Method of Studying Palatal Fusion using Static Organ Culture
Summary
Studies of palate development are motivated by the incidence of cleft palate, a birth defect that imposes a tremendous health burden and can leave lasting disfigurement. We demonstrate here a technique to culture palatal shelves that can be used to study different signaling pathways involved in palatal development and fusion.
Abstract
Cleft lip and palate are among the most common of all birth defects. The secondary palate forms from mesenchymal shelves covered with epithelium that adheres to form the midline epithelial seam (MES). The theories suggest that MES cells follow an epithelial to mesenchymal transition (EMT), apoptosis and migration, making a fused palate 1. Complete disintegration of the MES is the final essential phase of palatal confluence with surrounding mesenchymal cells. We provide a method for palate organ culture. The developed in vitro protocol allows the study of the biological and molecular processes during fusion. The applications of this technique are numerous, including evaluating responses to exogenous chemical agents, effects of regulatory and growth factors and specific proteins. Palatal organ culture has a number of advantages including manipulation at different stages of development that is not possible using in vivo studies.
Introduction
Orofacial clefts are the most predominant craniofacial birth defects. Also, taking in consideration all possible craniofacial defects, these are the second most common birth anomaly in newborns 2. Cleft palates occur at approximately 1 in every 700 births in the United States (US) every year, the incidence of cleft palate is equal to 475 children born with cleft palates per month or 15 children with clefts per day 3. Approximately 1% of kids born around the world each year exhibit some form of craniofacial dysmorphology.
Clefts of the palate and the lip need a very expensive and complicated procedure with lifelong implications for patients who have this anomaly. The estimated cost for each patient with oral cleft is approximately $100,0004. The treatment of a patient with cleft lip and palate requires a team of doctors including craniofacial surgeons, otolaryngologists, geneticists, anesthesiologists, speech-language pathologists, nutritionists, orthodontists, prosthodontists, psychologists, neurosurgeons, and ophthalmologists.
In palatogenesis, the secondary palate arises as paired outgrowths, which initially grow vertically and undergo palatal shelf elevation above the dorsum of the tongue. Following elevation, the paired palatal shelves grow towards the midline (at E14.5 –E15 in mice and week 9 in humans). The medial edge epithelium (MEE) that covers the shelf tip adheres forming the midline epithelial seam.
This is followed by epithelial to mesenchymal transition and/or apoptosis to allow mesenchymal confluence. Adhesion of opposing MEE is an essential event whose alteration causes cleft palate. However, only few studies investigated the initial adhesion of palatal shelves5. The hard palate forms by differentiation of mesenchymal cells into osteoblast. The abnormal development of the palate can produce cleft palates with or without involvement of the lip.
Palate organ culture techniques had been used widely for many labs over the past 30 years6,7.
In this protocol we describe in details a method of palatal dissection and static organ culture. The advantage of a motionless organ culture is that it allows palatal shelves to fuse. This technique had been used successfully in our laboratory for many fusion and signaling experiments8,9. However the scope of the technique is vast and can be used whenever static organ culture system is required, including the evaluation of responses to exogenous chemical agents, the effects of regulatory growth factors in different pathways and specific proteins.
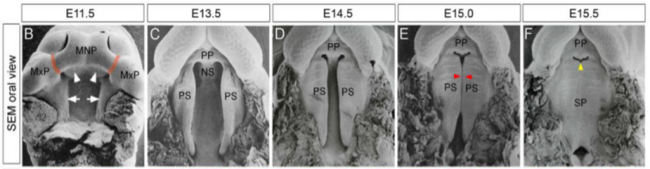
Figure 1. Mouse Palatogenesis. Developmental stages of the mice palate. (B-F) Scanning electron micrographs (SEM) of the secondary palate at representative developmental times. Red arrows: show the initial part of palatal adhesion and fusion. Yellow arrows: point to the space between the primary and secondary palates that will disappear after fusion (reprinted from Kaufman11 with permission from PLOS one).
Protocol
All procedures described must be performed in accordance with the guidelines and regulations for the use of vertebrate animals, including prior approval by the local Institutional Animal Care and Use Committee.
1. Preparation of Dissecting Instruments and Culture Media
- Prewash all instruments to be used in dissection with 3% hydrogen peroxide and then autoclave at 121 °C for 20 min. Filter BGJb media with antibiotic and antimycotic solution using a 0.22 µm pore-sized filter with vacuum suction in a hood.
