood brain barrier. For these reasons, we sought to optimize organotypic slice cultures specifically for postnatal neurogenesis research.
Introduction
Adult neurogenesis in the mammalian hippocampus represents a remarkable example of the brain’s innate capacity for adaptability and plasticity. Dentate granule cells (DGCs) are generated from a renewable pool of neural progenitor cells in the hippocampal dentate gyrus, which is one of the two presently well-characterized neurogenic regions in the mammalian brain, and is thought to be particularly important for learning and memory. The hippocampus is part of the limbic system and has a deep location within the mammalian brain, which makes it a difficult target for precise pharmacological manipulation. Additionally, aberrant neurogenesis has been implicated in conditions, such as epilepsy, schizophrenia, and Alzheimer’s disease, which has prompted interest in understanding the influence of various pharmacological agents during the maturation and survival of newborn neurons. The distinction between postnatal and adult neurogenesis is blurred and previous studies have shown that many features of in vivo neuronal development in the early postnatal period and adulthood are similar25. Here we emphasize postnatal neurogenesis and suggest possible applications to adult neurogenesis.
Organotypic slice cultures provide an efficient in vitro method for studying various physiological properties of the mammalian hippocampus. The value of slice cultures prepared from rodent brains can be summarized in three main qualities: 1) the protocol is straightforward and requires readily available materials; 2) slice cultures allow for pharmacological studies that eliminate complex variables such as the deep anatomic location of the hippocampus and circumvents the blood brain barrier1; and 3) the well characterized structure of the hippocampus and tri-synaptic circuit is preserved2. Previous investigators have used the organotypic hippocampal culture to study synaptic development and physiology3,4, gliogenesis5-7, ischemic brain damage8,9, neuroprotection and neurorepair10-12 as well as epilepsy13-15.The slice cultures could also provide a useful model system allowing for the monitoring of cell development in conjunction with labeling of cells with green fluorescent protein (GFP) or other vital markers.
Slice cultures have also been previously employed to study postnatal hippocampal neurogenesis16-19, but one important factor in the majority of these studies is the well-characterized degeneration that results from explanting tissue from adult animals after approximately 2 weeks in vitro20,21. For this reason, slice cultures are typically prepared from early post-natal (P5-P10) mice or rat pups, which utilizes the improved viability of early postnatal brain tissue for culturing22. While previous studies have shown that the early postnatal and adult hippocampus differ with regards to synaptic physiology and the expression of specific neuronal subtypes23,24, there is substantial conservation of the choreographed developmental program that newborn dentate granule cells proceed through during maturation25. Additionally, recent studies have suggested that the physiological characteristics of newborn DGCs in culture are very similar to immature neurons in the acute hippocampal slice preparation16.
Protocol
NOTE: All animal procedures conformed to the animal health and welfare guidelines of the Department of Comparative Medicine at the University of Toronto.
1. Preparation of Hippocampal Slices
- Sterilize the following instruments using the dry autoclave at 125 °C: Scalpel handle (#3) (2), Standard pattern forceps, large (1), Small dissector scissor (angled to side) (1), Micro spoon (spoon and flat spatula ends) (1), Micro-spatulas (rounded and rounded tapered ends) (2), Fine paintbrush (1), Fire polished Pasteur pipette (2), Gauze squares, 2 x 2 inches (5).
- When sterile, put the instruments into a sterile container and keep covered until use. Immediately before dissection, immerse instruments in a 70% ethanol solution.
- Prepare a 6-well culture plate with culture insert before beginning the dissection procedure by adding 1 ml of culture medium/well and storing the plate in the incubator at 35 °C and 5% CO2.
2. Arrange Dissection Tools in Sterile Laminar Flow hood
- Spray the laminar flow hood with 70% ethanol and remove sterilized dissection instruments from alcohol. Allow the instruments to dry while resting on a sterile Petri dish to avoid contact between the alcohol and the dissected brain tissue.
- Deposit 5-7 ml of sterilized ice-cold dissecting solution in 2 large sterile Petri dishes. One dish will chill and clean the head (dirty), the other one for cooling and rinsing the scooped out brain (clean). Place a sterilized filter paper in one of the Petri dish lids for dissecting out the brain.
- Place a small, sterilized filter paper in one of the small Petri dish lid for dissecting out the hippocampus. Deposit 3-5 ml of sterilized ice-cold dissecting solution in 2 small, sterile Petri dishes. One dish will hold the scooped out hippocampus, one will hold sections during separation of hippocampal sections under dissection microscope.
- Prepare the tissue chopper by taping a piece of sterilized filter paper to the cutting stage and mounting a sterile razor blade. Wet the filter paper with sterilized dissecting solution.
- Spray a clean bio-bag with alcohol and place it in laminar flow clean bench.
3. Hippocampal Dissection
- Spray the P7 Sprague Dawley rat pup with 70% ethanol outside of the laminar flow clean bench and quickly decapitate the animal using large sterile surgical scissors inside the laminar flow bench. Let the head drop into ice-cold dissecting solution in one of the Petri dishes.
- In the Petri dish, rinse off the blood and quickly transfer the head to sterilized filter paper, ventral side down.
