Isolation of Sertoli Cells and Peritubular Cells from Rat Testes
Summary
Primary Sertoli cells are required for studying testis-immune privilege, signal transduction during inflammation or infection, and utilization of their immunoprotective properties. Here we describe an enzyme-based protocol for the isolation of highly purified primary Sertoli cells and peritubular cells from rat testes.
Abstract
The testis, and in particular the male gamete, challenges the immune system in a unique way because differentiated sperm first appear at the time of puberty – more than ten years after the establishment of systemic immune tolerance. Spermatogenic cells express a number of proteins that may be seen as non-self by the immune system. The testis must then be able to establish tolerance to these neo-antigens on the one hand but still be able to protect itself from infections and tumor development on the other hand. Therefore the testis is one of a few immune privileged sites in the body that tolerate foreign antigens without evoking a detrimental inflammatory immune response. Sertoli cells play a key role for the maintenance of this immune privileged environment of the testis and also prolong survival of cotransplanted cells in a foreign environment. Therefore primary Sertoli cells are an important tool for studying the immune privilege of the testis that cannot be easily replaced by established cell lines or other cellular models. Here we present a detailed and comprehensive protocol for the isolation of Sertoli cells – and peritubular cells if desired – from rat testes within a single day.
Introduction
Testes produce male gametes and sexual hormones, i.e., androgens. The organ is composed of two compartments. In the interstitial compartment, that represents about 10-12% of total testicular volume1, steroidogenesis takes place within the Leydig cells. The tubular compartment represents about 60-80% of the testicular volume1 and contains germ cells and two types of somatic cells – peritubular cells and Sertoli cells. The testis is divided by connective tissue septa into 250-300 lobules, each containing 1-3 highly convoluted seminiferous tubules. These tubules are enclosed by a basal membrane, a sheet of collagen, and a circumferential layer of peritubular cells (Figure 1A).
The germinal epithelium is located on the luminal side of the basal membrane. Sertoli cells are large elongated cells that span the whole germinal epithelium from the basal membrane to the lumen. They are strongly attached to the basal membrane and form a continuous cellular sheet through basolateral organized tight junctions that occludes the germinal epithelium from the interstitium and represents the blood-testis barrier. Sertoli cells have prominent cytoplasmic projections and ramifications that enable them to get into tight morphological and functional contact with a species-specific but constant number of germ cells. Diploid germinal stem cells proliferate and differentiate into spermatogonia.
During meiosis I short-lived tetraploid spermatocytes are generated that develop further into four haploid spermatids during meiosis II. All germ cells are interconnected by cytoplasmic bridges so that they form a cellular net. The principal event during maturation of spermatids is the extrusion of large parts of the cytoplasm, forming residual bodies, in a process called spermiogenesis. Residual bodies are phagocytosed by Sertoli cells. Late spermatids are then released into the tubular lumen and transported into the epididymis for further maturation. Sertoli cells and germ cells appear to mutually coordinate spermatogenesis topographically and functionally.
Preparation of individual testicular cell types started almost a century ago when small testicular pieces were cultivated and cell types identified by microscopy2. By careful dissection of the tubules after opening the tunica albuginea using fine-tipped forceps it was later possible to separate tubular and interstitial compartments3. In 1975 Welsh and Wiebe introduced a collagenase treatment in order to free the tubules from adhering interstitial tissue and a pancreatin treatment for removal of the outer peritubular cell layer4. From early on young immature rats (around 20 days old) had been used in which the Sertoli cells comprise a large fraction of the tubular cell population because spermatogenesis has not begun yet. At this age rat Sertoli cells cease to divide, and tight junctions between neighboring cells form so that the blood-testis barrier is established5.
