A Simple and Inexpensive Method for Determining Cold Sensitivity and Adaptation in Mice
Summary
The Cold Plantar Assay (CPA) measures cold responsiveness between 30 °C and 5 °C, and can also measure cold adaptation. This protocol describes how to use the CPA to measure cold hypersensitivity, analgesia, and adaptation in mice.
Abstract
Cold hypersensitivity is a serious clinical problem, affecting a broad subset of patients and causing significant decreases in quality of life. The cold plantar assay allows the objective and inexpensive assessment of cold sensitivity in mice, and can quantify both analgesia and hypersensitivity. Mice are acclimated on a glass plate, and a compressed dry ice pellet is held against the glass surface underneath the hindpaw. The latency to withdrawal from the cooling glass is used as a measure of cold sensitivity.
Cold sensation is also important for survival in regions with seasonal temperature shifts, and in order to maintain sensitivity animals must be able to adjust their thermal response thresholds to match the ambient temperature. The Cold Plantar Assay (CPA) also allows the study of adaptation to changes in ambient temperature by testing the cold sensitivity of mice at temperatures ranging from 30 °C to 5 °C. Mice are acclimated as described above, but the glass plate is cooled to the desired starting temperature using aluminum boxes (or aluminum foil packets) filled with hot water, wet ice, or dry ice. The temperature of the plate is measured at the center using a filament T-type thermocouple probe. Once the plate has reached the desired starting temperature, the animals are tested as described above.
This assay allows testing of mice at temperatures ranging from innocuous to noxious. The CPA yields unambiguous and consistent behavioral responses in uninjured mice and can be used to quantify both hypersensitivity and analgesia. This protocol describes how to use the CPA to measure cold hypersensitivity, analgesia, and adaptation in mice.
Introduction
Measuring cold responsiveness in rodents is important for improving understanding of the potential mechanisms of cold sensitivity in humans under both normal and pathological conditions. The Cold Plantar Assay (CPA), originally developed several years ago1, is designed to generate reproducible, unambiguous murine behavioral responses to a cold stimulus delivered at RT. More recent enhancements of this assay have allowed the reproducible measurement of cold sensitivity at a wide range of temperatures2. Both versions are also designed to be relatively high-throughput, and inexpensive to use.
A great deal of progress has been made in understanding the mechanisms of cold sensitivity using other behavioral methods. One method is the acetone evaporation test, which involves dabbing or spraying acetone on the mouse paw and measuring the amount of time that the mouse spends flicking the paw3,4. Unfortunately, the responses to acetone evaporation are confounded by the wet sensation and the smell of the acetone. Also, the cold stimulus that is applied in the acetone evaporation test can vary based on the amount of acetone applied, and is difficult to quantify. Finally, uninjured mice have minimal responses to acetone at baseline, making it impossible to measure analgesia in the absence of hypersensitivity with this method.
Another classical assay for cold responses is the tail flick assay, where the latency to withdrawal is measured after the tail is immersed in cold water5,6. While the behavioral responses in this assay are unambiguous and the assay measures responses to a specific temperature, the animals must be restrained during testing, which can alter cold responsiveness through well-described stress-induced analgesic mechanisms7.
Another commonly used tool is the cold plate test, which measures the behavioral responses of mice after they are placed on a peltier-cooled plate8-10. While this tool provides information about animal responses at specific temperatures, it has also been inconsistently used; different groups have measured different types of responses including number of jumps8,11, the latency to first response8,11-13, and the number of paw lifts11,13,14 with very different results. The cold plate assay is also relatively low throughput as only one animal can be tested at a time, and it requires an expensive and fragile peltier device.
The 2-plate temperature preference test is a commonly used derivative of the cold plate test that measures the relative amount of time that animals spend on 2 connected plates of different temperatures9,15-17. Another similar commonly used assay is the thermal gradient assay, where the amount of time that mice spend in different temperature zones ranging between 5 °C and 45 °C on a long metal plate is measured16. While these assays allow comparison of temperatures, it is unclear whether the behavior represents temperature aversion or to temperature preference.
Finally, the dynamic cold plate assay has been used to measure how mice respond to changing ambient temperatures18. This method involves placing mice on a RT peltier device and ramping it down to 1 °C while measuring how much the mice jump or lick their paws at different plate temperatures. While this tests how mice adapt to a cooling environment, it does not provide a way to test how mice respond to a cold stimulus in the setting of a cooler ambient temperature. Additionally, it requires expensive equipment to perform and does not provide a way to acclimate mice to the testing equipment before measuring their cold sensitivity.
To complement these assays, the CPA tests the acclimated responses to a well-defined cold stimulus at a variety of temperature ranges, or during the process of adapting to cold ambient temperatures. It can test up to 14 mice at a time with our current apparatus, with the potential to be inexpensively scaled up for high-throughput testing.
Protocol
All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal Studies Committee of Washington University School of Medicine (St. Louis, MO).
1. Preparing the Testing Plate and Enclosures
- Clean off the glass surface.
- Secure the T-type filament thermocouple probe to the surface in the middle of the glass plate with laboratory tape.