- Using the scalpel, cut along the dorsal surface in the sagittal plane to expose the underlying skull. Cut through the skin, but not the underlying bone, which is soft and easily penetrable in rats of this age. Set aside this “dirty” scalpel and do not use on brain tissue.
- Using the small dissector scissors (angled to side) and forceps, cut open the skull along sagittal suture of the skull to bregma, the anatomical point on the skull where the coronal suture is intersected perpendicularly by the sagittal suture. Use forceps to pull skull flaps up and away from the midline of the skull.
- Place the micro spoon on the underside of the brain, beneath the brain stem, to gently lift the brain out of the skull. Lift the brain to expose the optic nerves and olfactory bulb on basal surface of brain. Cut these structures with small scissors to fully detach the brain from skull. Remove and transfer the intact brain to the other large Petri dish containing ice-cold dissecting solution.
- Using the micro spoon, transfer the brain to a small Petri-dish lid containing sterile filter paper. With a sterile Pasteur pipette, rinse the brain with a few drops of dissecting solution to keep tissue moist.
- Using a “clean” scalpel blade cut the two hemispheres apart. Transfer the left hemisphere back to large Petri dish with micro spoon and place hemisphere pia side down in ice-cold dissecting solution for subsequent use.
- View the medial face of the right hemisphere and identify the edge fornix, a prominent band of white matter along the medial edge of the hippocampus. Using a sterile scalpel, make a sagittal cut through the fornix, but take care because only 0.5 cm of the scalpel tip will be sufficient to cut the fornix.
- Using 2 micro-spatulas, remove the first hippocampus from right hemisphere by placing the right-hand-spatula on the brain stem and lifting the overlying cortex with the left-hand spatula. Gently lift the cortex to reveal the lateral ventricle and medial surface of the hippocampus. A white curved line, the fimbria, should now be visible.
- Align the curvature of spatula with the curvature of the fimbria and gently press the spatula under the fimbria. Slide the spatula left and ride along rostral-caudal axis and then lift spatula in dorsal direction to remove hippocampus.
- Transfer the hippocampus to a 2nd small Petri dish with ice-cold dissecting solution. Repeat the same procedure on the left hemisphere to remove the left hippocampus.
- Using a micro spatula, carefully transfer the hippocampi to the tissue chopper stage. Arrange them adjacent and parallel to each other and perpendicular to the axis of the chopper blade. Use a paintbrush to position the tissue and add a few drops of the dissecting solution on top of the hippocampi.
- Cut the tissue in 400 μm slices without pausing to remove individual slices (usually they will not adhere to the blade). After the whole hippocampus has been cut, use the paintbrush to gently transfer the sections to a 2nd small Petri dish with dissection solution.
- Under a dissecting microscope, carefully separate the floating slices using amicro-spatula and paintbrush.
- Remove the pre-prepared culture plate with culture insert from the incubator and place in a laminar flow hood.
- Using a fire-polished Pasteur pipette, draw 4-5 slices into the pipette and transfer slices to the apical surface of culture insert membrane. Next, adjust the positioning with a paintbrush and leave space between individual sections and the border of the culture insert.
- Using a sterile Pasteur pipette, remove the excess dissecting solution from apical surface of membrane.
NOTE: While removing solution avoid drawing tissue sections into the pipette. Alternatively, use a regular pipette (P200 or P1000) with sterilized pipette tips to slowly remove solution. - Place the culture plate with serum-containing culture medium and the hippocampal slices back into the incubator at 35 °C and 5% CO2.
NOTE: If experiment calls for generating cultures from multiple animals, thoroughly clean the laminar flow clean bench between dissections. Return instruments to alcohol solution and replace all Petri-dishes, filter papers and sterile razor blades prior to second dissection.
4. Feeding and Maintaining Organotypic Slices
- Feed cultures in a sterile laminar flow clean bench.
- Perform the first feeding of the cultured sections 2 days post-dissection. Aspirate old culture medium using sterilized glass pipette.
- Use sterile 5 ml serological pipette to add 1 ml of fresh, sterile, serum-containing medium to the wells.
- Gently replace the culture insert and take care to remove any air bubbles that may have formed underneath membrane surface.
- After the first feeding, change medium every other day.
5. Incubating Tissue Slices with Thymidine Analogues to Label Newborn Neurons
- In order to study the maturation and integration of dentate granule cells in the hippocampus, incubate the organotypic slices with a thymidine analogue, such as Bromodeoxyuridine (BrdU) or Chlorodeoxyuridine (CldU). Here, we use CldU because of its higher solubility in saline.
- After 3DIV, add 1 μl of 10 mg/ml CldU stock solution in saline to 1 ml of culture medium for a final concentration of 10 μg/ml. If BrdU is used, correct the concentration for differences in molecular weight. Add medium with CldU to culture wells and incubate tissue with CldU-containing medium for 2 hr at 35 °C.
- Following 2 hr of incubation at 35 °C, remove the CldU-containing medium and replace with regular feeding medium to resume normal feeding schedule (outlined above).
6. Tissue Fixation and Storage
- Establish a timeline for applying treatments and fixing tissue samples before starting culture experiments (Figure 2B).