Independent of Welsh and Wiebe Dorrington et al. introduced a combination of trypsin and deoxyribonuclease followed by soybeen trypsin inhibitor and collagenase treatment that was published in the same year6. Both groups also used mechanical force (drawing the digested tubular fragments repeatedly through a syringe needle or a Pasteur pipette respectively) in order to produce a homogeneous cell suspension for plating that contains approximately 70% Sertoli cells. After 3 days in culture, using a medium that is serum-free, the percentage of Sertoli cells increases to approximately 90%. This could be largely attributed to the death of contaminating germ cells. Residual peritubular cells (PTCs), however, stay firmly attached via their extracellular matrix. PTCs, but not Sertoli cells, are known to produce fibronectin that can serve as a marker protein for estimating contamination with PTCs. Therefore, Tung et al. reasoned that an additional hyaluronidase treatment might improve the purity of the Sertoli cell fraction7. Indeed they could show that an additional treatment was able to reduce contamination by PTCs approximately 20-fold, which becomes particularly evident when in comparison a serum-containing medium is used for cultivating purified Sertoli cells. From then on the improved procedure of Tung et al. became the prevailing protocol and was extensively used by other major groups in the field8 (Figure 1B).
During collagenase treatment the majority of PTCs are released and can be isolated in parallel to Sertoli cells. Whereas the PTCs vigorously proliferate and do not respond to follicle-stimulating hormone (FSH), Sertoli cells do not undergo mitosis anymore and respond to FSH by characteristic morphological changes and an increase in cyclic adenosine monophosphate (cAMP) concentration9. Very similar enzymatic digestion protocols can be used for the isolation of primary Sertoli cells from other animals like man10, mouse11,12, Siberian hamster13 or yak14. For the removal of large amounts of contaminating germ cells a hypotonic shock can be employed at the end of the isolation procedure15. This allows also the efficient isolation of Sertoli cells from adult rat testes16. An enriched Sertoli cell suspension can also be separated from germ cells by plating the suspension on lectin dishes coated with Datura Stramonium agglutinin17. Only Sertoli cells and a few residual PTCs adhere to the lectin plates.
Primary Sertoli cell cultures, mainly from the rat, have been used initially for investigating responsiveness to hormones or establishing cell lines like the mouse Sertoli cell line TM418. This cell line has been investigated in more than a hundred studies until today. In a translational approach Sertoli cells have been utilized for immuno-protection of co-cultured cells and tissues like in the co-transplantation of xeno- or allogeneic pancreatic islets for long-term graft survival without systemic immunosuppression19. Isolated Sertoli cells have been also used in co-culture experiments for studying epithelial-mesenchymal (Sertoli cells – PTCc) and somatic-germ cell (Sertoli cells – germ cells) interactions20,21. Recently primary Sertoli cells have been employed for investigating the expression of Toll-like receptors and the secretion of proinflammatory cytokines as well as the signal transduction cascades leading to cytokine expression following infection with nonpathogenic and uropathogenic E. coli22. Other recent investigations were using Sertoli cells for studying testicular immune privilege23 and demonstrated that testosterone pre-treatment suppresses the LPS-induced inflammatory response24.
Protocol
1. Animal Ethics Statement
Experiments described here were performed according to the guidelines of the Regierungspräsidium Giessen, Germany, as the local authority and confirm to the German Code of Practice for the Care and Use of Animals for Experimental Purposes (Permission no: GI 20/23 Nr. A 31/2012).
2. Preparation of Media, Enzyme Solutions and Animals
- Prepare 1% iodine alcohol by dissolving 1 g of iodine in 100 ml ethanol.
- Prepare 2x 500 ml Dulbecco´s phosphate buffered saline (PBS) without Ca2+ and Mg2+ supplemented each with 7.5 ml D-glucose (100 g/L) and 5 ml 100x Penicillin/Streptomycin stock solution (15 mg/ml D-glucose, 50 U/ml Penicillin and 50 µg/ml Streptomycin final).
- Supplement 1x 500 ml Roswell Park Memorial Institute (RPMI) 1640 medium with 5 ml 100x Penicillin/Streptomycin stock solution (50 U/ml Penicillin and 50 µg/ml Streptomycin final).
- Prepare a Trypsin-DNase I solution (2.5 mg/ml Trypsin and 10 µg/ml DNase I) by adding 25 mg Trypsin and 0.1 ml DNase I (1 mg/ml DNase I) to 9.9 ml PBS.
- Dissolve 50 mg Trypsin inhibitor in 5 ml PBS for Trypsin inhibitor A solution (10 mg/ml). Dissolve 25 mg Trypsin inhibitor in 10 ml PBS for Trypsin inhibitor B solution (2.5 mg/ml).