- Place the animal enclosures on the glass plate in a single line along the middle of the plate.
- Thread the thermocouple probe through the center animal enclosure and plug into the data logger. Turn the data logger on while deactivating the auto-shutdown feature, and attach the data logger to the computer with the provided cable.
- If recording the plate temperature during the experiment, open the data logger software to begin recording plate temperatures.
- If necessary, adjust the software to record the plate temperature once every second.
- Begin recording temperatures using the software packaged with the thermal data logger.
- Separate the enclosure with black inserts to prevent visual interaction between mice.
- Position mirrors underneath the glass such that the underside of the enclosures is visible from a comfortable seated position.
2. Warming/Cooling the Glass Plate
- Fill aluminum boxes with warmed water, wet ice, or dry ice and position them appropriately on the glass plate (aluminum foil packets filled with dry ice can also be used to cool the glass; Figure 1)2.
- For testing at 30 °C, position the aluminum boxes approximately 0.25’’ away from the animal enclosures (Figure 2B)2.
- Set a heated water circulator on either side of the glass plate. Set the circulator to 45 – 60 °C, and use it to fill the aluminum boxes with a steady stream of hot water (Figure 1C)2.
- Position the circulators such that the hot water from the aluminum boxes drains directly back into the reservoir of the circulator on each side (Figure 1C)2.
- For testing at RT, leave the boxes empty (Figure 2)2.
- For testing at 17 °C, position the boxes approximately 0.25’’ away from the animal enclosures on either side and fill with wet ice (Figure 2)2.
- For testing at 12 °C, position the boxes approximately 1.25’’ away from the enclosures on either side and fill with dry ice (Figure 2)2.
- For testing at 5 °C, position the boxes approximately 0.25’’ away from the enclosures on either side and fill with dry ice (Figure 2)2.
- When cooling the glass with dry ice, make sure there is sufficient ventilation to prevent CO2 buildup in the room.
- For testing at 30 °C, position the aluminum boxes approximately 0.25’’ away from the animal enclosures (Figure 2B)2.
- Wait for the glass to reach the desired temperature range.
- Add the mice to the enclosures on the plate.
NOTE: A white noise generator may be used to decrease noise disturbances. - Wait for the mice to acclimate.
NOTE: In our facility this takes roughly 2.5 hr, but this may vary significantly based on animal housing and handling conditions. - Maintain the glass at the desired temperature range by ensuring that the boxes are kept full of warmed water, wet ice, or dry ice.
NOTE: With our apparatus the boxes need to be refilled with ice roughly every 90 min.
NOTE: For the 17 °C condition, it is helpful to empty most of the water from the aluminum boxes through the drain holes before refilling it with ice. This will stabilize the temperature better, and prevent overflow
NOTE: The exact amount of the dry ice will vary seasonally, but in general keeping the boxes more than ¼ full along the entire length of the box will keep the temperature constant.
3. Testing the Mice at Fixed Temperatures
- Outside of the behavioral room, fill an ice bucket about half full of dry ice.
- Using a hammer or mallet, crush the dry ice into a fine powder.
NOTE: Overfilling the bucket will make it difficult to fully crush the dry ice into powder. - Using a straight razor blade or scissors, cut the top off a 3 ml syringe.
- Using a 21 G needle, poke 3 holes on opposing sides of the syringe (total of 6 holes).
NOTE: These holes will decrease the pressure generated by sublimation while compressing the dry ice. The cut-off syringe can be reused for multiple experiments. - Take the syringe, dry ice powder, and a hand-held stopwatch into the behavioral room.
- Fill the syringe chamber half full of dry ice powder. Hold the cut end of the syringe against a flat object, and firmly compress the powder using the plunger. Be careful; the plastic plunger may bend or break from the pressure. If this happens, replace the plunger from a new syringe.
- Extend the tip of the compressed dry ice pellet past the edge of the syringe.
- Test mice that are fully at rest.
- At 30 °C, 23 °C and 17 °C, test mice that have all 4 paws on the glass and not moving, but not fully asleep19.
- At 12 °C and 5 °C, test mice that are on 2 paws or 4 paws and not moving or jumping.
- Using the mirrors for targeting, gently but firmly press the flat pellet flush against the glass surface underneath the mouse hindpaw (Figure 1A)2. Start the hand-timer.
- Stop the timer and remove the pellet when the mouse moves away from the cooled glass.
NOTE: The withdrawal movement can be vertical or horizontal.- If the mouse very briefly moves the paw and then returns it to the cooling surface, continue timing and stimulating until the mouse makes a permanent move away.
NOTE: Our lab uses a maximum stimulus time of 20 sec for mice in the majority of cases.
- If the mouse very briefly moves the paw and then returns it to the cooling surface, continue timing and stimulating until the mouse makes a permanent move away.
- Repeat this testing procedure until at least 3 values on each paw of each animal are collected. Separate trials testing opposite paws on the same mouse by at least 7 min, and separate consecutive trials on any single paw by at least 15 min.
- If needed, use different thicknesses of glass to generate different rates of cooling (Figure 3)1.