- At a predetermined day post-dissection, prepare the following in a laboratory fume hood: a 10-50 ml beaker containing 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS); an empty beaker for discarded culture medium; small forceps; and a 1,000 ml pipette with disposable tips.
- Remove the culture plate(s) from incubation chamber and transfer to a fume hood. Tilt the individual well plate inserts with forceps. Next, use a pipette to remove culture medium and transfer to a disposal beaker. Tilt the culture plate at an angle to help ensure that all culture medium is removed.
- Finish removing medium for one culture plate at a time. When medium has been removed, add 1 ml of 4% PFA to each culture well and seal well plate with parafilm. Repeat for as many culture plates as necessary and transfer plates to refrigerator at 4 °C for 24 hr.
- After 24 hr, prepare the following in a fume hood: 10-50 ml beaker containing 0.1% Sodium Azide in PBS; an empty beaker for discarded PFA (follow safety precautions when discarding toxic substances); small forceps; and 1,000 ml pipette with disposable tips.
- Follow the procedure outlined in 6.4 for adding PFA, but add 1 ml of PBS with sodium azide instead. Once completely transferred, seal well plates for future use by wrapping edges in parafilm and storing in refrigerator at 4 °C.
7. Sectioning Tissue for Immunohistochemistry
- Perform tissue sectioning using a vibratome. The following series of steps help maximize the yield of usable tissue sections from organotypic cultures.
NOTE: Immediately following hippocampal dissection and plating of slices, the tissue has a thickness of approximately 400 μm. However, after 2-3 weeks in the incubation chamber, tissue slices will begin to flatten, resulting in a final section thickness of 150-300 μm. - Prepare the following items to section cultured tissue: a #11 scalpel blade and handle, a glass Petri dish containing ice cold PBS, a micro-dissection forceps, and a clean vibratome cutting stage to mount tissue.
- Use a scalpel to carefully cut along the perimeter of the circular insert membrane so that it can be detached from the plastic insert housing. Leave ample space between the cultured slice and the scalpel.
- Transfer the detached insert membrane to a Petri dish containing PBS. After rinsing in PBS, use forceps to transfer the membrane to the vibratome mounting stage.
- Next, use the scalpel to eliminate excess membrane surrounding cultured slices and cut away excess material to create clean edges. This step will ensure the membrane is flat and can easily adhere to the cutting surface.
- Place 1-2 drops of adhesive on the vibratome cutting stage and spread in even layer using a 22 G needle. Spread adhesive into a rectangular shape with the long edge parallel to the cutting blade of the vibratome. Perform this step quickly, to prevent the adhesive from drying.
- Use forceps to transfer the trimmed membrane containing hippocampal slices to the cutting stage and gently position the membrane on glue and ensure that there are no air bubbles.
- As the superglue dries, transfer the vibratome stage with the glued membrane back to the PBS containing Petri dish. Prepare the vibratome blade and a 48 well plate containing sodium azide to store tissue sections.
- Use the vibratome to generate 30 μm sections of the organotypic slice tissue and transfer to a 48 well plate containing sodium azide for storage and subsequent immunohistochemical staining.
- Refer to pre-existing protocols for immunohistochemical staining26,29.
Representative Results
Determining if organotypic cultures would be suitable for adult neurogenesis research required that they satisfy two main criteria: 1) that slices maintain characteristic morphological features of hippocampal slices after 10-21 days in vitro (DIV), and 2) that newborn DGCs can be quantified using standard immunohistochemical techniques commonly employed in adult neurogenesis research. Regarding the first criterion, Figure 1A and 1B highlight the preserved hippocampal morphology. Characteristic features such as the dentate gyrus (DG), CA1, and CA3 regions are easily identifiable.
Regarding the second criterion, Figure 1C (upper panel) provides a representative sample of newborn DGCs co-expressing the endogenous neuronal marker, Doublecortin (DCX) in green and the exogenous thymidine analogue, 5-Chloro-2′-deoxyuridine (CldU) in red. These neurons are located in the sub-granular zone of the hippocampal DG. In order to ensure correct birth dating of neurons, we identify CldU+ nuclei that co-express DCX. Confocal microscopy is needed at this stage to successfully identify double-labeled neurons because candidate cells must co-express the marker of interest throughout the Z-axis of the cell. Sample data obtained with such double-labeling yield approximately 17% of CldU+ cells that expressed DCX and 35% of CldU+ cells that expressed CaBP at 12 days after CldU application. The DCX value is very similar whereas the CaBP value is considerably lower than comparable figure obtained in vivo26. Standard tissue culture conditions may be responsible for a relatively low percentage of the CaBP+ cells.
Figure 2A presents the dissection steps that proceed from left to right (1-8): starting with decapitation of animal (1), removal of brain (2), transfer of brain to ice-cold dissection solution (3), dissection of hippocampus from left and right hemispheres (4), storage of dissected hippocampi in ice-cold dissection solution (5), transfer of both hippocampi to Stoelting tissue chopper and sectioning at 400 μm (6), separation of individual slices under dissecting microscope (7), and plating tissue on cell culture inserts (8). Proceeding in this manner helps maintain a sterile environment throughout the culture process.