- For a Collagenase-Hyaluronidase-Deoxyribonuclease I (DNase I) solution dissolve 10 mg Collagenase A (1 mg/ml final), 10 mg Hyaluronidase (1 mg/ml final) and 0.1 ml DNase I (1 mg/ml DNase I) (10 µg/ml final) in 10 ml PBS.
- Prepare a Hyaluronidase-DNase I solution by adding 10 mg Hyaluronidase (1 mg/ml final) and 0.1 ml DNase I solution (1 mg/ml) (10 µg/ml final) to 9.9 ml PBS.
- Order Wistar rats on time so that they are 19 days old on the day of Sertoli cell isolation.
Note: The described protocol is designed for 10 rats but can be modified for a minimum of 5 and a maximum of 20 rats. The volumes of all solutions have to be adjusted accordingly. - Prepare Oil Red O stock solution by dissolving 0.35 g Oil Red O in 100 ml isopropanol. Stir O/N, filter through 0.2 µm and store at RT for up to one year.
- Prepare Oil Red O staining solution by mixing 6 ml Oil Red O stock solution with 4 ml water (3:2). After 10-20 min filter through 0.2 µm. Use within the next 2 hr.
- Prepare a 4% (w/v) formaldehyde solution in a ventilated hood by adding 4 g paraformaldehyde powder (PFA) to 80 ml of 1x PBS while stirring gently. Heat to ~60 °C, add 1 ml of 1 M NaOH and continue stirring until the paraformaldehyde is dissolved. Let the solution cool down to RT, adjust the pH to 7.4 with 1 M HCl and the volume to 100 ml with 1x PBS. Filter the solution (0.45 µm) and use fresh or store in aliquots at −20 °C for several months.
Note: Polymerized formaldehyde (PFA) depolymerizes to monomeric formaldehyde in water. This process is catalyzed by moderate heating and a slightly alkaline pH. Prepare all enzyme solutions freshly and filter sterilize (0.2 µM) them on the day of use. If a different manufacturer for an enzyme (see Materials/Equipment Table) is used, carefully adjust its concentration/activity.
In the following protocol a "pipette" stands for a serological pipette.
3. Preparation of Seminiferous Tubules
- Anesthetize 10 male Wistar rats one by one in a desiccator using CO2 and a flow rate that displaces at least 20% of the chamber volume per minute. Wait until breathing stops, test anesthesia by squeezing a foot pad, and if there is no reaction euthanize each rat by cervical dislocation. Drain the blood by sectioning jugular vein and carotid artery (vagina carotica) under flowing water. Disinfect the abdomen by wiping with 70% ethanol.
- Using forceps lift the skin from the abdominal muscles and cut out an oval shaped skin lobe that is extending from the pubic symphysis to the sternum (Figure 2A) and flap it upwards onto the chest. Next, grab the abdominal muscles and make a medial incision from the pubic symphysis to the sternum.
- Squeeze the abdomen with the thumbs from pelvis upwards in order to push the testes out of the lower pelvis. Pick the epididymal fat pad (Figure 2B) with forceps for pulling up the testis further. Cut the spermatic cord (Figure 2B), leaving the tunica albuginea intact, and collect all testes in 20 ml PBS in a 50 ml conical tube (Figure 2C).
- After collecting all testes, disinfect them by adding an equal volume (20 ml) of 1% (w/v) iodine in ethanol. Invert the tube twice and decant the supernatant immediately. Quickly wash the testes twice with 25 ml PBS each (Figure 2D).
- Transfer the testes to a Petri dish containing 15 ml PBS (Figure 2E). Grasp each testis firmly by forceps at one end, make a small incision into the tunica albuginea at the opposite end, and squeeze out the tubules using closed scissor blades as a scraping tool.
Note: The tubules should still form a compact testicle-shaped bundle with only a few tubules extending into the solution in order to enable homogeneous enzymatic digestion (Figure 2F). - Transfer the de-capsulated testes to a 100 ml screw-cap bottle containing 10 ml Trypsin-DNase I solution. Perform the digestion in a shaking water bath at 32 °C (120 oscillations/min). After 4 min evaluate the tubules with the naked eye for dispersion of tubules. Once tubules start to disperse into the solution, stop the digestion immediately. If necessary, continue incubation for up to 2 min.