NOTE: The rate of cooling is inversely correlated with the thickness of the glass.
4. Testing the Mice During Cold Adaptation
NOTE: This is an alternate protocol which allows testing as the glass plate cools, rather than once the plate has stabilized and the mice have fully adapted to the cold environment.
- Follow the instructions listed in Section 1 to set up the apparatus.
- Follow the instructions listed in Section 3 to take baseline measurements at RT (Figure 7A)2.
- Pre-cool the aluminum boxes with dry ice.
- Once baseline withdrawal latencies have been measured, position the precooled boxes on the plate approximately 1.25’’ away from the enclosures on either side (Figure 7A, arrow labeled “Dry ice added”) 2.
- Follow the instructions listed in Section 3 to take measurements as the glass plate cools, taking measurements as often as possible.
Representative Results
The behavioral responses elicited from mice starting at 30 °C, 23 °C, 17 °C, and 12 °C are highly reproducible (Figure 4A)20. In order to measure the cold stimulus being generated under the hindpaw, mice were anesthetized with a ketamine/xylazine/acepromazine cocktail and their paws were secured on the glass on top of a T-type filament thermocouple (Figure 4B)20. The glass was cooled or warmed to the desired testing range. Although the plate is cooled uniformly along the length of the plate (Figure 5A)2, it should be noted that a cold gradient is generated across the behavior enclosures (Figure 5B)2. The parts of the enclosure that are closer to the dry ice on either side of the enclosures are cooler, while the central parts are slightly warmer (Figure 5B)2. In the coldest temperatures used, the mice spend the majority of their time in the central parts of the enclosure. Once the glass plate temperature had stabilized, a focal dry ice stimulus was placed on the glass underneath the paw/thermode. Based on the temperature traces recorded from this setup, it is clear that the cold stimuli generated using the CPA are highly reproducible at each temperature range (Figure 4C)20.
The cold stimulus generated in the CPA was also measured using three different thicknesses of glass to vary the intensity of the cooling (Figure 3). The rate of cooling is inversely related to the thickness of the glass, and any of these thicknesses can be used to measure cold sensitivity as needed (Figure 3).
Previous work has shown that the CPA can detect analgesia and hypersensitivity in mice. 30 min after subcutaneous injections of 1.5 mg/kg of morphine, mice have significantly longer latency to withdrawal than mice given subcutaneous injections of saline (Figure 6A: 2-way ANOVA main effect *p < 0.05 with Bonferroni post-hoc test; 30 min **p < 0.01; n = 12 per group)1. By 60 min after morphine/saline, there is no difference between the saline- and morphine-injected groups, which is consistent with the rate of morphine metabolism in mice.
Complete Freund’s Adjuvant (CFA) has previously been shown to cause inflammation and hypersensitivity after hindpaw injection21. After CFA injections, the CPA withdrawal latencies decrease 2 and 3 hr post-injection (Figure 6B: 2-way ANOVA main effect p < 0.001 with Bonferroni post-hoc test; 2 hr *p < 0.05, 3 hr **p < 0.01 n = 12 per group). 4 hr after CFA injection, the mice were given subcutaneous injections of 1.5 mg/kg morphine. 30 min after morphine injection, both CFA- and saline-injected mice had increased withdrawal latencies relative to their latencies at 3 hr (Figure 6B: 1-way ANOVA with Dunnett’s post-hoc test; CFA 3 hr vs. CFA 4.5 hr $$$p < 0.001, saline 3 hr vs. saline 4.5 hr $$$p < 0.001). An hour later, once the morphine had been metabolized, the CFA-injected mice once again had lower withdrawal latencies than the saline-injected control mice (Figure 6B: 2-way ANOVA with Bonferroni post-hoc test; **p < 0.01)1.
Most mammalian species have the ability to adapt their temperature sensitivity to match their environment. In vitro, studies have suggested that this adaptation process is dependent on PIP2 hydrolysis22-24, but previous behavioral tools were unable to validate this hypothesis in vivo. The CPA is capable of quantifying this adaptation in two different ways. By testing the withdrawal latency of mice as the glass cools (Figure 7A,C), the CPA can measure cold adaptation as it happens2. Under normal conditions the withdrawal latency is unchanged as the plate cools, suggesting that cold adaptation happens faster than can be quantified with the CPA (Figure 7B: 0 min = 12.13 ± 0.8 sec, 30 min = 12.1 ± 1.6 sec, 60 min = 13.2 ± 1.1 sec, 90 min = 10.8 ± 1.2 sec 1-way ANOVA with Bonferroni post hoc test p > 0.05, n = 6)2. However, when mice are given intraplantar injections of the phospholipase-C inhibitor U7312225 before the plate is cooled (Figure 7C) their withdrawal latencies are decreased, suggesting that adaptation is impaired (Figure 7D: baseline = 11.29 ± 0.53 sec, 30 min = 8.09 ± 1.17 sec; 1-way ANOVA with Dunnett’s post-hoc test, main effect p = 0.02, individual baseline vs. 30 min p = 0.02, n = 9).