2. Preparation of Culture Equipment
- Cut polycarbonate membrane filters that will support the palatal organs in small triangles, spray with 70% alcohol and allow them to dry. Prepare Center-Well Organ Culture Dish (60×15 mm) by placing a clean and sterilized equilateral triangular-shaped wire grid (20 mm) over the organ culture plate. Then fill the well with 1 ml of BGJb media culture media. NOTE: The media should be sufficient to reach the level of the grid without submerging the palates. Place the membrane filters, shiny side down, on top of the grid (3-4 membranes per grid).Depending on the experiment, treatment (proteins, antibodies or inhibitors) can be added to the media
3. Mouse Embryo Collection and Preparation for Culture
- To obtain wild type mouse embryos, use timed 13.5 pregnant CD-1 mouse genetic background strain. Clean the dissection area with 70% ethanol.
NOTE: Other strains of mice can be used. We prefer CD-1 mice because of the bigger litter number. - Euthanize pregnant mice at E13.5 dpc (days post coitus) with 5% Isoflurane followed by cervical dislocation to insure death using approved protocols.
- Spray 70% ethanol on the ventral abdominal surface to avoid having the abdominal hair stick to the instruments. Then open the abdominal cavity ventrally using a sharp-blunt operating scissors and micro-dissecting forceps to locate the uterus. Using the forceps, lift the entire uterus and separate it from the body by cutting at the uterine body and at the tips of the uterine horns with a light operating scissors.
- Place the uterus on a clean paper towel over ice (According to the protocol guide for care and use of laboratory animals). Cover the uterus with another clean paper towel to prevent contamination. Sterilize the instruments using 70% ethanol spray before further use. The uterus and the embryos can be transported to the laboratory on ice.
- In an open laminar flow hood, carefully make a small opening in the yolk sac using sharp-blunt operating scissors and micro-dissecting forceps. Make the opening wider and then gently push it to exteriorize the embryo from the yolk sac.
- Transfer the embryos immediately to a Petri dish with cold phosphate-buffered Saline 1X (PBS) pH 7.4. To assure the proper stage, examine the embryo under a dissecting microscope for the body, head size and limb morphology.
NOTE: Embryos that are not at the right stage of development are not used for the experiment.
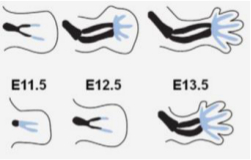
Figure 2. Limb Bud Morphology. The fore and hind limbs are checked for the degree of webbing indentation between the digits. At E13.5, all the digit condensations are prominent, with the webbing and distal indentations between digits appearing very distinct. The hind digits are behind by a half-day. 12 Upper row: forelimb, lower row: hind limb.
4. Palate Dissection
- Place one embryo at a time in a petri dish with PBS under the dissecting microscope. Using small dissecting scissors and tweezers separate the head from the body of the embryos.
- Carefully holding the mouse cranium above eye level with forceps (avoiding the maxillary process) make two incisions on both sides of the lip line. Then remove the mandible and tongue. Isolate the maxillary process region by making a cut at eye level and remove the brain, eyes, nasal septum and adjoining tissue.
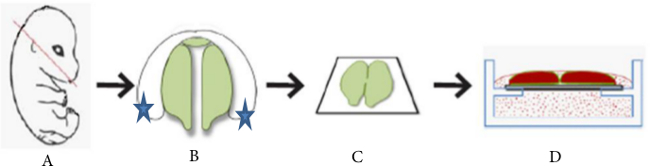
Figure 3.Palate tissue collected from 13.5 embryos, when the palate shelves had elevated but not made contact. The palatal shelves were placed in organ culture on a filter at the air media interface for 72 hr in the static model. (A) Level of dissection on E13.5 embryo. (B) Blue stars demarcate areas to hold the tissue with forceps during dissection. (C) View of dissected palatal shelves placed on a trapezoid filter membrane. (D) Side view of culture dish after placement of the dissected palates and addition of culture media.
- Place the palatal oral side up and remove the nasal septum and the tissue around it, keeping the primary palate attached to help orient it from anterior to posterior. Use a spoon spatula and very carefully transfer the palatal shelves (nasal side down) to the prepared filters on the grids in the culture dishes. NOTE: Adjust volume of media to allow proper air-media interface.
- Under the stereo microscope, dissect the primary palate and use tweezers to place the palatal shelves close to each other. The palatal shelves will not fuse if they are not placed in contact. Cut the triangular membrane filter where the anterior part of the palate is located. The trapezoid shape will aid in localizing the anterior portion of the palate.
- Culture the palatal shelves individually or with two or three in the same organ culture dish using BGJb culture media.
5. Culturing Mouse Palatal Shelves
- Incubate the tissues at 37 °C in a humidified gas mixture (5% CO2 and 95% air) for 72 hr, and change the medium every 24 hr.