- Stop trypsin digestion by adding 5 ml of trypsin inhibitor solution A. Thoroughly mix the solution by pipetting up and down 3-4 times using a 10 ml pipette and transfer it to a 50 ml conical tube.
- After 5 min of incubation observe the tubules settle by unit gravity, carefully remove the supernatant by using the 10 ml pipette from step 3.6 and add 10 ml of trypsin inhibitor B solution in order to fully stop trypsin digestion. Resuspend the tubules by pipetting up and down 2-3 times.
- In order to remove contaminating interstitial cells wash the tubules 9 times with 25 ml PBS each. Resuspend tubules in each wash by using a 25 ml pipette and allow them to settle by unit gravity for 10 min. Always use the same 25 ml pipette for pipetting PBS, resuspension and removal of the supernatant.
4. Removal/Isolation of Peritubular Cells (PTCs)
- Transfer all tubules to a 100 ml screw-cap bottle and add the Collagenase-Hyaluronidase-DNase I solution (Figure 3A). Perform digestion for 10 min in a shaking water bath at 32 °C (120 oscillations/min).
- Pipet a drop of the suspension on a glass slide and quickly check the tubules under an inverted light microscope for routine cell culture at 100x magnification. If digestion is not advanced enough continue incubation for additional 2 min (do not count the time needed for the microscopical check).
Note: During this step all tubules get shortened in length and tubule edges should become rough (Figure 3B and C). Rough edges are indicative for the release of peritubular cells. - Transfer the suspension with digested tubules to a 50 ml conical tube using the 25 ml pipette from step 3.8. Wash the 100 ml bottle with 10 ml PBS and add it to the tubules in the conical tube. Allow the digested tubules to settle by unit gravity for 10 min, and carefully aspirate the supernatant, containing the PTCs, using the 25 ml pipette again, and transfer it to a new conical tube. For aspiration of the last few milliliters use a 5 ml pipette in order to prevent any carry-over of tubules.
- Add 20 ml RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) to the collected tubule supernatants, mix and centrifuge at 300 x g for 10 min at RT without using the break.
- Carefully decant the supernatant and resuspend the pelleted PTCs in 20 ml RPMI 1640 medium supplemented with 10% heat-inactivated FBS and seed 4 ml each on five T75 flasks containing 16 ml medium each. Incubate at 37 °C in 5% CO2 atmosphere (Figure 4A).
- On the 3rd day of culture trypsinize the PTCs and split them 1:2 on T75 flasks containing 16 ml RPMI 1640 medium supplemented with 10% heat-inactivated FBS each (yields 10 flasks altogether) (Figure 4B). Continue incubation at 37 °C in 5% CO2 atmosphere.
- On the 5th day of culture trypsinize the PTCs again and plate them on 6-well or 24-well plates depending on the experiment (one T75 flask per plate). Experiments can be performed on day 6.
5. Isolation of Sertoli Cells (SCs)
- Resuspend the settled tubules from step 4.3 thoroughly in 25 ml of PBS by using a 25 ml pipette and allow them to settle by unit gravity for 12 min. Use the same 25 ml pipette for pipetting PBS, resuspension of tubules and removal of supernatants. Repeat the wash 3 times (4 washes in total).
- After the last wash transfer the digested tubules to another 100 ml screw-cap bottle containing the Hyaluronidase-DNase I solution. Perform the digestion in a shaking water bath at 32 °C (120 oscillations/min).
- After 5 min check the tubules again by light microscopic inspection at 100X magnification as described in step 4.2.
Note: Short tubules, tubular aggregates and released cells should be visible (Figure 3D).- Allow the tubular aggregates settle for 10 min, and aspirate the supernatant using a 25 ml pipette. Wash aggregates 4 times using 25 ml PBS and a sedimentation time of 10 min for each washing step (Figure 3E).
- Add 20 ml of RPMI 1640 medium and pass the suspension 10 times through an 18 G needle mounted on a 20 ml syringe. Avoid air bubbles.
