Preparation and Reactivity of Gasless Nanostructured Energetic Materials
Summary
This protocol describes the preparation of gasless nanostructured energetic materials (Ni+Al, Ta+C, Ti+C) using the short-term high-energy ball milling (HEBM) technique. It also describes a high-speed thermal imaging method to study the reactivity of mechanically fabricated nanocomposites. These protocols can be extended to other reactive nanostructured energetic materials.
Abstract
High-Energy Ball Milling (HEBM) is a ball milling process where a powder mixture placed in the ball mill is subjected to high-energy collisions from the balls. Among other applications, it is a versatile technique that allows for effective preparation of gasless reactive nanostructured materials with high energy density per volume (Ni+Al, Ta+C, Ti+C). The structural transformations of reactive media, which take place during HEBM, define the reaction mechanism in the produced energetic composites. Varying the processing conditions permits fine tuning of the milling-induced microstructures of the fabricated composite particles. In turn, the reactivity, i.e., self-ignition temperature, ignition delay time, as well as reaction kinetics, of high energy density materials depends on its microstructure. Analysis of the milling-induced microstructures suggests that the formation of fresh oxygen-free intimate high surface area contacts between the reagents is responsible for the enhancement of their reactivity. This manifests itself in a reduction of ignition temperature and delay time, an increased rate of chemical reaction, and an overall decrease of the effective activation energy of the reaction. The protocol provides a detailed description for the preparation of reactive nanocomposites with tailored microstructure using short-term HEBM method. It also describes a high-speed thermal imaging technique to determine the ignition/combustion characteristics of the energetic materials. The protocol can be adapted to preparation and characterization of a variety of nanostructured energetic composites.
Introduction
Classical energetic materials, i.e., explosives, propellants and pyrotechnics are a class of material with a high amount of stored chemical energy that can be released during rapid exothermic reaction1-5. For example, explosives are usually generated by combining fuel and oxidizer groups into one molecule. The energy density of those materials is very high. For example, upon decomposition trinitrotoluene (TNT) releases 7.22 kJ/cm3 and forms 8.36 moles of gases per 100 g (Table 1) in a very short period of time. These materials are composed of micrometer-scale organic and inorganic species (fuels and oxidizers).
Thermite systems, where reactions take place between the inorganic compound, i.e., reducing metals (e.g., Al) and oxides (Fe2O3, CuO, Bi2O3), belong to another type of energetic materials. The energy density (15-21 kJ/cm3) of such systems exceeds that of TNT, however the amount of gas products (0.15-0.6 moles per 100 g) is typically much less than for explosives (Table 1). Also, the nano-thermites may show extremely high velocity of combustion wave propagation (>1,000 m/sec)2-5.
It was recently shown6-12 that a number of gasless heterogeneous reactive systems (Ni+Al, Ti+C, Ti+B) that form intermetallic or refractory compounds could also be considered as energetic materials. The energy densities (kJ/cm3) of those systems are closer or higher than that of TNT (Table 1). At the same time, the absence of gas products during the reaction makes such materials excellent candidates for a variety of applications including synthesis of nanomaterials, reactive bonding of refractory and dissimilar parts, gasless micro power generators, etc.11-17. However, the relatively high ignition temperature of those systems (900-3,000 K, see Table 1) compared to thermites (~1,000 K) hinders their applications. The preparation of engineered nanostructured composites could significantly enhance the ignition and combustion characteristics of gasless heterogeneous systems12-14, 17.
Many methods have been developed to fabricate the engineered energetic nanocomposites, such as ultrasonic mixing18,19, self-assembly approaches5, sol-gel20-22, vapor deposition techniques16,17,23,24, as well as high-energy ball milling (HEBM)1,5. The disadvantage of ultrasonic mixing of nano-powder is that a thick (5-10 nm) oxide shell on metal nanoparticles reduces energy density and degrades the combustion performance of reactive mixtures. Also, the distribution of fuel and oxidizer is not uniform, and the interfacial contact between reactants is not intimate. Sol-gel and self-assembly strategies were developed for preparation of specific thermite nanocomposites. Despite being low-cost techniques, those strategies are not green from an environmental standpoint. Moreover, large amounts of impurities are introduced into prepared composites. Vapor deposition or magnetron sputtering is used to prepare reactive multi-layer foils and core-shell energetic materials. It provides a pore-free and well-defined geometry of composites that simplifies theoretical modeling and enhances accuracy. However, this technology is expensive and difficult to scale up. Furthermore, the prepared layered nanocomposites are unstable in certain conditions.
High-Energy Ball Milling (HEBM) is an environmentally friendly, easily scalable approach that allows effective fabrication of nanostructured energetic composites5,9-14. HEBM is inexpensive and can be used with various reactive material compositions (e.g., thermites, reactions that form intermetallics, carbides, borides, etc.).