Lastly, since the time-course of development is an important feature of hippocampal neurogenesis, we chose to incubate the cultured slices with CldU for exactly 2 hr after 3 DIV to label dividing neural stem cells. The narrow time window for CldU administration was chosen to improve the likelihood that labeled neurons constituted a homogeneous population of cells at approximately the same maturational stage (Figure 2). With regards to CldU labeling, one critical feature of neurogenesis for hippocampal function is that at a given time there is a heterogeneous population of dentate granule cells at various maturational stages27,28.
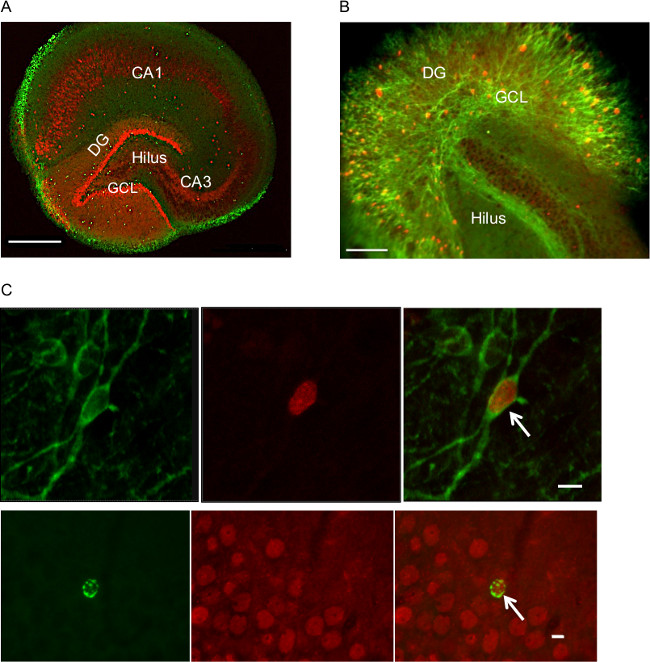
Figure 1. Sample fluorescence microscope photographs highlighting preserved hippocampal morphology. (A) Organotypic slice from 12 days post dissection immunolabeled for CldU (green) and CaBP (red), 20x air composite image (Scale bar= 500 µm). (B) Sample micrograph of slice from 21 days post dissection immunolabeled for CldU (red) and DCX (green) (opposite color-scheme of Figure 1A), 20x air (Scale bar= 100 µm). (C) Upper panel. Representative confocal microscope photograph of cells co-expressing ClXdU and endogenous immature neuronal marker, DCX. DGCs co-expressing DCX (green) and CldU (red) are counted as newborn neurons. Arrow indicates a double-labeled cell at early stage of development, 40X oil-immersion (Scale bar = 10 µm). Comparable cells have been observed 10 days post-labeling in vivo26. Lower panel. Representative fluorescent images of cells co-expressing CldU (green) and CaBP (red). Arrow indicates a double-labeled cell, 40X fluorescent micrograph (Scale bar = 10 μm). DG-dentate gyrus. GCL-granule cell layer. Please click here to view a larger version of this figure.
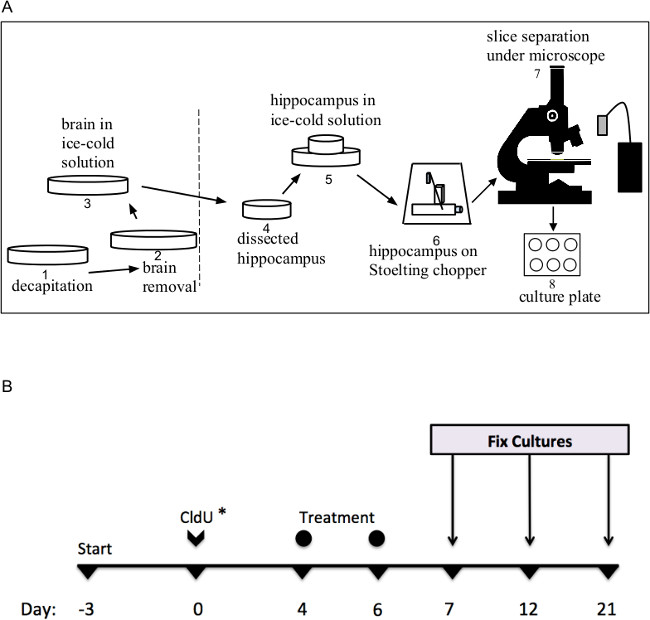
Figure 2. (A) Illustration of the sequential steps for hippocampal dissection in laminar flow clean bench. Dotted line indicates “unsterile” (left) and “sterile” (right) zones of the dissection area. (B) Timeline for organotypic slice cultures prepared from P7 rat pups (start). Notations indicate application of thymidine analogue, CldU*, and “treatment,” which can include various pharmacological agents suited to the experimental question. Cultures are fixed with paraformaldehyde at desired dwell times from CldU application (Fix Cultures). Please click here to view a larger version of this figure.