The CPA can also measure the ability to adapt to cold ambient temperatures over long periods of time. When wild-type mice are tested using the CPA after being acclimated for 3 hr at 30 °C, 23 °C, 17 °C, or 12 °C the withdrawal latency is the same at all starting temperatures, suggesting that the wild-type mice adapted to the colder ambient temperature (Figure 2A: WT 30 °C = 13.23 ± 0.5 sec, 23 °C = 12.8 ± 0.7 sec, 17 °C = 12.3 ± 0.9 sec, 12 °C = 12.8 ± 0.5 sec, 1-way ANOVA with Bonferroni post-hoc test, p > 0.05 n = 6 for 30 °C, n = 15 for 23 °C, 17 °C, and 12 °C)20. Unlike the wild-type mice, as the starting temperature decreases the withdrawal latencies of TRPM8-KO mice decrease, suggesting that they are unable to adapt their response threshold to suit their environment (Figure 8: 1-way repeated measures ANOVA with Bonferroni post-hoc test; males main effect p = 1.5 x 10-5, 12 °C vs. 23 °C p = 6 x 10-5, 17 °C vs. 23 °C p = 0.004; females main effect p = 3.6 x 10-5, 12 °C vs. 23 °C p = 9.25 x 10-5, 17 °C vs. 23 °C p = 0.0005; df = 1, n = 11 males and 11 females)20.
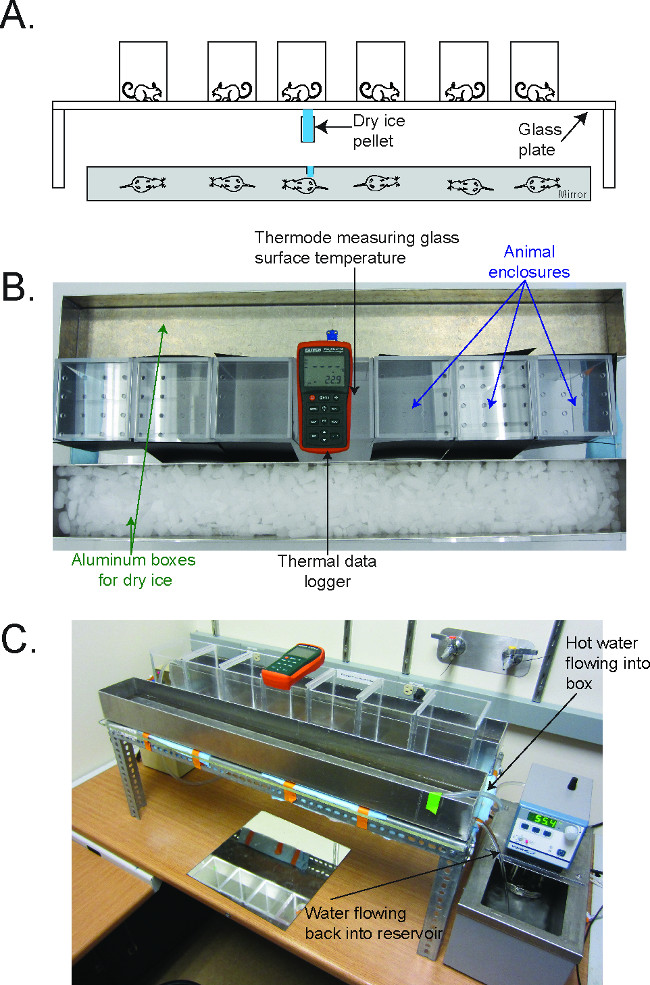
Figure 1. The Cold Plantar Assay (CPA) apparatus2. (A) Schematic for performing the CPA. Mice are acclimated on a glass plate in plastic behavioral enclosures until they are at rest. A dry ice pellet is applied to the underside of the glass underneath the hindpaw, and the latency to withdraw from the cooling glass is measured. (B) Picture of the CPA apparatus, in configuration to cool the plate to 5 °C. The thermal data logger is in the center of the enclosures, and the aluminum boxes flank the enclosure on either side. (C) Picture of the CPA apparatus, in configuration to warm the plate to 30 °C. The water circulator flows hot water into the aluminum box, which then flows out the drain on the side back into the reservoir of the circulator. Reused with permission from Brenner et al. 2014 2. Please click here to view a larger version of this figure.
