Functional Human Liver Preservation and Recovery by Means of Subnormothermic Machine Perfusion
Summary
We describe a method of ex vivo machine perfusion of human liver grafts at subnormothermic temperature (21 °C).
Abstract
There is currently a severe shortage of liver grafts available for transplantation. Novel organ preservation techniques are needed to expand the pool of donor livers. Machine perfusion of donor liver grafts is an alternative to traditional cold storage of livers and holds much promise as a modality to expand the donor organ pool. We have recently described the potential benefit of subnormothermic machine perfusion of human livers. Machine perfused livers showed improving function and restoration of tissue ATP levels. Additionally, machine perfusion of liver grafts at subnormothermic temperatures allows for objective assessment of the functionality and suitability of a liver for transplantation. In these ways a great many livers that were previously discarded due to their suboptimal quality can be rescued via the restorative effects of machine perfusion and utilized for transplantation. Here we describe this technique of subnormothermic machine perfusion in detail. Human liver grafts allocated for research are perfused via the hepatic artery and portal vein with an acellular oxygenated perfusate at 21 °C.
Introduction
Liver transplantation is the only curative treatment for tens of thousands of patients who suffer from end-stage liver disease. To facilitate successful transplantation, optimal preservation of the liver from the time it is procured from the donor until the time it is implanted in the recipient is necessary to prevent rapid deterioration of the graft. The current standard for liver preservation is termed ‘static cold storage’: the liver is cooled in an ice-cold preservation solution, thereby reducing the metabolism of the liver and slowing down the deleterious effects of ischemia. Although this cold storage technique has allowed for successful transplantation, organs of marginal quality such as DCD organs damaged by warm ischemia or steatosis show inferior patient outcomes 1. There is a rapidly expanding body of evidence that ex vivo machine perfusion of liver grafts as an alternative preservation modality can potentially improve outcomes for these marginal organs 2,3.
Liver transplantation has become a victim of its own success. Far more patients are referred for transplantation than there are livers available, and thousands die on the waiting list in the United States every year. Given the reality of the donor liver shortage and the increasing utilization of liver grafts of suboptimal quality for needy recipients, it is widely held that ex vivo machine perfusion of liver grafts prior to implantation holds the promise of a paradigm shift in liver transplantation. There has been a marked increase in research interest in this topic in recent years 4-8. In various European and North American centers hypothermic machine perfusion has made a clinical introduction 8 and normothermic machine perfusion at physiological temperatures has recently been applied to discarded human livers and is being translated to clinical use as well 9. Extensive development has led to the development of various protocols, while continuous optimization identifies the optimum perfusion parameters 10-12. Use of marginal-quality grafts has increased more than 10-fold over the past decade 13. When compared with the current standard for liver preservation (static cold storage), ex vivo machine perfusion provides numerous potential benefits, all of which result in much-needed expansion of the organ pool and a potentially decrease in the incidence of post-transplant complications. In particular, the biliary complications that currently plague suboptimal-quality liver grafts after transplantation remain a substantial issue 14-18.
Machine perfusion at subnormothermic conditions provides a time window to assess graft function objectively as to suitability for transplantation 19. While the liver is being perfused in an ex vivo circuit, both the perfusate and the bile produced during perfusion can be sampled for measurement of markers of organ function. In this way ‘severely compromised’ livers that are discarded for transplantation under the current criteria can be objectively assessed as to their suitability for transplant. Viability assessment potentially allows for many of these organs to be utilized for transplantation. An equally powerful benefit of machine perfusion is repair and improvement of livers that have been damaged by warm/cold ischemia. ATP is depleted very rapidly during warm and subsequent cold ischemia and can be repleted during a period of machine perfusion prior to implantation of the liver 20. The liver, with its energy stores and metabolic state replenished, is preconditioned and better prepared for the injurious effects of reperfusion injury after implantation in the recipient.
This work describes a method of ex vivo machine perfusion of human liver grafts in the laboratory, which will be useful for researchers who wish to study both the technique and beneficial effects of ex vivo machine perfusion. We make use of human donor livers that have been declined for transplantation and are then allocated for research purposes.