Note: Pulling the suspension in and out through the needle is considered as one pass. The disruption and homogenization of cells by hydrodynamic shearing is a standard technique in the preparation of cells that tend to aggregate. The passing of cells through a hypodermic needle of small inner diameter is unlikely to have an impact on their functional properties25. Figure 5C and D shows the normal ultrastructure of purified Sertoli cells on day 4 of culture.- Pipet a drop of the suspension on a glass slide and quickly check the tubules under an inverted light microscope for routine cell culture at 10X magnification if the aggregates are all dispersed (Figure 3F). Filter the cell suspension through a 70 µm cell strainer in order to obtain a pure single cell suspension in the filtrate.
- Centrifuge the filtrate of step 5.4 at 200 x g for 10 min at RT without using the break. Carefully aspirate the supernatant using a 25 ml pipette, and resuspend the Sertoli cells in 40 ml RPMI 1640 medium (serum-free).
- Mix 100 µl cell suspension with 100 µl of Trypan Blue Stain and count cells with a Neubauer Improved chamber. Adjust the cell concentration to 3 x 106 cells/ml according to the counting result. Seed 1 ml per well on a 6-well plate (=day 1). If an Oil Red O staining (step 6) is planned put a cover slip into one or a few wells before seeding Sertoli cells.
Note: Sertoli cells in suspension settle quickly so that the tube should be briefly shaken every time before a cell aliquot is taken with the pipette. Until day 4 they firmly attach to the substratum. - In order to remove floating or adherent germ cells on day 4 (Figure 4C) wash each well of the 6-well plates used for plating isolated Sertoli cells (step 5.6) with PBS (Figure 4D). Aspirate the medium, add 1-2 ml of PBS to each well and shake the 6-well plates horizontally on a table vigorously but without spilling PBS. Repeat the wash 3 times and perform the same washing procedure on day 5 and 6 (Figure 4E).
Note: On day 4 Sertoli cells have firmly attached and show a cobblestone like pattern under the inverted light microscope (Figure 4C). Experiments can be performed from day 7 onwards.- (Alternative for step 5.7) For the removal of floating and adherent germ cells perform a hypotonic shock 3 days after seeding on 6-well plates. Remove the medium and add 1 ml of 20 mM Tris pH 7.5 taken directly out of the fridge. Aspirate the Tris buffer after 90 to 120 sec but not later. Wash cells twice with PBS, and add 2 ml of RPMI 1640 medium (serum-free). Optionally, perform experiments the next day.
Note: The longer the duration of the hypotonic shock is the more germ cells can be removed but the more Sertoli cells get lost as well. The first PBS wash should be performed quickly but with care in order not to displace any Sertoli cells. Directly after hypotonic treatment numerous vacuoles of different sizes can be observed in the cytoplasm by phase contrast microscopy. Vacuoles disappear within a few hours after hypotonic treatment.
- (Alternative for step 5.7) For the removal of floating and adherent germ cells perform a hypotonic shock 3 days after seeding on 6-well plates. Remove the medium and add 1 ml of 20 mM Tris pH 7.5 taken directly out of the fridge. Aspirate the Tris buffer after 90 to 120 sec but not later. Wash cells twice with PBS, and add 2 ml of RPMI 1640 medium (serum-free). Optionally, perform experiments the next day.
6. Oil Red O Staining
- Perform all steps at RT. On day 7 wash Sertoli cells grown on a cover slip (step 5.6) with PBS twice and fix them with 10% formalin in PBS. After 10 min replace formalin with fresh solution, and continue fixation for another 60 min.
Note: All solutions are added to the well(s) with a cover slip and aspirated before the next step. - Aspirate formalin, wash cells with water twice and add 60% isopropanol. After 5 min allow cells to air dry for a few minutes.
- Add 1 ml Oil Red O staining solution per well and incubate for 10 min. Aspirate the solution, and wash cells immediately 4 times with water and mount cover slips in glycerol-PBS (9:1). Take bright field images with a routine light microscope (Figure 4F).
7. Immunofluorescence Staining
- Wash PTCs (from step 4.5) or Sertoli cells (from step 5.5) grown on a cover slip with PBS twice and fix them with 4% PFA for 10 min at RT.
- Incubate with 5% BSA in PBS for 1 hr at RT. Discard the BSA-PBS solution and add fresh BSA-PBS solution containing actin (smooth muscle) (for PTCs) or vimentin (for Sertoli cells) primary antibody (dilution 1:100 for both antibodies) and incubate at 4 °C O/N.