| Name of Reagent/ Equipment | Company | Catalog Number | Comments/Description |
| 5-chloro-2'-deoxyuridine (CldU) | MP Biomedicals | 105478 | Hazardous, Carcinogenic |
| Cell culture inserts, 30 mm diameter, 0.4 µm pore size | Thermo scientific | 140660 | Nuclon delta coating on these inserts provides better tissue adhesion and improves slice quality. |
| Conical Centrifuge tubes (sterile) | Fisher Scientific | 14-432-22 | |
| Dissector scissors (angled to side) | Fine Science Tools | 14082-09 | |
| Minimum essential medium (MEM) | Gibco | 11095; liquid | Store at 4 °C |
| Eclipse Ni-U fluorescent microscope | Nikon | ||
| Glue for tissue | Krazy Glue | KG585 | Use minimum amount of glue to achieve adhesion as any tissue exposed to glue will be unusable for IHC. |
| Hank’s Balanced Salt Solution (HBSS) (500 ml) | Gibco | 14025-092 | Store at 4 °C |
| Horse Serum Heat Inactivated (500 ml) | Gibco | 16050-122 | Make 50 ml aliquots and store at -20 °C |
| Kimwipes | Kimberly-Clarke | TW 31KYPBX | |
| Modified glass pipettes (bottom of Pasteur pipette removed and edge smoothed with Bunsen flame) | |||
| Petri Dish (100 mm x 15 mm) and (60 mm x 15 mm) | Fisher Brand | FB0875712 and FB0875713A | |
| Scalpel blades #11 | Fine Science Tools | 10011-00 | |
| Scalpel handle #3 | Fine Science Tools | 10003-12 | |
| Serological Pipettes | Sorfa Medical Plastic Co. | P8050 | |
| Standard Pattern forceps | Fine Science Tools | 11000-12 | |
| Sterile vacuum filter | Thermo-Scientific | 565-0020 | |
| Surgical Scissors | Fine Science Tools | 14054-13 | |
| Syringe driven filter unit | Millipore-Millex | SLGP033RS | |
| Tissue chopper with moveable stage | Stoelting | 51425 | |
| Fine tip paintbrush |
Table 1. Supplies and Reagents
| Solution | Ingredients and Instructions |
| Dissection solution | a) 500 ml of Hank's Balanced Salt Solution (HBSS) (Gibco-14025-092). |
| b) Add 2.2 g D-glucose. | |
| c) Add 0.5 g Sucrose. | |
| d) Add 1.787 g HEPES. | |
| e) Mix for 30 min with magnetic stir plate. | |
| f) Use pH meter to ensure solution has a final pH= 7.4. | |
| g) Use osmometer to ensure final osmolality= 320-330 mOsm. | |
| h) Sterilize solution in sterile laminar flow hood using vacuum filtration through 0.2 μm filter. | |
| Serum-containing culture medium: 100 ml Minimum Essential Medium (MEM) (Gibco 11095), 50 ml Horse serum (Gibco 16050-122), 50 ml HBSS. | a) Add the following to 50 ml HBSS in beaker and dissolve in 37°C water bath. Mix with magnetic stirrer. |
| b) 1.3 g D-glucose. | |
| c) 36 mg MgSO4. | |
| d) 17.6 mg Ascorbic acid. | |
| e) 5 μl of 2M CaCl2 stock solution. | |
| f) Add 50 μl Antibiotic-Antimycotic (100x stock, sterile; Gibco 15140-062). | |
| g) 1 μg/ml Insulin. | |
| h) Sterilize above solution by filtration through a 0.2 μm filter. | |
| i) Mix filtered solution with 100 ml MEM and 50 ml Horse serum in laminar flow hood. | |
| j) Make 50 ml aliquots in sterile conical centrifuge tubes (Fisher Scientific-14-432-22) and store at 4°C. | |
| 4% Paraformaldehyde fixative solution. | a) Prepare phosphate buffered saline (PBS) by adding the following to 300 ml of distilled H2O and mixing on magnetic stir plate. |
| b) Add 2.7 g sodium phosphate monobasic (NaH2PO4). | |
| c) Add 11.5 g sodium phosphate dibasic (NaHPO4). | |
| d) Add 9.0 g sodium chloride (NaCl). | |
| e) Heat approx. 700 ml of distilled H2O to 55 °C and turn off heat. | |
| f) Add 40 g paraformaldehyde (PFA) and stir into 700 ml of water using magnetic stir plate. | |
| g) Combine the PBS (a,b,c,d) and PFA (e,f) solutions, adjust the pH to 7.4 and top up to final volume of 1,000 ml. | |
| 0.1% Sodium Azide Solution | a) Add 1g of powdered sodium azide (NaN3) to 1 L of PBS solution. |
| b) Mix using magnetic stir plate and store at 4°C. |
Table 2. Solutions and Recipes
Discussion
Following CldU (or BrdU) administration, the timeline of application of pharmacological agents can be chosen to target newborn DGCs during particular developmental windows. For example, a hypothetical agent can be applied during the second week post-CldU injection, which is proposed to coincide with the age of immature neurons that are at a developmental stage where GABA is depolarizing. Future studies using this protocol could adapt the pharmacological agent and the window of exposure to “tailor” the approach to the specific experimental question of interest.