The protocol provides a detailed description for preparation of reactive energetic (Ni+Al, Ti+C, Ta+C) nanocomposites with tailored microstructure by using the short-term HEBM method. It also describes a high-speed thermal imaging technique to determine the ignition/combustion characteristics of as-fabricated energetic materials. Finally it shows the analysis of the microstructure of the nanocomposites using Field Emission Scanning Electron Microscope (FESEM) Equipped by Focused Ion Beam (FIB). The protocol is an important guide for the preparation of different energetic nanomaterials (gasless and thermite systems) that could be used as either high energy density sources or for synthesis and processing of advanced nanomaterials by combustion-based approaches.
Protocol
1. High-energy Ball Milling
- Prepare 35 g of the initial 1:1 molar ratio Ni+Al mixture. In this case, weigh 11.02 g of Al and 23.98 g of Ni powders.
- Use a steel milling jar for HEBM of this system. Ensure that the jar has a higher hardness than the powders to be added, otherwise the powders will damage the jar and contamination will arise. Note: Typical jar choices include steel, zirconium oxide, or tungsten carbide.
- Use a 5:1 ball:powder (charge ratio) for this system, i.e., 175 g of 10 mm steel balls. Ensure that the balls are made of the same material as the jar otherwise either the balls or the jar will be damaged.
Note: The charge ratio defines the intensity of interaction between powder and the milling agents. - Add balls and powders to the jar.
- Seal the jar and pump the atmospheric gas from the jar by mechanical pump and purge by argon. Conduct four cycles of filling and purging with Ar gas (this ensures that there is no oxygen remaining in the jar). Finally, fill the jar with argon gas slightly above (0.13 MPa) atmospheric pressure.
- Insert the jar into a planetary ball mill.
- Choose 650 revolution per minute (rpm) for the rate of revolution of jar and 1,400 rpm for the internal rotation (sun wheel).
Note: In some cases, the rotation ratio (k) of sum wheel (1,400 rpm) and milling jar (from 700 to 1,300 rpm) was varied to regulate the microstructure of composite particles. - Run the HEBM procedure for 15 min. Note: Systems have a critical time, which, for the described conditions, equals 17 min. There is a finite amount of milling that can be conducted on the system before the reaction occurs in the jar. If HEBM is conducted longer than the critical time, a reaction will occur in the ball-milling jar, ruining the experiment.
- Following completion of milling time, cool the jar to RT, and then move the jar to a fume hood.
- Vent the jar to remove excess gas pressure from initial pressurization and possible gas released during milling.
- Remove the lid from the jar under the fume hood. Take caution when opening the jar, as the powder formed is very reactive. Open the jar wearing heat resistant gloves and safety goggles.
- Before collecting powder, expose it to air for at least 5 min for “passivation”.
Note: This prevents spontaneous reaction that may occur while handling the mixture.
2. Reactivity Characterization of Energetic Materials
- Collect the powder from the jar. Do not use a metallic spatula for this procedure.
- If classification and separation of the particles is desired, utilize sieves. To ensure that proper separation is done, use a sieve shaker for an extended period of time (12+ hr). Classify the powder into various size bins (under 10 µm, 10-20 µm, 20-53 µm, above 53 µm). From this point onwards, use 20-53 µm sized particles.
- Press the sieved powders into a pellet using a uniaxial press set to 1,100 kg on a 5 mm stainless steel press die (1,360 MPa) for a dwell time of 2.0 min. Record the height (h) and the diameter (d) of the pellet with a micrometer. Record the weight of the sample (m) with a scale. From here, determine the density of the pellet. Calculate the theoretical max density percent (TMD%) by the following formula:
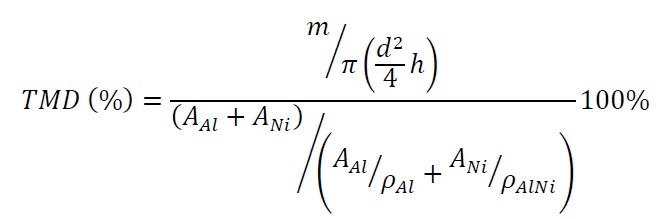
where AAl, ANi — atom weight of Al and Ni; ρAl and ρNi — density of Al and Ni. Assume that the stoichiometric ratio of the powders retains the ratio of the initial powders added.- If the cylindrical pellet is being used to determine a combustion front propagation velocity and temperature profile in the reaction front, ensure that it is tall enough, determined by the ratio between height and diameter which should be ≥2 (e.g., d = 5 mm; h ≥ 10 mm).
- If the pellet is being used to define ignition parameters, use a thin disk (e.g., diameter = 5 mm, thickness = 1 mm).
- To define combustion characteristics, place the sample onto a graphite plate.
- Make a coiled tungsten wire attached to a variable transformer.
- Position the W coil such that the coiled portion of the wire rests on the top of the pellet. If the reactive system is oxygen sensitive, do this in an oxygen-free reaction chamber, otherwise perform the reaction in open air.