Standard liver procurement technique involves in situ arterial flush of the liver following aortic cross-clamping in brain dead donors (DBD) or after circulatory arrest in circulatory death donors (DCD), described in more detail elsewhere 20. Additionally, the liver is cooled during the procurement by filling the donor's abdominal cavity with ice. Flush solution preferences vary between regions, with the majority of procurements using the University of Wisconsin or Histidine-tryptophan-ketoglutarate (HTK) solution. An additional back-table flush of the portal vein improves the washout of residual blood. Livers are often procured leaving an aortic segment surrounding the celiac trunk. The gallbladder is incised, bile is aspirated, and the bile duct is flushed. The livers are packaged in sterile bags containing ice-cold preservation solution and transported in designated boxes or coolers. For representative results warm and cold ischemia time should be limited to 60 min and 12 hr, respectively. Despite routine serological screening for transmissible pathogens, standard precautions must be taken when handing human organs, samples obtained from human organs, and any waste products.
The protocol here describes subnormothermic machine perfusion using a commercially available liver perfusion device. Use of such a device allows for more rapid translation to the clinical setting and cross-validation of different protocols and device settings amongst research groups and transplant centers.
Protocol
The use of human tissue must be reviewed by an institutional review board (IRB) or equivalent. The work described here was approved and declared exempt by the Massachusetts General Hospital Institutional Review Board (No. 2011P001496).
1. Solution Preparation
- Aseptically add supplements to phenol red-free Williams’ medium E as outlined in Table 1. The solution should be prepared fresh before use. Insulin should be added just before use.
2. Back Table Preparation of the Liver
- Place an ice-filled organ bowl on a sterile, draped surface. Remove the liver from the box, leaving it in the bag of cold preservation solution. Keep the liver mostly submerged.
- Identify the hepatic artery (HA), which will be located distal to the aortic patch. Dissect free the artery to reveal various cut branches along the length using Metzenbaum scissors. Carefully dissect the entire length of the artery to prevent severing a vessel that supplies the liver. Do not cut or tie branches that do not have a visible end.
- Tie off all arterial branches not supplying the liver using silk suture ranging from size 0 to 4–0, depending on the size of the vessel. Close branches that are too short to tie or holes in the artery with a stitch of 7–0 prolene. Tie and cut the splenic and left gastric arteries close to their origin on the celiac trunk.
- Remove the aortic patch by cutting the celiac trunk directly under the patch. Cannulate the celiac trunk using the aortic cannula.
- Identify the portal vein (PV) and bluntly dissect it free. Tie off any branches and cannulate the PV with the prepared segment of size 24 tubing.
- Remove sections of diaphragm from the suprahepatic vena cava, without cutting the vein itself. Outflow from the vena cava drains directly into the organ chamber.
- Cut 2 full-circumference tissue samples (2-3 mm length) from the end of the common bile duct; snap freeze one in liquid nitrogen (store at -80 °C) and store the other in 10% buffered formalin, for tissue and histological analysis, respectively. Cannulate the common bile duct with the vessel cannula and a drain tube made of membrane oxygenator tubing.
- Identify and ligate the cystic duct with a 0 silk tie. The cystic duct is found between the common bile duct and the gall bladder.
- Connect the flush tubing set to ice-cold bags of Lactated Ringer’s (LR) solution and prime the tubing, removing all air.
- Set the flow regulator on the flush tubing to a slow trickle. Prior to connecting the flush tubing to the portal vein cannula, occlude the portal vein with fingers at the hilum and fill the cannula and vein with flush to remove the air from the portal vein. Do not elevate the bag more than 20 cm over the height of the liver during cold flushing to avoid excessive pressure on the vein.
- During the flush briefly occlude the PV at the lowest point. Examine the PV for leaks. Vessel branches can be closed off as described above. Flush the liver through the PV with a total of 2 L of ice-cold LR.
- Repeat steps 2.10, 2.11 for the HA with 1 L of LR.