An important criterion for determining that slice cultures are a valid model for postnatal neurogenesis research is the ability to stain and quantify newborn neurons in the hippocampus. The two main findings in support of this hypothesis were that microscopic analysis revealed immunohistochemical reactivity for CldU and endogenous protein markers in the same neurons. When used in combination with endogenous neuronal markers, thymidine analogues such as BrdU and CldU are powerful tools for neurogenesis research.
The application of thymidine analogues, such as BrdU, via intraperitoneal injections is commonly utilized in neurogenesis research to label neurons undergoing S-phase of mitosis29. Similar approaches can be employed in organotypic cultures with certain modifications. For example, previous studies administered BrdU (0.5 μM for 3 days) to slice cultures after ~14 DIV18. Reviewing the data presented in that paper reveals that some of the metrics used for quantifying neurogenesis do not employ the standard techniques used in the neurogenesis field, i.e., stereological quantification30. For example, when reporting the co-expression of BrdU and Neuronal-nuclei (NeuN) positive cells, they indicate a total number of cells “per culture” instead of providing information regarding tissue area or volume.
Subsequent studies improved on this method by sectioning the cultured slices to individual 10 μm sections, which improved visualization and immunohistochemistry protocols by allowing antibodies to more readily permeate the tissue samples31. Bunk et al.28 reported double labeling with BrdU (10 μM for 3 days) as the number of co-labeled cells per 10 μm section, but did not provide information about the comparative area or specific hippocampal region studied i.e., CA1, CA3 or DG. Additionally, analysis of the confocal and fluorescence microscopy images does not convincingly show that hippocampal morphology was successfully maintained.
Importantly, both studies used a BrdU exposure period of 3 days, which has associated drawbacks. BrdU labeling has greatly aided neurogenesis studies by allowing investigators to track newly divided cells in various brain regions. However, BrdU toxicity has also been well characterized. Its use has been shown to cause morphological and behavioral abnormalities32,33 and negative effects on cell cycle, differentiation, migration and survival of neural stem cells34-36. The prolonged administration of BrdU in the previously mentioned studies may have introduced confounding variables that altered hippocampal physiology and while some side effects from BrdU administration may be unavoidable, our experimental protocol was designed to limit some of these complications by incubating the tissue with thymidine analogues for 2 hr. Additionally, we chose to use CldU instead of BrdU because it showed better solubility than BrdU when preparing the incubating solution. Although the 3 day protocol may be useful for certain experimental designs e.g., maximizing the labeling of proliferating cells, this 2 hr protocol has an advantage of pulse-labeling of a relatively small population of cells which can be studied at desired survival times (see Figure 2B).
By comparing the level of neuronal production following two different methods of BrdU application, Namba et al. made an important contribution to labeling techniques in organotypic slice cultures37. The authors compared intraperitoneal (I.P.) injection of BrdU (50 mg/kg) in postnatal day 5 (P5) rats with in vitro cultures that received culture medium containing 1 μM BrdU for 30 min immediately following explantation of tissue. They report no statistically significant difference between in-vivo and early in vitro BrdU injection in cultured tissue. The authors did not present clear images outlining the hippocampal structure but they report BrdU immunoreactivity as percentages of total cells in the granule cell layer. While they employ stereological counting, providing a measure by area or volume would be valuable. In general, the cited studies present organotypic cultures as a thorough detailing of postnatal hippocampal slice cultures, with applications for the study of neurogenesis and pharmacological perturbations. Using this technique, hippocampal slices can be maintained for up to 21 days in vitro (DIV) and drugs can be added to the medium at any point in the culture period to study the effect on neurogenesis.
Our aim was to label a discrete, relatively homogenous population of DGCs by providing a brief ‘pulse’ application of CldU for 2 hr. One commonly employed strategy for studying neurogenesis involves identification of a neuron’s maturational stage via immunohistochemical staining for various endogenous markers with a thymidine analogue. Confocal and fluorescence microscopy confirmed the presence of nuclei that incorporated the thymidine analogue CldU and were therefore actively undergoing mitosis during the culture period. Figure 2 provides evidence that immunohistochemical protocols commonly used for in-vivo tissue analysis can be adapted for slice cultures.
Specifically, a commonly used method in neurogenesis research is to perform immunohistochemical staining for the microtubule associated protein, DCX, which is predominantly expressed in immature neurons from day 3-21 and the mature neuronal marker, Calbindin (CaBP), which is fully expressed following 28 days post-mitosis. The phenotype of CldU+ cells was determined using these endogenous markers26.
Improved methods for maintaining slice cultures for longer periods may have the additional benefit of allowing more CldU+ neurons to reach the mature, CaBP+ stage. At present, one limitation of the organotypic culture approach is that the tissue is continuously changing during the culture period. For example, immediately following hippocampal dissection and plating of slices, the tissue has a width of approximately 400 μm. However, after 2-3 weeks in the incubation chamber, tissue slices will begin to thin, which results in a final width between 250-350 μm. This limits the amount of tissue that can be used for immunohistochemistry and should be considered when planning how many animals to use for a project. Additional experiments will help characterize the functional changes in hippocampal physiology that occur in vitro.