- In order to determine the combustion wave velocity, use the recording from the high-speed camera. Position and focus the high-speed thermal camera on the tested sample and start recording. This will enable accurate temperature and combustion velocity information to be gathered.
- Slowly turn the variable transformer on, causing the tungsten wire to heat up. The reaction will initiate, and then propagate.
- To obtain the desired parameters of the combustion process, conduct frame by frame analysis of the recorded IR movie.
- Plot the position of the reaction front propagation vs. time. Obtain the average combustion velocity from the slope of the plot.
- Plot the temperature changes in a spot in the middle of the sample. Use the obtained graph to gain information about the temperature time profile of the reaction wave.
- To define ignition characteristics (ignition temperature and ignition delay time) put the thin disk on a hot plate preheated to the desired temperature (e.g., 800 K). Note that the exact values obtained from this experiment will vary significantly if any parameters are changed, whether they are size of the pellet, temperature of the hotplate, or TMD. This analysis is useful for determination of trends.
- In order to determine the ignition parameters use the high-speed camera. Position and focus the high-speed thermal camera on the area where the sample will be placed on the hot plate and start recording.
Note: This will enable accurate temperature information during the process.- If the reaction is oxygen sensitive, perform this in an oxygen free reaction chamber. IMPORTANT: Run this experiment multiple times to gain a good statistical dataset.
- Put the pellet into the zone of focus. Do this in a way that the particle could be seen on every frame — it is important to see the first frame that the pellet touches the hot plate.
- To obtain the desired ignition parameters, conduct frame by frame analysis of the recorded IR movie.
- To determine the ignition delay time, determine the time between the first frame, when the pellet touches the surface of the hotplate, to the reaction initiation.
- To determine the ignition temperature, plot the highest temperature spot on the particle. When the time-temperature profile switches from that of a preheating temperature profile to that of a thermal explosive regime, the point of inflection matches the ignition temperature.
- In order to determine the ignition parameters use the high-speed camera. Position and focus the high-speed thermal camera on the area where the sample will be placed on the hot plate and start recording.
3. Microstructure Analysis Using Field Emission Scanning Electron Microscope (FESEM) Equipped by Focus Ion Beam (FIB)
- Suspend 0.1 g of the fabricated particles in 10 ml ethanol and deposit one drop of the suspension onto a surface of scanning electron microscopy (SEM) sample holder.
- Dry the sample holder at 90 °C for 5 min.
- Insert the sample into a dual beam FIB/SEM system.
- Conduct sample plasma cleaning for 5 min. Note: This reduces the amount of damage that the sample will experience from exposure to the electron beam (E-beam).
- Turn on the E-beam (5 kV, 3.5 nA) and focus on a single particle. Link the z-height to the working distance, then raise the sample to eucentric height.
- Using the E-beam with the gas injection needle, deposit an initial layer of platinum (70 nm) onto the sample to protect from degradation from use of the gallium ion-beam (I-beam).
- Tilt the sample to 52°, and then turn on the I-beam. Using the I-beam (5 kV, 0.28 nA), again with the gas injection needle, deposit an additional layer of platinum (0.5 µm) onto the sample for protection.
- Cut fiduciary marks on the sample. Mill the particle into a rectangular shape. This vastly increases the chance that there will be an adequate fiduciary, since there will be multiple cuts and corners to use.
- With the aid of a program, slice the particle with the I-beam.
- Select “File” then “Image Save Location” to choose a directory where the images will be stored.
- Depending on the individual particle, select the appropriate width, length, and depth; choose these to completely mill through the entire volume of the particle. Additionally, select the number of slices, as well as the number of slices per image. These options can be found in the “Slice” tab.
- Set the beam current by selecting “Utilities” then “Suggest Currents”. Note: This will allow the program to select the appropriate beam current to mill the sample in a reasonable time while guarding against sample damage.
- Click “Show” and the software will provide a visual milling grid that shows what portion of the particle will be milled; ensure that the milling grid is placed accurately over the particle in the portion that is to be milled.
- After each slice take a high-quality e-beam image for later reconstruction. To select the appropriate e-beam parameters, select the “Setup” menu and choose “EBeam Image Scan Parameters”.
Note: This will give a grid to select resolution and dwell time. The higher the dwell time, the more time it takes to collect the image.
- Utilizing a 3D reconstruction software package, reconstruct the set of images collected from the FIB/SEM as described previously25. Note: This yields a complete 3D virtual copy of the particle, which can then be used to calculate surface area contact, porosity of the individual particles, diffusive layer thickness, as well as countless other useful parameters.
Representative Results
To prepare nanostructured energetic composites, a mixture of desired powdered components (typically micrometer-sized) is mechanically treated under preset milling conditions. Processing time (typically minutes) is accurately controlled to generate the compositionally homogenized nanocomposite particles but not permitting the self-sustaining chemical reaction to initiate during milling.