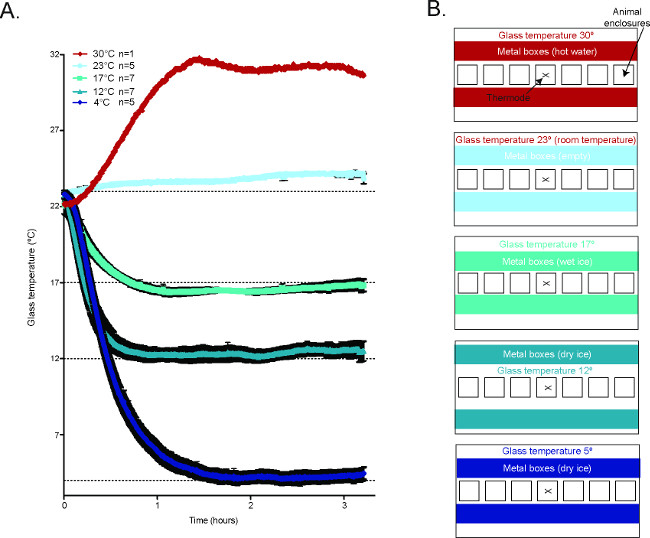
Figure 2. Temperature of the glass plate during the CPA2. (A) Averaged temperature tracings of the glass plate during behavioral experiments in the CPA. 30 °C n = 1, 23 °C n = 5, 17 °C n = 7, 12 °C n = 7, 4 °C n = 5. (B) Schematic diagrams demonstrating how to generate the different temperature conditions in the CPA. Reused with permission from Brenner et al. 2014 2. Please click here to view a larger version of this figure.
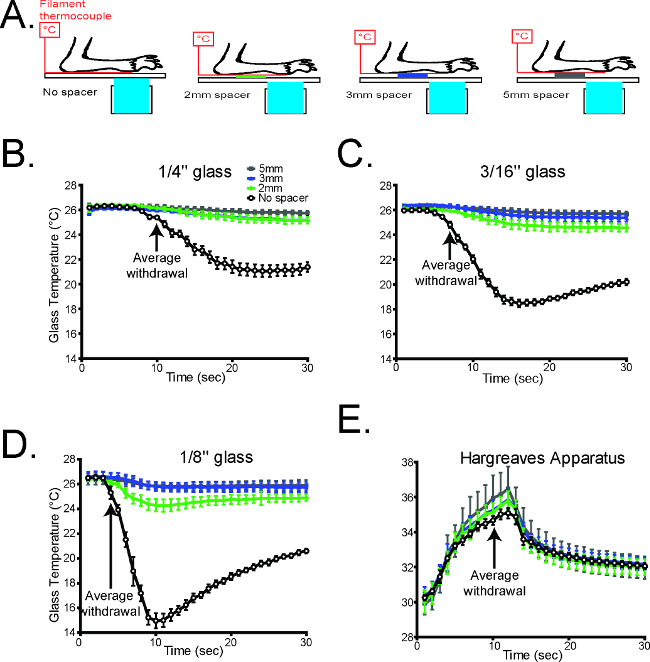
Figure 3. The glass thickness is inversely correlated with the rate of cooling1. (A) Schematic diagramming the experimental design (B-D). The temperature during cold plantar stimulus underneath the paw was measured on all three glass thicknesses under normal conditions, and which Styrofoam spacers propping the paw away from the glass surface. In all cases, propping the paw away from the glass caused a dramatic decrease in the cold stimulus measured at the paw (n = 6 per glass thickness). (E) The temperature underneath the paw was measured during Hargreaves radiant heat stimulation with the paw propped up with Styrofoam. Unlike the cold plantar assay, the thermal stimulus in the Hargreaves assay is largely unaltered when the paw is propped away from the glass (n=6). Reused with permission from Brenner et al. 20121 Please click here to view a larger version of this figure.
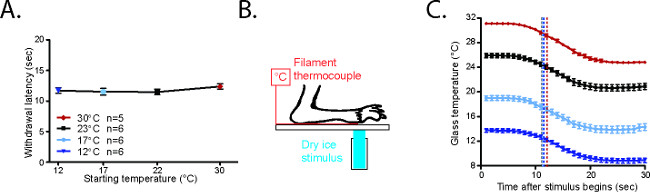
Figure 4. CPA withdrawal latencies are consistent20. (A) Average withdrawal latency for mice starting from 23 °C, 17 °C, or 12 °C. (B) Configuration to measure CPA cold stimulus. The paw of an anesthetized mouse is secured on the glass plate with laboratory tape on top of a T-type filament thermocouple. The CPA stimulus is placed on the underside of the glass underneath both paw and thermocouple. (C) Temperatures generated in the CPA starting from 30 °C, 23 °C, 17 °C, or 12 °C. The black arrows represent the average withdrawal latencies for awake mice in each condition. Reused with permission from Brenner et al. 20142. Please click here to view a larger version of this figure.
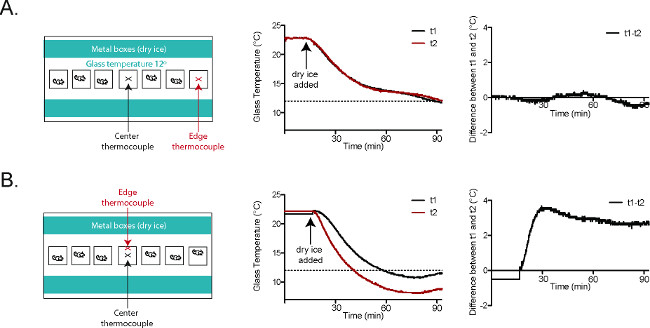
Figure 5. Glass plate temperatures are consistent in the CPA2. (A) Thermocouple t1 (black) was placed at the center of the plate. Thermocouple t2 (red) was placed in the behavioral enclosure closest to the right edge of the plate. The temperature tracings and the graph at the far right (t1-t2) show nearly identical temperatures at t1 and t2 throughout the course of the experiment. (B) Thermocouple t1 (black) was placed at the center of the plate. Thermocouple t2 (red) was placed in the central behavioral enclosure, at the wall closer to the dry ice-filled aluminum boxes. The temperature tracings and the graph at the far right (t1-t2) show that there is a roughly 3 °C difference between t1 and t2 once the plate has reached a stable temperature. Reused with permission from Brenner et al. 20142. Please click here to view a larger version of this figure.