3. Priming the SNMP System
- Prime the perfusion device by adding 2 L of perfusate (21°C) to the organ bowl and starting the device to prime the tubing. Follow the device’s instruction to prepare for perfusion, setting the temperature to 21 °C. Begin with pressures of 3 mmHg and 30 mmHg on the PV and HA, respectively. Open the gas tank and set to a flow of 3 L/min.
- Take a blood gas sample from both the HA and PV inflow by drawing a 0.3 ml sample from the sample ports and running it in the blood gas analysis machine according to the manufacturer’s instructions. Confirm adequate oxygenation (pO2 >700 mmHg) and pH (7.35-7.45).
- Before the liver is connected take a 1.0 ml sample of the perfusate as a t=0 measurement in an eppendorf tube and store at -80 °C. Cut two ± 250 mg wedge biopsies from the liver using a single-edged steel blade; snap freeze one in liquid nitrogen (store at –80 °C) and store the other in 10% buffered formalin. Weigh the liver before perfusion.
4. Human Liver Perfusion
- Transfer the liver to the device. Connect the PV inflow to the PV cannula, after removing air from the PV as in step 2.10. Connect the HA in a similar fashion. Set PV and HA pressure to 3 and 30 mmHg. The liver should be nearly submerged by perfusate. Cover any dry surfaces, including the inflow vessels, with wet sterile gauze to prevent dehydration
- Let the bile tubing drain into a collection container. Make sure the opening of the bile drain is at the level of the liver or lower to allow bile to run out freely.
- Target flow rates are 275–325 ml/min.kg and 50–100 ml/min.kg for the PV and HA respectively once the liver has warmed to 21 °C. Since every liver reacts differently to perfusion, monitor the flow closely during the first minutes. Increase or decrease the pressure on either of the vessels if the target flow rates are not reached. Do not exceed 50 mmHg on the HA and 5 mmHg on the PV.
- Samples can be taken from the liver tissue, perfusate and bile at the investigators preference. We recommend at a minimum the following sample collection regimen during perfusion.
- Tissue biopsies, n = 2 x 250 mg, every hour. Storage: snap freeze one in liquid nitrogen and store at –80 °C long-term. Additionally, take another biopsy before and after perfusion and fix in 10% buffered formalin (n = 1)
- Perfusate samples, n = 2x 1 ml, every 15 min for the first hour and every 30 min thereafter. Draw samples from the PV inflow sample port. Storage: –80 °C long-term.
- Blood gas analysis of PV and HA inflow, and vena cava outflow. n = 3 x 0.3 ml, every 30 min. Draw samples from both the PV and HA sample ports. Draw a 0.3 ml sample from the vena cava by inserting a syringe into the vein and directly run in the blood gas analyzer. Use the output to ensure adequate oxygenation and pH.
- Bile production, n = 1 x 1 ml, every hr. Visually quantify bile production every hour and take a sample from the collection container. Renew the container after sampling. Storage: dry ice and –80 °C long-term.
- Continue perfusion for 3 hr. Monitoring the pressure, pH and oxygenation and taking samples throughout. Adjust the he pH by adding sodium bicarbonate to the perfusate.
- At the end of perfusion take the final samples while the liver is being perfused. Disconnect the liver and remove the bile duct cannula. Take 2 post-perfusion tissue samples of the bile duct as described before for storage at –80 °C and in 10% buffered formalin.
- Discard the human liver following proper biohazard disposal guidelines.
Representative Results
A number of observations and analyses can be performed on the liver during perfusion, including direct real time observations, such as flow rates and bile production; real time measurements, such as gas analysis of the perfusate, and post-hoc measurements that are made after sample collection including biochemical analysis of the perfusate and tissue and histological analysis. Results mentioned here are from 22 perfused human livers. Livers were rejected for transplantation for various reasons, including donor age, excessive warm ischemic time, biopsy results (steatosis, inflammation, fibrosis) and for logistical reasons. 18 livers were procured following cardiac death, and 4 following brain death. In both cases, donors were pretreated with 30,000 units of heparin and flushed in situ and on the back table with UW solution. Mean cold ischemic time was 531 ± 237(SD) min and the mean warm ischemic time was 27 ± 10(SD) min, measured from withdrawal of life support to cold flush. Real time observations and measurements can be used to assess the liver during perfusion, while post-hoc measurements are revealed after the perfusion.