Figure 1 and Video 1 show that contact surface area between the reactants in composite particles increases by orders of magnitude compared to the initial mixture. After HEBM each component is incorporated into the matrix of another component. In most cases, the obtained nanostructured energetic composites are fully dense with high contact area between reactants (Figure 2). Moreover, the reactants can be mixed on a scale of less than 100 nm. It is also important that tuning HEBM conditions allows regulation of the internal microstructure of composites. It is seen in Figure 2 that different mixing degrees between the reactant may be achieved in the same system. Moreover, HEBM forms fresh (oxygen-free) contacts between reactants. Figure 3 illustrates that HEBM effectively removes the protective oxide layer on the initial metal (e.g., Al) particles. Dark field (DF) image of transmission electron microscopy (TEM) analysis coupled with energy-dispersive X-ray spectroscopy (EDX) in Ni/Al composite particles clearly indicate new boundaries between the reactants that are oxygen-free.
Despite tuning of the internal microstructure of composite particles HEBM enables the regulation of the size of the particles. For example, this could be achieved by changing the rotation ratio (k) of sun wheel (1,400 rpm) and milling jar (from 700 to 1,300 rpm). Video imaging show that several HEBM regimes may occur depending on k ratio. The mixture of balls and powder k ≤ 1.5 is “sliding” on the surface of jar (Video 2). In 1.85 ≤ k < 1.5 interval intensive collisions of balls take places (Video 3). Figure 4 indicate that such different HEBM regimes significantly influence the sizes of particles, i.e., coarse particles (100-150 µm) are formed in the “sliding” regime, while many fine particles (10-50 µm) could be prepared in the collision regime.
Along with preparation of energetic composite particles, the protocol describes their characterization techniques. Such an approach reveals the important links between preparation of materials, their microstructure and reactivity of composite particles. For example, detailed microstructural investigation of Ti/C composite particles revealed that, after 3 min of HEBM, a carbon-rich layer formed between the flattened titanium layers11 due to the cold welding. TEM images in Figure 5 indicate that the carbon layer contains uniformly distributed titanium nanoparticles and titanium carbide (TiC) nuclei.
Temperature-time profiles recorded by infrared imaging for Ti/C composite particles are shown in Figure 5C.The particles were placed on a hot plate with a temperature of ~600 K. A High speed Thermo-Vision system was used to monitor the time-temperature history of the particle. The selected temperature measurement range was 600-1,200 K. It can be seen that the materials, after 2 min of mechanical treatment, cannot be self-ignited under the investigated conditions. The self-ignition temperature after 3 min of HEBM is about 600 K, while after 5 and 7.5 min of treatment Tig is well below 600 K. It is interesting that the ignition temperature is again above 600 K for a milling time of 9 min. This effect is explained by the formation of an amount of the TiC phase in the milling jar. It should be noted that that during combustion of a conventional Ti + C mixture, the microstructure of the reaction medium remains unchanged upon appearance of a liquid metal phase (1,941 K) and the exothermic reaction initiates at ~2,000 K. These results indicate that a direct link exists between the microstructure formed during the HEBM and ignition temperature. Formation of intimate oxygen-free contacts between reactants and product nuclei makes the Ti/C composites extremely reactive as the ignition temperature drops from 2,000 to 600 K. HEBM also significantly influences the ignition delay time, i.e., the time after immersing the particle in a furnace and until reaction initiation, as well as combustion front propagation velocity. Temperature-time profiles in Figure 5C shows that the ignition delay time also decreases with an increase of milling time.
It is worth noting that combustion of mechanically fabricated composite shows great advantages for synthesis of nanostructured materials. During the combustion of conventional media, control over the microstructure of product is extremely difficult. For example, the reaction onset temperature of conventional Ni+Al coincides with the lowest eutectic temperature of the system (~910 K). The liquid phase formed during the reaction significantly changes the microstructure of the initial mixture (Figure 6). In the mechanically fabricated composites, the reactions proceed below the eutectic temperature of the system, which completely eliminates formation of liquid phases, i.e., a true solid-state combustion, so-called solid flame, takes place. This is evidenced by an onset reaction temperature as low as 470 K, whereas the lowest eutectic temperature in this system occurs at 910 K; this implies that a significant conversion must occur due to a purely solid-state reaction. Samples prepared from such composite particles retain their shape and microstructure (Figure 6).