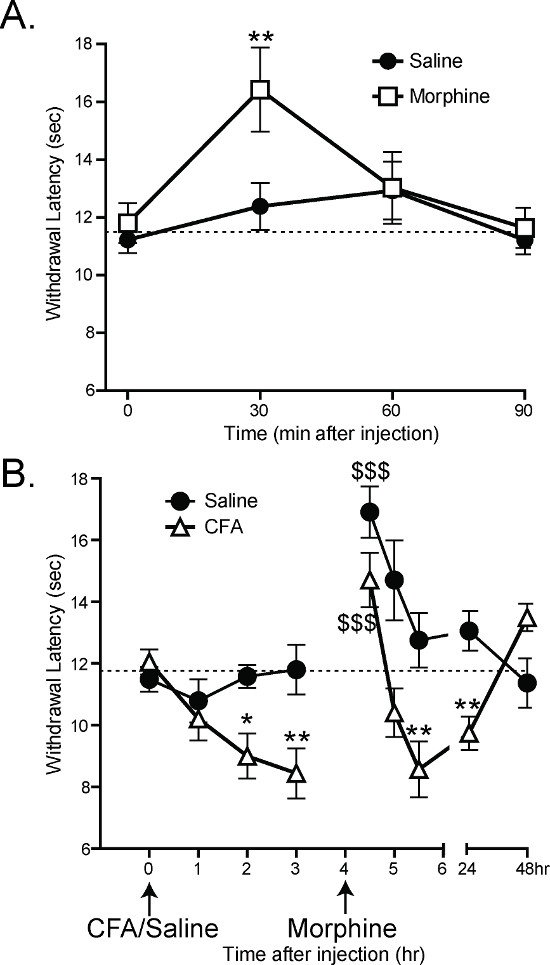
Figure 6. The CPA can measure analgesia and hypersensitivity1. (A) Subcutaneous injection of 1.5 mg/kg morphine increases the withdrawal latency of mice 30 min after injection (2-way ANOVA with Bonferroni post-hoc test; 30 min post injection **p < 0.01). 60 min after injection, there is no significant difference between morphine-injected and saline-injected mice. (B) Intraplantar injection of 10 ul Complete Freund’s Adjuvant (CFA) decreases the withdrawal latency of mice 2 and 3 hr post injection (2-way ANOVA with Bonferroni post-hoc test; *p<0.05, **p<0.01). All mice were given subcutaneous injections of morphine at 4 hr, and all withdrawal latencies at 4.5 hr were significantly higher compared with 3 hr (1-way ANOVA with Dunnet’s post-hoc test; $$$p<0.001). 5.5 hr after injection of CFA (1.5 hr after morphine injection), CFA-injected mice still had lower withdrawal latencies than saline-injected mice (2-way ANOVA with Bonferroni post-hoc test; **p<0.01). Reused with permission from Brenner et al. 20121. Please click here to view a larger version of this figure.
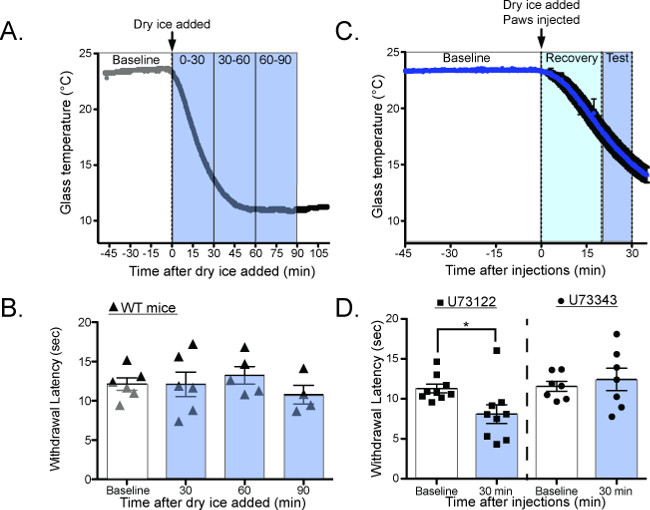
Figure 7. Measuring cold adaptation as the glass plate dynamically cools20. (A) Schematic for performing the CPA as the glass plate is cooling. Baseline temperatures are measured at RT, the dry ice containers are added to the plate, and the withdrawal latency is measured as the glass plate cools. (B) Wild-type mice have the same withdrawal latency as the glass plate cools, suggesting that they adapt to cooling temperatures faster than can be measured with the CPA (Baseline = 12.8 ± 0.3 sec, 30 min = 13.67 ± 0.9 sec, 60 min = 11.03 ± 1.0 sec, 90 min = 11.31 ± 0.6 sec, n = 3 mice; 1-way ANOVA with Bonferroni post-hoc test, no significant differences between any groups). (C) Schematic for performing the CPA as the glass plate is cooling, after intraplantar injections of the PLC inhibitor U73122 or the control compound U73343. (D) Mice have significantly lower withdrawal latencies while the plate is cooling after U73122 injection, suggesting that U73122 interferes with the ability to adapt to cooling ambient temperatures. Reused with permission from Brenner et al. 2014 20. This Figure has been reproduced with the permission of the International Association for the Study of Pain (IASP). The figure may NOT be reproduced for any other purpose without permission. Please click here to view a larger version of this figure.