Real time observations
Flow through the liver begins lower than the target flow rates, as a result of a higher resistance in the cold liver. Using a pressure of 3 mmHg on the PV and 30 mmHg on the HA the target flows can generally be achieved once the liver has warmed up to 21 °C after 60 min of perfusion (Figure 1A). Bile flow can generally be observed within 10 min of perfusion and is produced steadily during perfusion (Figure 1B). Bile quantity depends on the quality of the liver and ranges from 0.3 ml/ hr/ kg liver to 18 ml/min/kg. In livers with longer warm ischemic time, bile flow will tend to taper off, while shorter warm ischemic time results in a more steady or even increasing bile production.
Real time measurements
Direct and frequent measurement of the perfusate by blood gas analysis in essential for both experimental purposes as well as maintaining adequate perfusion conditions, importantly oxygenation and pH. Dissolved oxygen partial pressure should be greater than 700 mmHg on the inflow of both the PV and HA. Outflow oxygen pressure, measured in the vena cava, generally decreases with longer perfusion, reflecting an increasing oxygen uptake. Oxygen uptake rates can be calculated as described previously 13 and ranged from 0.5–2.2 ml O2/ min/ kg at the beginning of perfusion to 2.4-9.7 ml O2/ min/ kg at t =3 hr (Figure 1C). A drop in pH is observed in the first 30 min (Figure 1D), primarily as a result of lactate release in the perfusate. This can be supported by supplementation with 8.4% sodium bicarbonate and after about 90 min the pH falls back into normal range. Commonly, 30-50 ml of 8.4% sodium bicarbonate is required. Lactate concentration increases rapidly in the first 15-30 min, but begins to decease after the first hour (Figure 1E).
Post-hoc measurements
Hepatic transaminases such as ALT can be measured in the perfusate. In the first 30 min a large increase of ALT is generally observed which reflects the washout of ALT that was released during ischemia (Figure 1F). ALT was shown to correlate well with warm ischemic time 13. Machine perfusion increased ATP content 2.8 fold, reflecting a recovery energy status (Figure 1G). H&E histological analysis reveals no additional injury sustained during machine perfusion (Figure 1H, I). It should be noted that the biopsy regimen proposed in this protocol is for research purposes and may not be applicable for clinical purposes.
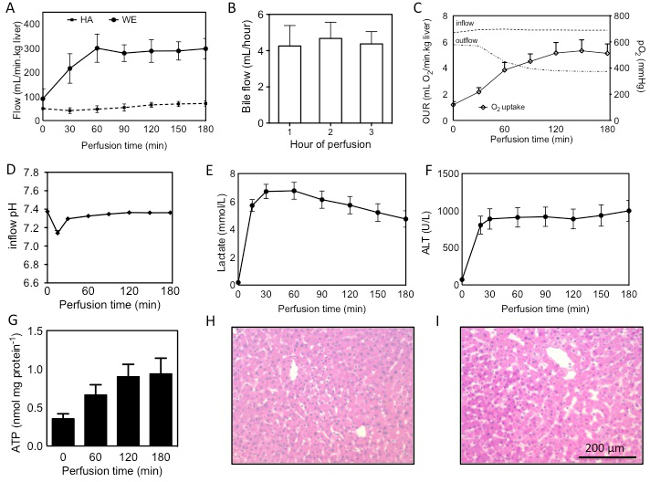
Figure 1: Assessment of human livers during machine perfusion. Flow through the PV and HA during SNMP (A), bile production, quantified per hour of perfusion (B), oxygen uprate rate (OUR), calculated from the difference in inflow (PV+HA) and outflow (vena cava), interrupted lines show partial oxygen pressures in the in- and outflow during perfusion (C), pH and lactate during perfusion (D, E), release of ALT into the perfusate (F), ATP content measured in tissue from hourly biopsies (G) and H&E stains of the liver (54 year old DCD, 19 min warm ischemia, 559 min cold ischemia) before (H) and after (I) perfusion. Results are presented as mean ± SEM. Please click here to view a larger version of this figure.