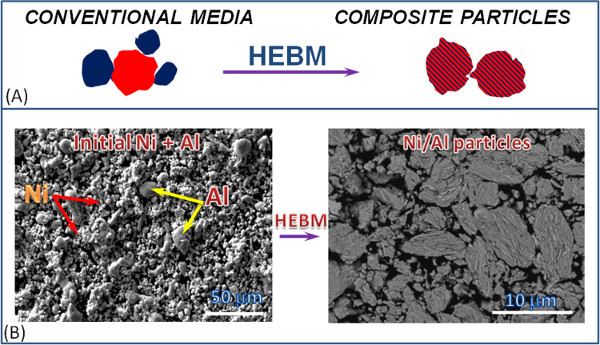
Figure 1. The transformation of the heterogeneous reactive media microstructure during high-energy ball milling: Schematic representation of the transformation of micrometer size particles of individual reactants to a layered composite particles (A), and the formation of Ni/Al composite particles by using HEBM of nickel and aluminum reactants (B). Please click here to view a larger version of this figure.
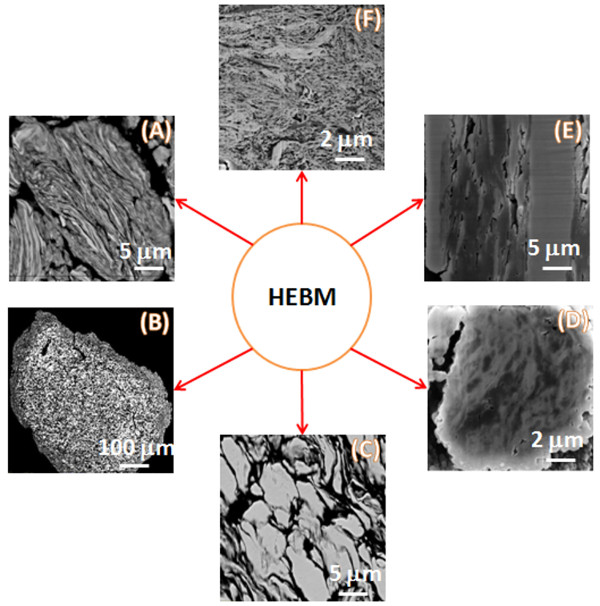
Figure 2. Tuning the contact area between the reactants by varying HEBM conditions for different systems: Ni/Al (A–C), Ti/C (D,E) and Ta/C (F). Please click here to view a larger version of this figure.
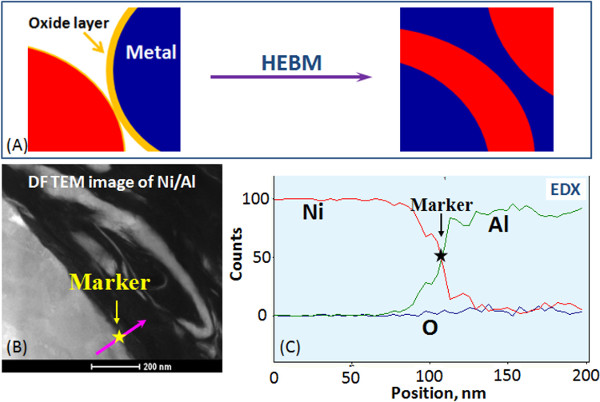
Figure 3. Formation of oxygen-free contacts between the reactants: Schematic representation (A), Bright field image of a Ni/Al boundary formed by HEBM (B) and Energy dispersive X-ray Spectroscopy (EDS) profiles of nickel, aluminum and oxygen (C). Please click here to view a larger version of this figure.
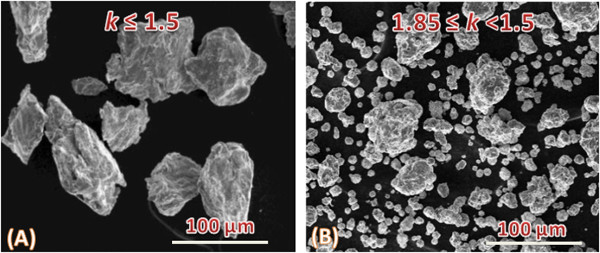
Figure 4. Preparation of composite particles with different sizes by tuning the ratio (k) of sun wheel and milling jar rotation speeds: k ≤ 1.5 (A) and 1.8 ≤ k < 1.5 (B). Please click here to view a larger version of this figure.