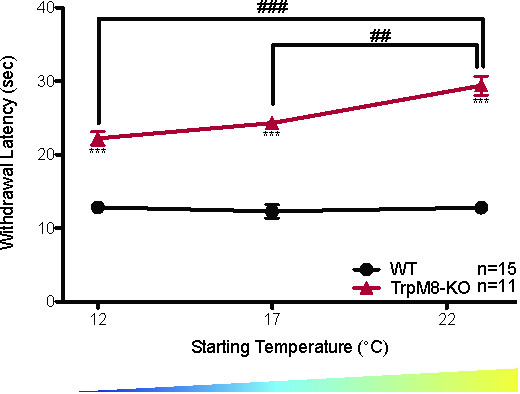
Figure 8. TRPM8-KO mice do not adapt to environmental cooling20. TRPM8-KO mice have higher withdrawal latencies than wild-type littermates at all starting temperatures measured (2-way ANOVA with Bonferroni post-hoc test; ***p < 0.001). The withdrawal latency of TRPM8-KO mice also decreases as the starting temperature decreases (1-way ANOVA with Bonferroni post-hoc test; ##p < 0.01, ###p < 0.001), while there is no significant change in the withdrawal latency of wild-type littermates as the starting temperature decreases. Reused with permission from Brenner et al. 2014 20. This Figure has been reproduced with the permission of the International Association for the Study of Pain (IASP). The figure may NOT be reproduced for any other purpose without permission. Please click here to view a larger version of this figure.
Discussion
The CPA can be used to assess cold sensitivity and cold adaptation in mice. It provides an affordable, efficient way to measure cold responses in unrestrained, acclimated animals at a wide variety of temperature ranges. It also provides an unambiguous behavioral response with an easily quantified and analyzed output variable. It has already been used to assess changes in cold sensitivity induced by inflammation1, neuropathic injury1, analgesics1, genetic knockouts20, and genetic neuron ablation studies26. It can be modified to measure responses to a variety of rates of cooling using different thicknesses of glass, and the size of the cold stimulus applied can be altered using different sized syringes.
The most critical step for performing this assay properly is cooling or warming the glass plate evenly during testing. Carefully arranging the aluminum boxes so that they are evenly spaced on either side of the animal enclosures will help generate more uniform temperatures. Marking the glass plate to standardize aluminum box placement between experiments will also improve the reproducibility of the assay, although these markings may have to be periodically recalibrated to account for seasonal variations in building temperature.
It can also be challenging to maintain a consistent glass plate temperature over the course of the experiments. Ensuring that the aluminum boxes are always evenly filled with dry ice/wet ice/warmed water will yield experiments with stable plate temperatures. Additionally, under the 17 °C testing condition it may become more difficult to maintain the temperature as the wet ice melts. We recommend draining excess water from the melted ice out of the aluminum boxes before refilling with more wet ice.
The main limitation is that the mouse paw must be in contact with the glass surface in order to ensure a consistent cold stimulus. This can be an issue in some neuropathic models, such as the chronic constriction injury, which can cause paw curling. Other challenges to obtaining reproducible results with this assay include that the mice must be fully at rest, which requires adequate acclimation periods. If the mice are too active for testing, it may help to allow longer acclimation periods. Finally, groups that are planning to utilize this assay must also ensure that there is sufficient ventilation in the room in order to prevent CO2 buildup when using large amounts of dry ice.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge contributions from the entire Gereau Lab for manuscript editing. This work is supported by NINDS funds 1F31NS078852 to DSB and NINDS fund NS42595 to RWG.