6. Palate Processing for Histological Analysis
- Fix the palates after 72 hr in culture with 4% formaldehyde/phosphate-buffered saline O/N at 4° C. Then, gently with a transfer pipette wash the tissue in the culture dish with PBS. Wrap the palates in a piece of laboratory wipes and place in embedding cassettes.
NOTE: Wrapping the palates in laboratory wipes prevent their loss during the dehydration process. - Dehydrate the tissue following 70%, 80% and up to 100% ethanol washes for 1 hr, followed by the regular paraffin embedding procedure. For embedding, place the anterior part of the palate facing the tray.
- Cut Serial 6 µm sections in the coronal orientation from anterior to posterior. A complete section palate will yield 350-450 sections. Stain the sections with hematoxylin and eosin (H&E).10 Score every 20th section using the previously described Mean Fusion Score (MFS) scale (Table 1). 8
Representative Results
The technique can be applied to different kinds of experiments.The following study was designed to test the teratogenic effect of nicotine in vitro on palatal fusion. Two strains of mice were utilized for this experiment, CD1 and C57.Palatal tissue was treated with 0.06 and 6 mM concentrations of nicotine hemisulfate. It was observed that palatal fusion patterns and timing were different in the two different strains. In CD1 mice, palatal shelves from the control group continued fusion in time dependent manner. However, in the treated group nicotine delayed fusion especially at 6mM nicotine concentration (Figure 4A). Histology sections of CD1 mouse palates showed total fusion after 72 hr in the control specimens. At lower doses of nicotine the palatal shelves adhere but the epithelial cells remain after 3 days in culture. Palatal shelves incubated at high doses of nicotine did not make contact after 72 hr of incubation.
In C57 mice, palatal shelves from the control group continued fusion in time dependent manner. However, palatal shelves of C57 mice showed higher score at 6 mM concentration when the specimens were incubated for 48 hr in comparison with the specimens incubated at 0.6 mM concentration of nicotine for the same period of time (Figure 4B). Histology sections of C57 mice palates showed that in treatment groups a continuous epithelial seam persisted in the midline. Fusion was observed just in the control group at 72 hr.
We observed that palatal fusion in both kinds of mice was affected by high doses of nicotine. However, palates from C57 were more sensible to nicotine. In conclusion, our results demonstrate that genetic background of mice influences nicotine responses in palatal tissues.
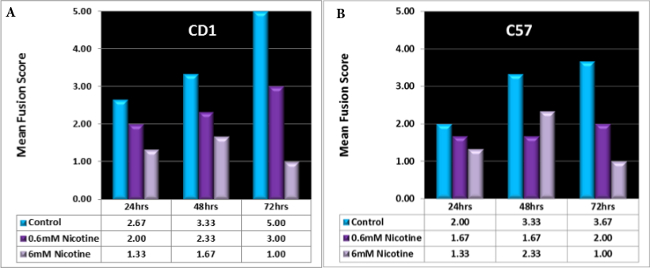
Figure 4. Two strains of mouse palates cultured in the presence of nicotine for 24, 48, or 72 hr. The mouse strains responded to the nicotine in different degrees. All palates exposed to the high dose of nicotine (6 mM) failed to fuse and had low mean fusion score (MFS) .Note that at 24 hr, none of the palates had closed and the MFS ranged from 1 (palatal shelves not touching) to 3 (partial fusion) in all groups. By 48 hr, many of the control palates had partially fused (scores over 3) and by 72 hr, the CD1 palates were completely fused (score of 5), but the C57 controls had not completely fused (3.67). (Unpublished data from Maria Serrano and Dr Kathy Svoboda).
| Score | Criteria |
| 1 | Non-fusion with no adhesion. |
| 2 | Non-fusion with some apparent adhesion. |
| 3 | Adhesion with some disintegration of MEE layers and clear partial mesenchymal confluence. |
| 4 | Complete fusion with some traces of MEE cells or seam remaining. |
| 5 | Complete fusion with no evidence of MEE cells or seam visible. |
Table 1:Mean Fusion Score (MFS) scale (Kang and Svoboda, 2002). Calculate the scores for each palate for the anterior (sections 1-150), middle (sections 150-300) and posterior (sections 300-450). 8
Discussion
The protocol in this article provides a method of dissecting palatal shelves from embryos at embryonic day 13.5. Palatal shelves from mouse embryos were cultured in a serum-free culture media in an atmosphere of 95% O2 5% CO2 at 37 °C. Successful palatal dissection is critically dependent on multiple factors at each step during the procedure from the embryonic time of the euthanized mice to the completion of the culture. One of the most important factors influencing palatal fusion is the time taken to start the organ culture; embryos need to be at embryonic day 13.5 as the secondary palate shelves are horizontal, but have not started to fuse (Figure 1).