The protocol for sectioning and staining hippocampal slices was developed to analyze cellular and morphological changes taking place during the culturing period. Slice cultures provide an opportunity to test the effect of various pharmacological agents as hippocampal DGCs pass through distinct developmental stages during maturation and represent a valuable tool for future adult neurogenesis studies.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by a research grant MOP 119271 to JMW by the Canadian Institute of Health Research. The authors would like to thank Ms. Yao Fang Tan for her outstanding technical assistance.
Materials
| Name of Reagent/ Equipment | Company | Catalog Number | Comments/Description |
| 5-chloro-2'-deoxyuridine (CldU) | MP Biomedicals | 105478 | Hazardous, Carcinogenic |
| Cell culture inserts, 30mm diameter, 0.4µm pore size | Thermo scientific | 140660 | Nuclon delta coating on these inserts provides better tissue adhesion and improves slice quality. |
| Conical Centrifuge tubes (sterile) | Fisher Scientific | 14-432-22 | |
| Dissector scissors (angled to side) | Fine Science Tools | 14082-09 | |
| Minimum essential medium (MEM) | Gibco | 11095; liquid | Store at 4°C |
| Eclipse Ni-U fluorescent microscope | Nikon | ||
| Glue for tissue | Krazy Glue | KG585 | Use minimum amount of glue to achieve adhesion as any tissue exposed to glue will be unusable for IHC. |
| Hank’s Balanced Salt Solution (HBSS) (500 mL) | Gibco | 14025-092 | Store at 4°C |
| Horse Serum Heat Inactivated (500 mL) | Gibco | 16050-122 | Make 50 mL aliquots and store at -20°C |
| Kimwipes | Kimberly-Clarke | TW 31KYPBX | |
| Modified glass pipettes (bottom of Pasteur pipette removed and edge smoothed with Bunsen flame) | |||
| Petri Dish (100mm x 15mm) and (60mm x 15mm) | Fisher Brand | FB0875712 and FB0875713A | |
| Scalpel blades #11 | Fine Science Tools | 10011-00 | |
| Scalpel handle #3 | Fine Science Tools | 10003-12 | |
| Serological Pipettes | Sorfa Medical Plastic Co. | P8050 | |
| Standard Pattern forceps | Fine Science Tools | 11000-12 | |
| Sterile vacuum filter | Thermo-Scientific | 565-0020 | |
| Surgical Scissors | Fine Science Tools | 14054-13 | |
| Syringe driven filter unit | Millipore-Millex | SLGP033RS | |
| Tissue chopper with moveable stage | Stoelting | 51425 | |
| Fine tip paintbrush |
References
- Buchs, P. A., Stoppini, L., Muller, D. Structural modifications associated with synaptic development in area CA1 of rat hippocampal organotypic cultures. Brain research. Developmental Brain Research. 71 (1), 81-91 (1993).
- Stoppini, L., Buchs, P. A., Muller, D. A simple method for organotypic cultures of nervous tissue. Journal of Neuroscience Methods. 37 (2), 173-182 (1991).
- Opitz-Araya, X., Barria, A. Organotypic hippocampal slice cultures. Journal of Visualized Experiments. (48), (2011).
- Muller, D., Buchs, P. A., Stoppini, L. Time course of synaptic development in hippocampal organotypic cultures. Developmental Brain Research. 71 (1), 93-100 (1993).
- Rio, J. A., Heimrich, B., Soriano, E., Schwegler, H., Frotscher, M. Proliferation and differentiation of glial fibrillary acidic protein-immunoreactive glial cells in organotypic slice cultures of rat hippocampus. Neurosciences. 43 (2-3), 335-347 (1991).
- Ziemka-Nalecz, M., Stanaszek, L., Zalewska, T. Oxygen-glucose deprivation promotes gliogenesis and microglia activation in organotypic hippocampal slice culture: involvement of metalloproteinases. Acta Neurobiologiae Experimentalis. 73 (1), 130-142 (2013).
- Subramanian, L., et al. Transcription factor Lhx2 is necessary and sufficient to suppress astrogliogenesis and promote neurogenesis in the developing hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 108 (27), E265-E274 (2011).
- Strassburger, M., Braun, H., Reymann, K. G. Anti-inflammatory treatment with the p38 mitogen-activated protein kinase inhibitor SB239063 is neuroprotective, decreases the number of activated microglia and facilitates neurogenesis in oxygen-glucose-deprived hippocampal slice cultures. European Journal Of Pharmacology. 592 (1-3), 55-61 (2008).
- Sadgrove, M. P., Chad, J. E., Gray, W. P. Kainic acid induces rapid cell death followed by transiently reduced cell proliferation in the immature granule cell layer of rat organotypic hippocampal slice cultures. Brain Research. 1035 (2), 111-119 (2005).
- Wise-Faberowski, L., Robinson, P. N., Rich, S., Warner, D. S. Oxygen and glucose deprivation in an organotypic hippocampal slice model of the developing rat brain: the effects on N-methyl-D-aspartate subunit composition. Anesthesia and Analgesia. 109 (1), 205-210 (2009).
- Cho, S., Wood, A., Brain Bowlby, M. R. slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Current Neuropharmacology. 5 (1), 19-33 (2007).
- Noraberg, J., et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Current Drug Targets. CNS And Neurological Disorders. 4 (4), 435-452 (2005).