Note: Incubation with BSA blocks non-specific binding of the primary antibody. - Wash cover slips 3 times for 5 min in PBS-0.1% Tween 20 and incubate with green-fluorescent dye conjugated goat anti-mouse secondary antibody (dilution 1:1,000) at RT for 1 hr in the dark.
- Wash cover slips 3 times for 5 min in PBS-0.1% Tween 20 and mount them in DAPI mounting medium.
8. Ultrastructural Analysis
- Wash Sertoli cells (from step 5.6) grown on a 6 cm cell culture plate with PBS and fix them for 2 hr at 4 °C in a mixture of 2.5% glutaraldehyde, 2.5% paraformaldehyde and 0.05% picric acid in 67 mM cacodylate buffer (pH 7.4) according to Ito and Karnovsky26.
- Perform post-fixation in 1% osmium tetroxide followed by an O/N incubation in 0.3% uranyl acetate dissolved in 50 mM maleate buffer (pH 5). Embed samples in embedding media according to standard procedures. Cut thin sections, contrast them with lead citrate and examine with an electron microscope.
Representative Results
The described procedure allows isolation of approximately 12 x 107 Sertoli cells from 10 rat testes. 3 x 106 cells are plated per well on a 6-well plate so that six to seven 6-well plates are available for experiments on day 7. The Trypsin-DNase I digestion is the most critical step during Sertoli cell isolation. If digestion at this point is advancing too far, the ratio of germ cells/Sertoli cells will disproportionally increase up to the end. During day 3-6 of culture it is important to carefully wash away as many non-adhering or loosely attached germ cells as possible. As an alternative to extensive washings over 4 days germ cells can be removed by hypotonic treatment on day 327. An advantage of this method is that the time of Sertoli cell culture is kept to a minimum so that Sertoli cell-specific functions are potentially better retained than after a prolonged period of culture.
Although the Hyaluronidase treatment serves to remove most residual peritubular cells, a few cells will survive the treatment. Purity of the isolated Sertoli cells is >95% as can be demonstrated by vimentin immunolabeling (Figure 5B). Because peritubular cells have a strong proliferative potential, Sertoli cell culture has to be performed in the absence of serum. In the presence of 10% serum PTCs would constitute approximately 20% of all cells after 6 days of culture whereas its fraction can be expected to be below 1% in the absence of serum7. Virtually all isolated Sertoli cells are viable as judged by Trypan blue staining (not shown). Around day 7 of culture the isolated Sertoli cells are least contaminated with germ cells and peritubular cells (Figure 4), so that experiments should be performed around this day if possible. For good reproducibility it is important to repeat experiments always on the same day of culture. If transfections are planned they should be performed on day 4 or 5, and cell extracts should be made on day 5 or 6.
Over at least two weeks in serum-free conditions Sertoli cells are responsive to FSH and produce more androgen-binding protein, plasminogen activator and transferrin upon FSH treatment. For longer culture a feeder layer of peritubular cells or serum is required. After four weeks cultures deteriorate, and cells detach from the substratum7.
On day 6 of culture most Sertoli cells have acquired lipid droplets. In the majority of cells a single large vacuole has formed (Figure 5C and 5D). The neutral lipid content can be easily visualized by Oil Red O staining (Figure 4F).
In addition to Sertoli cells the removed PTCs can be cultured as well (Figure 4A and 4B). PTCs from 10 testes yield five T75 flasks that can be expected to form a confluent layer after 2 days of culture. Like isolated Sertoli cells freshly isolated PTCs are contaminated by germ cells that can be largely removed by passaging. Flasks are split 1:2 by standard trypsinization so that 10 flasks will be confluent on day 5 after isolation. Purity of isolated PTCs is >95% and can be confirmed using α-smooth muscle actin immunolabeling (Figure 5A). From this day onwards PTCs can be used for experiments. One flask is sufficient for approximately one 6-well plate, and wells should be confluent the next day. Cells can be passaged many times, but experiments should be performed always at the same passage in order to make sure that cells behave in a reproducible manner.