Another critical point during the procedure is the exteriorization of embryos from the yolk sac. This step requires great care to prevent damage to the facial structures. Once the embryo is removed from the sac, the head is removed from the body and placed in a petri dish to be viewed with a stereomicroscope with fiber optic illumination from both sides. The mandible and the tongue are removed, exposing the palate. The brain and residual tissue are removed at eye level. At this point it is important to consider the epithelial layer around the shelves. It is necessary to manipulate the tissue very carefully to keep the integrity of these cells. Epithelial cells are responsible for adherence and formation of the MES.
Each dissected palate is turned oral side down to clean any cartilage from the nasal septum. This tissue is on the anterior side of the palate and appears whiter and more condensed. Palatal shelves free of unwanted tissue are moved to a culture dish and placed on top of the filter membrane. The position of the shelves is checked again under the stereo dissecting microscope. They must be pushed together to allow adherence and further on palatal fusion.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors do not have any acknowledgements.
Materials
| BGJb | Invitrogen | ||
| Penicillin/Streptomycin | Lonza Inc. | 17-602E | 100X |
| Petri dishes | VWR | 25384-088 | 100x15mm |
| Center-well organ culture dish | Falcon | #353037 | 60×15 mm |
| Wire triangular grid | Custom made with stainless steel wire mesh to fit the organ culture dish well. | ||
| Polycarbonate filter | GE& Water and Process Technologies | K04BP04700 | Black 0.4 micron, 47 mm |
| Stereo microscope | ZEISS Stemi SR | ||
| Culture hood | NUAIRE Laminar Flow | ||
| Microdissecting forceps | Dumont Medical | #5 | |
| Microdissecting scissors | Kent Scientific Corporation | INS14003-G | Vannas Scissors, straight, 8cm long, 0.1mm tips, 5mm blades, German made |
| Microdissecting scissors | Kent Scientific Corporation | INS500086 | Vannas Scissors, straight, 8.5cm long, 0.025mm x 0.015mm tips, 7mm blades |
| Microdissecting scissors | Kent Scientific Corporation | INS14127-G | Spring scissors curved curved, 10.5cm long, 8mm blades, German made |
| Surgical currete (spoon spatula) | Hu-friedy | CM 2/4 | |
| Protector laboratory hood | Labconco | ||
| Incubator | Thermo Scientific | Farma | |
| Series11 water Jacket | |||
| Co2 incubator | |||
| PBS | GIBCO | 10010-023 | 1X |
| Fiber optic Light source | Fiber-light Dolan-Jenner | PL-750 | |
| Embedding cassette | Statlab | EC301 | |
| Kim Wipes | VWR | 470173-504 | |
References
- Nawshad, A. Palatal seam disintegration to die or not to die that is no longer the question. Developmental dynamics an official publication of the American Association of Anatomists. 237, 2643-2656 (2008).
- Strong, E. B., Buckmiller, L. M. Management of the cleft palate. Facial plastic surgery clinics of North America. 9, 15-25 (2001).
- Witt, P. D., Marsh, J. L. Advances in assessing outcome of surgical repair of cleft lip and cleft palate. Plastic and reconstructive surgery. 100, 1907-1917 (1997).
- 4miloro, . principles of oral and maxillofafcial surgery. 209, 231-249 (1984).
- Mima, J. Regulation of the epithelial adhesion molecule CEACAM1 is important for palate formation. PloS one. 8, e61653 (2013).
- Carette, M. J., Ferguson, M. W. The fate of medial edge epithelial cells during palatal fusion in vitro an analysis by DiI labelling and confocal microscopy. Development (Cambridge, England). 114, 379-388 (1992).
- Ferguson, M. W., Honig, L. S., Slavkin, H. C. Differentiation of cultured palatal shelves from alligator chick and mouse embryos. The Anatomical record. 209, 231-249 (1984).
- San Miguel, S. Ephrin reverse signaling controls palate fusion via a PI3 kinase dependent mechanism. Developmental dynamics an official publication of the American Association of Anatomists. 240, 357-364 (2011).
- Kang, P., Svoboda, K. K. PI 3 kinase activity is required for epithelial mesenchymal transformation during palate fusion. Developmental dynamics an official publication of the American Association of Anatomists. 225, 316-322 (2002).
- RD, L. . Histopathologic Technic and Practical Histochemistry. , (1965).
- Kaufman, M. H. . The Atlas of Mouse Development. , (1992).
- Taher, L. Global gene expression analysis of murine limb development. PloS one. 6, e28358 (2011).