Discussion
In an attempt to recover livers injured during ischemia we developed a SNMP system that can be employed after a period of cold storage. Subnormothermic machine perfusion offers a viable alternative to conventional cold storage, as well as hypothermic and normothermic machine perfusion modalities. Various different systems exist; all offer different advantages and disadvantages 3,9,20. SNMP allows for perfusion without an oxygen carrier, as metabolic oxygen demands at 21 °C are met by active oxygenation of the perfusate.
Although reduced under subnormothermic conditions, metabolism is substantial and requires the support of a nutrient-rich perfusion solution. Traditional perfusion solutions, such as the Belzer machine perfusion solution, are generally minimal in composition and are designed for cold perfusion. Williams’ Medium E has been used as a hepatocyte culture medium for many years, and contains components that are universal for supporting cellular function, in particular under warm ex vivo conditions.
Measurements made during machine perfusion are reflective of the function of the organ. Directly observable parameters such as bile production and oxygen uptake are real time measurements that can be used to assess the liver pre-transplantation. Similarly, markers of cellular injury and ischemia (K+, lactate release) can be measured directly in the perfusion solution and may be indicative of organ function 20. As machine perfusion technology develops further and achieves more widespread clinical application, accurate correlations between ex vivo function and clinical outcome can be made and perfusion parameters will be useful in aiding decisions to transplant or reject marginal-quality livers. Moreover, as point-of care analytical tools advance, more sophisticated analysis will become available directly during machine perfusion 21.
In this work we show that livers can be supported in the SNMP system with minimal injury to the liver, reflected by histology and release of ALT. Functional recovery of the liver is best reflected by ATP, which has been shown to correlate to liver viability and is strongly suggestive of transplant success in animal models 22. Ex vivo and pre-transplant recovery of liver grafts would allow a significant expansion of the donor liver pool, correcting the disparity between supply and demand of donor livers in transplantation.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Funding from the US National Institutes of Health (grants R01EB008678, R01DK096075, R01DK084053, R00DK088962 and F32 DK103500), CIMIT Project No. 12-1732 and the Shriners Hospitals for Children is gratefully acknowledged. We would like to gratefully acknowledge the New England Organ Bank for supporting this work.
Materials
| Liver Assist perfusion device | Organ Assist B.V. | Liver Assist | |
| Liver Assist disposable set | Organ Assist B.V. | Liver Assist disposable | |
| Williams’ Medium E | Sigma | W1878–6x500ml | |
| Insulin | Eli Lilly & Co | Humulin R U-100 | |
| Penicillin/Streptomycin 5,000 U/ml | Life Technologies | 15070-063 | |
| L-glutamine | Invitrogen | 25030-156 | |
| Hydrocortisone | MGH pharmacy | 7750500 | |
| Carbogen gas tank 95% O2/5% CO2 | Airgas | ZO2OX9522000043 | |
| Specialty gas regulator | Airgas | Y11244D580 | |
| Lactated Ringer’s solution | Baxter | 2B2324X | |
| 10% neutral buffered formalin | Fischer Scientific | 316-155 | |
| Toothed Adson forceps | Roboz | RS-5234 | |
| Debakey tissue forceps, 7.75”, 2.25 mm | Roboz | RS-7562 | |
| Metzenbaum Scissors 7" Curved SureCut Tungsten | Roboz | RS-6965SC | |
| Castroviejo Needle holder 5.5–7” | Fine Science Tools | 12565-14 | |
| 0 blackbraided silk sutures | Ethicon | SA66G | |
| 4-0 nylon suture, Nurolon RB1 | Ethicon | C554D | |
| Blood gas analysis machine | Siemens | RapidPoint 500 | |
| Balance scale | Cole Parmer | EW-10000-12 | |
| Pressure display box | Medtronic | 66000 | |
| Disposable pressure display sets | Medtronic | 61000 | |
| Handheld thermocouple thermometer and probe | Cole Parmer | EW-91500-04 and EW-08516-55 | |
| Acorn-tipped vessel cannula, 4 mm | Medtronic | 30005 | |
| Irrigation set flush tubing | Hospira | 06543-01 | |
| Mixing bowl, 4L | Cole Parmer | EW-07300-40 | |
References
- Merion, R. M., Pelletier, S. J., Goodrich, N., Englesbe, M. J., Delmonico, F. L. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 244 (4), 555-562 (2006).