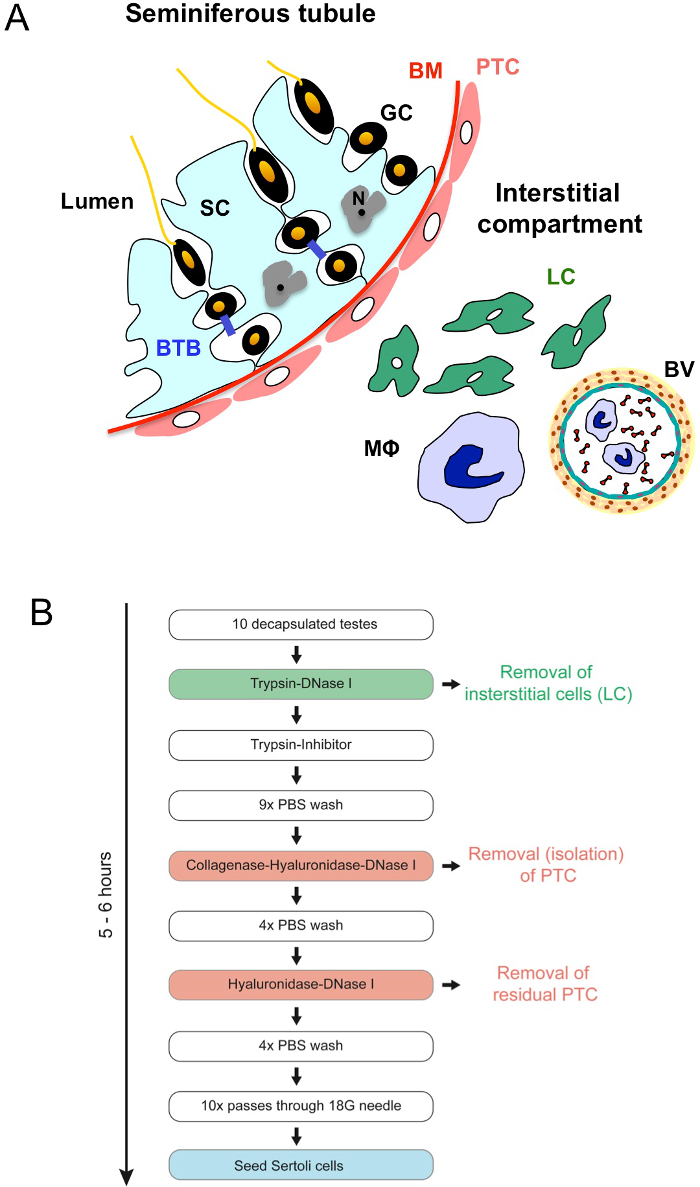
Figure 1. Morphology of the testis and workflow of the procedure. (A) A sector of a seminiferous tubule with adjacent interstitial tissue is schematically shown. Seminiferous tubules are enclosed by a basal membrane (BM) and a circumferential layer of myoid peritubular cells (PTC). The germinal epithelium is located on the luminal side of the basal membrane. Sertoli cells (SC), spanning the whole germinal epithelium are strongly attached to the BM and interconnected via basolateral positioned occluding junctions representing the blood-testis barrier (BTB). Their irregular nucleus (N) shows one or more fissure-like indentations and contains a nucleolus. Germ cells (GC) are in intimate contact with SCs at all stages of their development. The interstitial compartment between the tubules contains Leydig cells (LC) and immune cells such as macrophages (MΦ) as well as blood vessels (BV). Figure adapted from Fijak and Meinhardt28. (B) Workflow of the procedure; color coding is like in (A). Please click here to view a larger version of this figure.