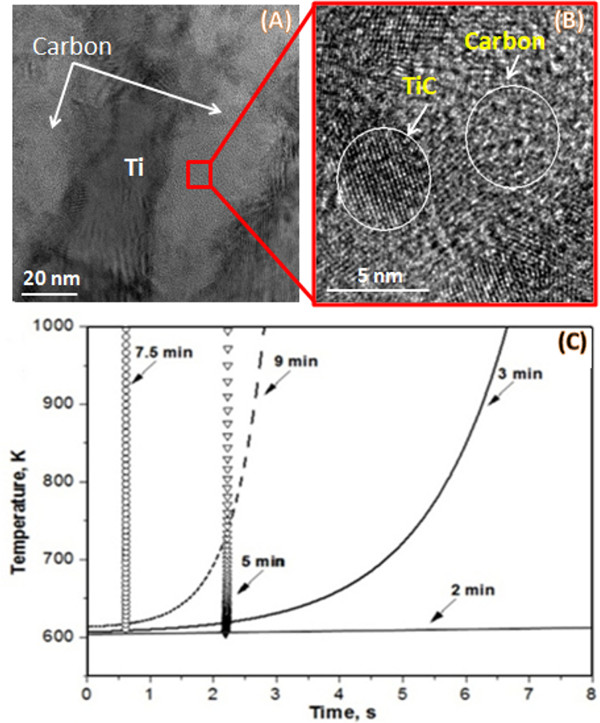
Figure 5. The relationship between microstructure and reactivity of composite particles: A TEM image of Ti/C composite particle (A), high resolution TEM image of TiC nanoparticles (B) and time-temperature ignition profiles (C) of Ti/C composite particles various milling times (2, 3, 5, 7.5, 9 min). Please click here to view a larger version of this figure.
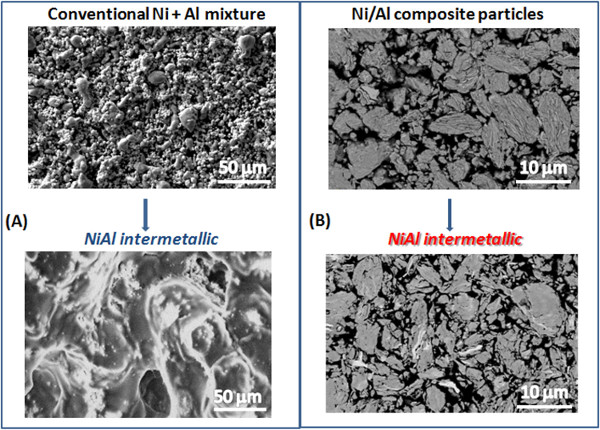
Figure 6. Synthesis of materials with pre-designed microstructure by using combustion of nano-structured composite particles: Microstructures of NiAl intermetallics using conventional media (A) and mechanically fabricated composite particles (B). Please click here to view a larger version of this figure.
| Mole gas released per 100 g | Energy densities per volume, kJ/cm3 | Ignition temperature, K | |
| Trinitrotoluene (TNT) | 8.36 | -7.22 | 510 |
| Termites | |||
| 2Al + 3CuO | 0.54 | -20.8 | 900-1,100 |
| 2Al + Fe2O3 | 0.14 | -16.4 | |
| 2Al+ Bi2O3 | 0.47 | -15.2 | |
| Gasless Systems | |||
| Al + Ni | 0 | -7.13 | 910/520 |
| Ta + C | 0 | -10.9 | 3,000/1,500 |
| Ti + C | 0 | -15.2 | 2,000/900 |
Table 1. Some characteristics of energetic materials.
Video 1. “Slice and view” imaging of a Ni/Al composite particle.
Video 2. “Sliding” regime of HEBM at k ≤ 1.5.
Video 3. Intensive collisions of balls in 1.85 ≤ k < 1.5 interval.
Discussion
The protocol provides a detailed description for preparation of reactive energetic (Ti+C, Ta+C, Ni+Al) nanocomposites with tailored microstructure by using the short-term HEBM method. HEBM of gasless heterogeneous mixtures involve their processing in a high-speed planetary ball mill, where the particles of the mixture are subjected to mechanical impact with a force sufficient for breakdown of brittle components (e.g. graphite) and deformation of plastic components (e.g., Al, Ti, Ta, Ni). Brittle reactants are milled to finer particles and may become amorphous, while plastic metals are subjected to multiple deformations and cold welding, forming composites particles. Small fragments of brittle components are often found inside the particles of plastic reactants. Fine tuning of the HEBM conditions allow for control of the composite particle sizes and their intrinsic microstructure. It should be noted that such a degree of control in microstructure cannot be achieved in most other techniques currently available for preparation of nanostructured energetic composites. Thus the energy released in mechanically fabricated energetic composites could be precisely controlled by their microstructure through fine tuning of HEBM conditions.
The unique HEBM conditions also allow one to produce the metastable non-equilibrium supersaturated solutions, which enable reactions to occur at significantly lower temperatures than conventional powder mixtures. Moreover, in some cases the reactions proceed below the eutectic temperature of the system, which completely eliminates formation of liquid phases. Samples prepared from such composite particles retain their shape and microstructure.
One use of HEBM is in production of highly reactive, energetic nanocomposites. This process is simple, highly economical, and easily scaled. There are two major issues with this process, however. The first is safety issues; this process creates nanocomposites that are highly reactive, and as such, the operator must follow all safety procedures. This includes general safety procedures relating to the operation of the machine itself and to more specific safety procedures relating to the compounds being utilized. Because of the highly reactive nature of these nanocomposites; a limited quantity of this material should be produced until knowledge about the safety of the specific system is ascertained. Finally, impurities relating to the vessel can be introduced. This can lead to simple contamination or even undesired side reactions. Third, preparation of pore-free composites (e.g., coatings, films) is difficult and requires additional steps (cold spraying or rolling) 26.