- Guarrera, J. V., et al. Hypothermic machine preservation facilitates successful transplantation of ‘orphan’ extended criteria donor livers. Am J Transplant. 15, 161-169 (2015).
- Dutkowski, P., Schlegel, A., de Oliveira, M., Müllhaupt, B., Clavien, P. -. A. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 60 (4), 765-772 (2013).
- Dutkowski, P., Clavien, P. -. A. Solutions to Shortage of Liver Grafts for Transplantation. Br J Surg. 101 (7), 739-774 (2014).
- Vogel, T., Brockmann, J. G., Friend, P. J. Ex-vivo Normothermic Liver Perfusion: An Update. Curr Opin Organ Transplant. 15 (2), 167-172 (2010).
- Monbaliu, D., Brassil, J. Machine Perfusion of the Liver: Past, Present, and Future. Curr Opin Organ Transplant. 15 (2), 160-166 (2010).
- Matsuno, M., Uchida, K., Furukawa, H. Impact of Machine Perfusion Preservation of Liver Grafts From Donation After Cardiac Death. Transplant Proc. 46 (4), 1099-1103 (2014).
- Schlegel, A., Dutkowski, P. Role of hypothermic machine perfusion in liver transplantation. Transplant Int. , (2014).
- Op den Dries, S., et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 13, 1327-1335 (2013).
- Bruinsma, B. G., et al. Antibiotic prophylaxis in (sub)normothermic organ preservation: In vitro efficacy and toxicity of cephalosporins. Transplantation. 95 (8), 1064-1069 (2013).
- Post, I. C., Dirkes, M. C., Heger, M., Bezemer, R., van’t Leven, J., van Gulik, T. M. Optimal flow and pressure management in machine perfusion systems for organ preservation. Ann Biomed Eng. 40 (12), 2698-2707 (2012).
- Post, I. C., et al. Endothelial cell preservation at hypothermic to normothermic conditions using clinical and experimental organ preservation solutions. Exp Cell Res. 319 (17), 2501-2513 (2013).
- Klein, A. S., et al. Organ Donation and Utilization in the United States, 1999-2008. Am J Transplant. 10 (4), 973-986 (2010).
- Jay, C., et al. The Increased Costs of Donation After Cardiac Death Liver Transplantation. Ann Surg. 251 (4), 743-748 (2010).
- Seehofer, D., Eurich, D., Veltzke-Schlieker, W., Neuhaus, P. Biliary Complications After Liver Transplantation: Old Problems and New Challenges. Am J Transplant. 13, 253-265 (2013).
- Morrissey, P., Monaco, A. Donation After Circulatory Death: Current Practices, Ongoing Challenges and Potential Improvement. Transplantation. 97 (3), 258-264 (2014).
- Verdonk, R., Buis, C., Porte, R., Haagsma, E. Biliary complications after liver transplantation: A review. Scand J Gastroenterol. 41, 89-101 (2006).
- Pine, J., et al. Liver Transplantation Following Donation After Cardiac Death: An Analysis Using Matched Pairs. Liver Transpl. 15 (9), 1072-1082 (2009).
- Sutton, M. E., et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 11, e110642 (2014).
- Bruinsma, B. G., et al. Subnormothermic Machine Perfusion for Ex Vivo Preservation and Recovery of the Human Liver for Transplantation. Am J Transplant. 14, 1400-1409 (2014).
- Bruinsma, B. G., Yarmush, M. L., Uygun, K. Organomatics and organometrics: Novel platforms for long-term whole-organ culture. Technology. 02 (1), 13-22 (2014).
- Berendsen, T. A., et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res. 1 (1), 6 (2012).