- Berdichevsky, Y., et al. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. The Journal Of Neuroscience : The Official Journal Of The Society For Neuroscience. 33 (21), 9056-9067 (2013).
- Koyama, R., et al. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nature Medicine. 18 (8), 1271 (2012).
- Staley, K. J., White, A., Dudek, F. E. Interictal spikes: harbingers or causes of epilepsy. Neuroscience Letters. 497 (3), 247-250 (2011).
- Lee, H., Lee, D., Park, C. H., Ho, W. K., Lee, S. H. GABA mediates the network activity-dependent facilitation of axonal outgrowth from the newborn granule cells in the early postnatal rat hippocampus. The European Journal Of Neuroscience. 36 (6), 2743-2752 (2012).
- Raineteau, O., et al. Conditional labeling of newborn granule cells to visualize their integration into established circuits in hippocampal slice cultures. Molecular and Cellular Neurosciences. 32 (4), 344-355 (2006).
- Raineteau, O., Rietschin, L., Gradwohl, G., Guillemot, F., Gahwiler, B. H. Neurogenesis in hippocampal slice cultures. Molecular And Cellular Neurosciences. 26 (2), 241-250 (2004).
- Kamada, M., et al. Intrinsic and spontaneous neurogenesis in the postnatal slice culture of rat hippocampus. The European Journal Of Neuroscience. 20 (10), 2499-2508 (2004).
- Kim, H., Kim, E., Park, M., Lee, E., Namkoong, K. Organotypic hippocampal slice culture from the adult mouse brain: a versatile tool for translational neuropsychopharmacology. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 41, 36-43 (2013).
- Legradi, A., Varszegi, S., Szigeti, C., Gulya, K. Adult rat hippocampal slices as in vitro models for neurodegeneration: Studies on cell viability and apoptotic processes. Brain Research Bulletin. 84 (1), 39-44 (2011).
- Sadgrove, M. P., Laskowski, A., Gray, W. P. Examination of granule layer cell count, cell density, and single-pulse BrdU incorporation in rat organotypic hippocampal slice cultures with respect to culture medium, septotemporal position, and time in vitro. The Journal of Comparative Neurology. 497 (3), 397-415 (2006).
- Mielke, J. G., et al. Cytoskeletal, synaptic, and nuclear protein changes associated with rat interface organotypic hippocampal slice culture development. Developmental Brain Research. 160 (2), 275-286 (2005).
- Fabian-Fine, R., Volknandt, W., Fine, A., Stewart, M. G. Age-dependent pre- and postsynaptic distribution of AMPA receptors at synapses in CA3 stratum radiatum of hippocampal slice cultures compared with intact brain. European Journal of Neuroscience. 12 (10), 3687-3700 (2000).
- Laplagne, D. A., et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biology. 4 (12), e409 (2006).
- McDonald, H. Y., Wojtowicz, J. M. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neuroscience Letters. 385 (1), 70-75 (2005).
- Stone, S. S., et al. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus. 21 (12), 1348-1362 (2011).
- Wang, S., Scott, B. W., Wojtowicz, J. M. Heterogenous properties of dentate granule neurons in the adult rat. Journal of Neurobiology. 42 (2), 248-257 (2000).
- Wojtowicz, J. M., Kee, N. BrdU assay for neurogenesis in rodents. Nature Protocols. 1 (3), 1399-1405 (2006).
- Fritsch, R. S. E. R., Weibel, E. R. Stereological Methods, Vol. 1: Practical Methods for Biological Morphometry. Zeitschrift für Allgemeine Mikrobiologie. 21 (8), 630-630 (1981).
- Bunk, E. C., Konig, H. G., Bonner, H. P., Kirby, B. P., Prehn, J. H. NMDA-induced injury of mouse organotypic hippocampal slice cultures triggers delayed neuroblast proliferation in the dentate gyrus: an in vitro model for the study of neural precursor cell proliferation. Brain Research. 1359, 22-32 (2010).
- Kolb, B., Pedersen, B., Ballermann, M., Gibb, R., Whishaw, I. Q. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. The Journal Of Neuroscience : The Official Journal Of The Society For Neuroscience. 19 (6), 2337-2346 (1999).
- Morris, S. M. The genetic toxicology of 5-bromodeoxyuridine in mammalian cells. Mutation Research. 258 (2), 161-188 (1991).
- Bannigan, J., Langman, J. The cellular effect of 5-bromodeoxyuridine on the mammalian embryo. Journal Of Embryology And Experimental Morphology. 50, 123-135 (1979).
- Breunig, J. J., Arellano, J. I., Macklis, J. D., Rakic, P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 1 (6), 612-627 (2007).
- Duque, A., Rakic, P. Different effects of bromodeoxyuridine and [3H]thymidine incorporation into DNA on cell proliferation, position, and fate. The Journal Of Neuroscience : The Official Journal Of The Society For Neuroscience. 31 (42), 15205-15217 (2011).
- Namba, T., Mochizuki, H., Onodera, M., Namiki, H., Seki, T. Postnatal neurogenesis in hippocampal slice cultures: early in vitro labeling of neural precursor cells leads to efficient neuronal production. Journal of Neuroscience Research. 85 (8), 1704-1712 (2007).