The protocol also provides in-depth information on the characterization of mechanically fabricated nanostructured energetic composites. The use of high-speed infrared techniques allows for accurate spatial (2 μm), thermal (5 K), and temporal resolution (15,000 fps). This enables accurate characterization of the composite particles, including their time-temperature history, ignition temperature, delay time, and propagation velocity.
The protocol is an important guide for the preparation of different energetic nanomaterials (gasless) that could be used as either high energy density sources or for synthesis and processing of advanced nanomaterials by combustion-based approaches. It can be easily modified to apply to thermite systems, and other energetic materials such as metal-polymer composites.
Critical steps within the protocol include the initial preparation of the nanocomposites, starting from the weighing of the powders and choosing the appropriate charge ratio. Additionally, it is of key importance to ensure that the internal atmosphere of the jar is inert through Ar purging. Selection of the ball milling parameters, including the revolution speed and total milling time are necessary for tailoring the microstructure. Finally, the exposure, collection, and classification of the powder with a safe procedure are important, lest the experiment is ruined. Preparation of the powders for the experimentation by pressing determines the data that can be collected, followed by accurate analysis of the data. Use of the FIB S&V program to generate a 3D dataset for analysis is also of importance.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This material is based upon work supported by the Department of Energy, National Nuclear Security Administration, under Award Number DE-NA0002377. Funding from the Defense Threat Reduction Agency (DTRA), Grant Number HDTRA1-10-1-0119. Counter-WMD basic research program, Dr. Suhithi M. Peiris, program director is gratefully acknowledged. This work was also supported by the Ministry of Education and Science and Education of the Russian Federation in the framework of Increase Competitiveness Program of NUST “MISIS” grant No. K2-2014-001.
Materials
| Titanium | Alfa Aesar | 42624 | Particle size: -325 mesh | Purity, 99.5% |
| Graphite | Alfa Aesar | 46304 | Particle size: 7-11 micron | Purity, 99% |
| Nickel | Alfa Aesar | 10256 | Particle size: 3-7 micron | Purity, 99.9% |
| Aluminum | Alfa Aesar | 11067 | Particle size: -325 mesh | Purity, 99.5% |
| Tantalum | Materion advanced chemicals | T-2017 | Particle size: 325 mesh | Purity, 99.9% |
| Carbon lampblack | Fisher scientific | C198-500 | Particle size: 0.1 micron | Purity, 99.9% |
| Tungsten wire | Mcmaster Carr | n/a | 0.032" diameter | n/a |
| Planetary Ball Mill | Retsch GmbH, Germany | n/a | n/a | n/a |
| Uniaxial press | Carver Hydraulic | n/a | n/a | n/a |
| Sieve shaker | Gilson performer | n/a | 5mm diameter | n/a |
| Cylindrical stainless steel press die | Action Machine | n/a | n/a | n/a |
| Stainless steel sieves | Mcmaster Carr | Type 304 | n/a | n/a |
| High-speed thermal camera (SC6000) | FLIR | n/a | n/a | n/a |
| Helios NanoLab 600, Field Emission Scanning Electron Microscope (FESEM) Equipped by Focus Ion Beam (FIB) | FEI | n/a | n/a | n/a |
| Cylindrical reactor with a vacuum pomp | Action Machine | n/a | n/a | n/a |
| Autoslice and View (S&V) | FEI | n/a | n/a | n/a |
| Avizo Fire | FEI | n/a | n/a | n/a |
References
- Fried, L. E., Manaa, M. R., Pagoria, P. F., Simpson, R. L. Design and Synthesis of Energetic Materials. Annual Review of Materials Research. 31, 291-321 (2001).
- Dlott, D. D. Thinking Big (and Small) about Energetic Materials. Materials Science and Technology. 22 (4), 463-473 (2006).
- Dreizin, E. L. Metal-Based Reactive Nanomaterials. Progress in Energy and Combustion Science. 35 (2), 141-167 (2009).
- Yetter, R. A., Risha, G. A., Son, S. F. Metal Particle Combustion and Nanotechnology. Proceedings of the Combustion Institute. 32 (2), 1819-1838 (2009).
- Zhou, X., Torabi, M., Lu, J., Shen, R., Zhang, Z. Nanostructured Energetic Composites: Synthesis, Ignition/Combustion Modeling, and Applications. ACS Applied Materials and Interfaces. 6 (5), 3058-3074 (2014).
- Mann, A. B., et al. Modeling and Characterizing the Propagation Velocity of Exothermic Reactions in Multilayer Foils. Journal of Applied Physics. 82 (3), 1178 (1997).