Materials
| Name | Company | Catalog Number | Comments |
| T-type thermocouple probe | Physitemp | IT-24p | Used to measure the surface temperature of the glass (http://www.physitemp.com/products/probesandwire/) |
| Glass plate | Local glass company (in St. Louis, Stemmerich Inc) | N/A | We use pyrex glass (borosilicate float). Our lab generally uses 1/4'', but 3/16'' and 1/8'' are also useful |
| Thermal Data logger | Extech | EA15 | Thermologger to keep track of glass temperature (http://www.extech.com/instruments/product.asp?catid=64&prodid=408) |
| 3mL Syringe | BD | 309657 | The top is cut off, and dry ice is compressed in the syringe to generate a cold probe |
| Computer | If using Extech logger, any Pcwill work | N/A | |
| Aluminum boxes | Washington University in St. Louis machine shop | N/A | boxes are 3' long, 4.5'' wide, and 3'' tall with a sealed lid. There is a 1/2'' hole drilled into one short side of each box, near the bottom. These holes are filled with rubber stopcocks when the boxes are filled with wet ice or hot water. |
| Heated water circulator | VWR | Any water circulator model with a pump will work | |
| 21 gauge needle | BD | 305165 | The exact needle size is not important |
| Hand timer | N/A | Any hand timer will work | |
| Mirror | N/A | Any flat mirror will work |
References
- Brenner, D. S., Golden, J. P., Gereau, R. W. A Novel Behavioral Assay for Measuring Cold Sensation in Mice. Plos ONE. 7 (6), 8 (2012).
- Brenner, D. S., Vogt, S. K., Gereau, R. W. A technique to measure cold adaptation in freely behaving mice. Journal of Neuroscience Methods. , (2014).
- Choi, Y., Yoon, T. W., Na, H. S., Kim, S. H., Chung, J. M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 59 (3), 369-376 (1994).
- Gauchan, P., Andoh, T., Kato, A., Kuraishi, Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neuroscience letters. 458 (2), 93-95 (2009).
- Carlton, S. M., Lekan, H. A., Kim, S. H., Chung, J. M. Behavioral manifestations of an experimental model for peripheral neuropathy produced by spinal nerve ligation in the primate. Pain. 56 (2), 155-166 (1994).
- Pizziketti, R. J., Pressman, N. S., Geller, E. B., Cowan, A., Adler, M. W. Rat cold water tail-flick: A novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. European Journal of Pharmacology. 119 (1-2), 23-29 (1985).
- Pinto-Ribeiro, F., Almeida, A., Pego, J. M., Cerqueira, J., Sousa, N. Chronic unpredictable stress inhibits nociception in male rats. Neuroscience letters. 359 (1-2), 73-76 (2004).
- Karashima, Y., et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 106 (4), 1273-1278 (2009).
- Knowlton, W. M., Bifolck-Fisher, A., Bautista, D. M., McKemy, D. D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 150 (2), 340-350 (2010).
- Allchorne, A. J., Broom, D. C., Woolf, C. J. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Molecular pain. 1, 36 (2005).
- Colburn, R. W., et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 54 (3), 379-386 (2007).
- Dhaka, A., Murray, A. N., Mathur, J., Earley, T. J., Petrus, M. J., Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron. 54 (3), 371-378 (2007).
- Bautista, D. M., et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 448 (7150), 204-208 (2007).
- Obata, K., et al. TrpA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. The Journal of Clinical Investigation. 115 (9), 2393-2401 (2005).
- Tang, Z., et al. Pirt functions as an endogenous regulator of TRPM8. Nature communications. 4, 2179 (2013).
- Lee, H., Iida, T., Mizuno, A., Suzuki, M., Caterina, M. J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. The Journal of neuroscience the official journal of the Society for Neuroscience. 25 (5), 1304-1310 (2005).
- Pogorzala, L. A., Mishra, S. K., Hoon, M. A. The cellular code for Mammalian thermosensation. The Journal of neuroscience the official journal of the Society for Neuroscience. 33 (13), 5533-5541 (2013).
- Yalcin, I., Charlet, A., Freund-Mercier, M. -. J., Barrot, M., Poisbeau, P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. The journal of pain official journal of the American Pain Society. 10 (7), 767-773 (2009).
- Callahan, B. L., Gil, A. S., Levesque, A., Mogil, J. S. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. The journal of pain: official journal of the American Pain Society. 9 (2), 174-184 (2008).
- Brenner, D. S., Golden, J. P., Vogt, S. K., Dhaka, A., Story, G. M., Gereau, R. W. A dynamic set point for thermal adaptation requires phospholipase C-mediated regulation of TRPM8 in vivo. Pain. , (2014).
- Patwardhan, A. M., Scotland, P. E., Akopian, A. N., Hargreaves, K. M. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 106 (44), 18820-18824 (2009).
- Fujita, F., Uchida, K., Takaishi, M., Sokabe, T., Tominaga, M. Ambient Temperature Affects the Temperature Threshold for TRPM8 Activation through Interaction of Phosphatidylinositol 4,5-Bisphosphate. Journal of Neuroscience. 33 (14), 6154-6159 (2013).
- Rohacs, T., Lopes, C. M., Michailidis, I., Logothetis, D. E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nature neuroscience. 8 (5), 626-634 (2005).
- Daniels, R. L., Takashima, Y., McKemy, D. D. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. The Journal of biological chemistry. 284 (3), 1570-1582 (2009).
- Zhang, H., et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: a novel pathway for heat hyperalgesia. The Journal of neuroscience the official journal of the Society for Neuroscience. 27 (44), 12067-12077 (2007).
- Wang, H., Zylka, M. J. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. The Journal of neuroscience the official journal of the Society for Neuroscience. 29 (42), 13202-13209 (2009).