- Jayaraman, S., Mann, A. B., Reiss, M., Weihs, T. P., Knio, O. M. Numerical Study of the Effect of Heat Losses on Self-Propagating Reactions in Multilayer Foils. Combustion and Flame. 124 (1-2), 178-194 (2001).
- Rogachev, A. S. Exothermic Reaction Waves in Multilayer Nanofilms. Russian Chemical Reviews. 77 (1), 21-37 (2008).
- White, J. D. E., Reeves, R. V., Son, S. F., Mukasyan, A. S. Thermal Explosion in Al-Ni System: Influence of Mechanical Activation. The Journal of Physical Chemistry A. 113 (48), 13541-13547 (2009).
- Shteinberg, A. S., Lin, Y. -. C., Son, S. F., Mukasyan, A. S. Kinetics of High Temperature Reaction in Ni-Al System: Influence of Mechanical Activation. The Journal of Physical Chemistry A. 114 (20), 6111-6116 (2010).
- Manukyan, K. V., Lin, Y. L., Rouvimov, S., McGinn, P. J., Mukasyan, A. S. Microstructure-reactivity relationship of Ti reactive nanomaterials. Journal of Applied Physics. 113 (2), 024302 (2013).
- Mukasyan, A. S., Lin, Y. C., Rogachev, A. S., Moskovskikh, D. M. Direct Combustion Synthesis of Silicon Carbide Nanopowder from the Elements. Journal of the American Ceramic Society. 96 (1), 111-117 (2013).
- Lin, Y. C., Nepapushev, A. A., McGinn, P. J., Rogachev, A. S., Mukasyan, A. S. Combustion joining of carbon/carbon composites by a reactive mixture of titanium and mechanically activated nickel/aluminum powders. Ceramics International. 39 (7), 7499-7505 (2013).
- Manukyan, K. V., et al. Tailored Reactivity of Ni+AlNanocomposites: Microstructural Correlations. The Journal of Physical Chemistry C. 116 (39), 21027-21038 (2012).
- Qiu, X., Wang, J. Bonding Silicon Wafers with Reactive Multilayer Foils. Sensors and Actuators A. 141 (2), 476-481 (2008).
- Wang, J., et al. Joining of Stainless-Steel Specimens with Nanostructured Al/Ni Foils. Journal of Applied Physics. 95 (1), 248-256 (2004).
- Rogachev, A. S., Mukasyan, A. S. Combustion of Heterogeneous Nanostructural Systems (Review). Combustion, Explosion, and Shock Waves. (Engl. Transl). 46 (3), 243-266 (2010).
- Granier, J. J., Pantoya, M. L. Laser Ignition of Nanocomposite Thermites). Combustion and Flame. 138 (4), 373-383 (2004).
- Bockmon, B. S., Pantoya, M. L., Son, S. F., Asay, B. W., Mang, J. T. Combustion Velocities and Propagation Mechanisms of Metastable Interstitial Composites. Journal of Applied Physics. 98 (6), 064903-064907 (2005).
- Tillotson, T. M., et al. Nanostructured Energetic Materials Using Sol−Gel Methodologies. Journal of Non-Crystalline Solids. 285 (1-3), 338-345 (2001).
- Cervantes, O. G., Kuntz, J. D., Gash, A. E., Munir, Z. A. Heat of Combustion of Tantalum−Tungsten Oxide Thermite Composites. Combustion and Flame. 157 (12), 2326-2332 (2010).
- Leventis, N., Chandrasekaran, N., Sadekar, A. G., Sotiriou-Leventis, C., Lu, H. One-Pot Synthesis of Interpenetrating Inorganic/Organic Networks of CuO/Resorcinol-Formaldehyde Aerogels: Nanostructured Energetic Materials. Journal of the American Chemical Society. 131 (13), 4576-4577 (2009).
- Zhang, K., Rossi, C., Ardila Rodriguez, G. A., Tenailleau, C., Alphonse, P. Development of a Nano-Al/CuO Based Energetic Material on Silicon Substrate. Applied Physics Letters. 91 (11), 113117-113113 (2007).
- Xu, D., Yang, Y., Cheng, H., Li, Y. Y., Zhang, K. Integration of Nano-Al with Co3O4Nanorods to Realize High-Exothermic Core-Shell Nanoenergetic Materials on a Silicon Substrate. Combustion and Flame. 159 (6), 2202-2209 (2012).
- Shearing, P. R., et al. Exploring Microstructural Changes Assicuated with Oxidation in Ni-YSZ SOFC Electrodes Using High Resolution X-ray Computed Tomography. Solid State Ionics. 216 (28), 69-72 (2012).
- Bacciochini, A., Bourdon-Lafleur, S., Poupart, C., Radulescu, M., Ni-Al, J. o. d. o. i. n. B. Nanoscale Energetic Materials: Phenomena Involved During the Manufacturing of Bulk Samples by Cold Spray. Journal of Thermal Spray Technology. In press, (2014).