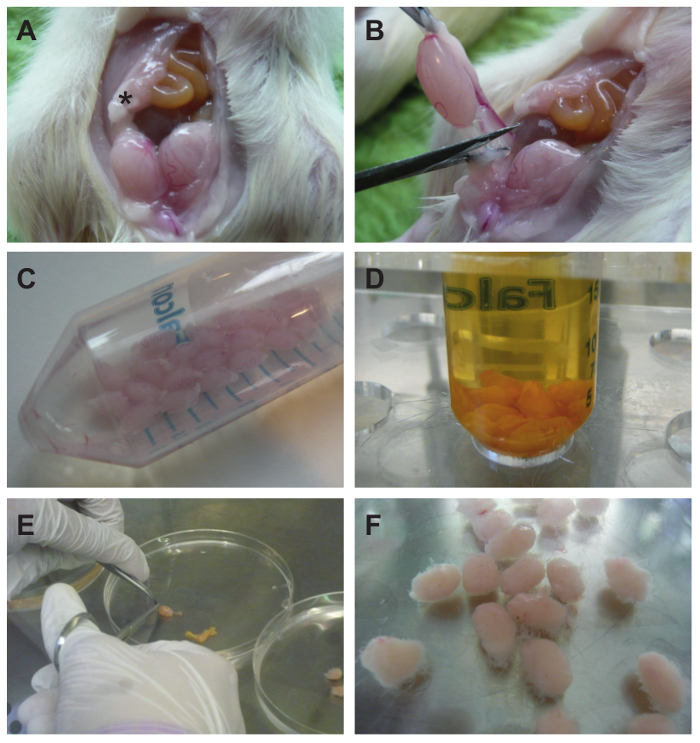
Figure 2. Testes from Wistar rats are excised and decapsulated. (A) The peritoneal cavity is opened through a longitudinal incision as described in the text. An asterisk (*) indicates the epidydimal fat pad. (B) Testes are removed by cutting the spermatic cord and (C) collected in a 50 ml conical tube containing 20 ml PBS. When all testes have been collected they are disinfected in 20 ml of 1% (w/v) iodine in ethanol. For removal of the iodine they are quickly washed twice with 25 ml PBS each. (D) Testes after first PBS wash. (E) Testes are transferred to a Petri dish (on the right), and seminiferous tubules are squeezed out of the opened tunica albuginea by means of closed scissor blades (on the left) as described in the text. (F) The decapsulated testes still form a compact testicle-shaped mass with single tubules protruding. Please click here to view a larger version of this figure.
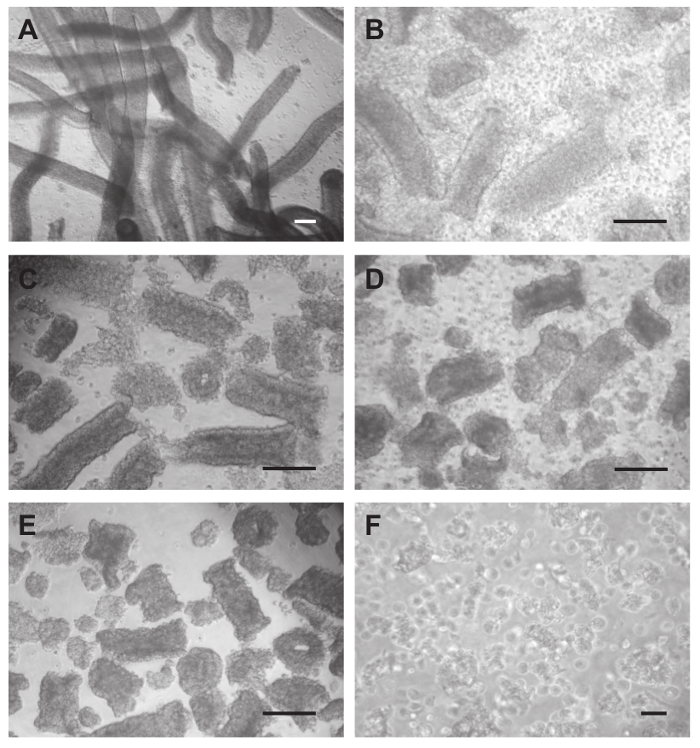
Figure 3. Isolation of Sertoli cells. (A) After removal of interstitial cells by Trypsin-DNase I digestion and washing 9x with PBS tubules become mobilized and look clean. (B) During collagenase-hyaluronidase-DNase I treatment peritubular cells from the outer layer and germ cells are released. The tubules get shortened and obtain a rough appearance. (C) Tubular fragments after washing 4x with PBS. (D) Hyaluronidase-DNase I treatment releases residual peritubular cells as well as germ cells. Tubules get further shortened, and tubular aggregates form. (E) Tubular aggregates after washing 4x with PBS. (F) Single Sertoli cells are produced by passing the aggregates 10x through an 18G needle. Scale bars in (A-E) = 200 µm, in (F) = 50 µm. Please click here to view a larger version of this figure.
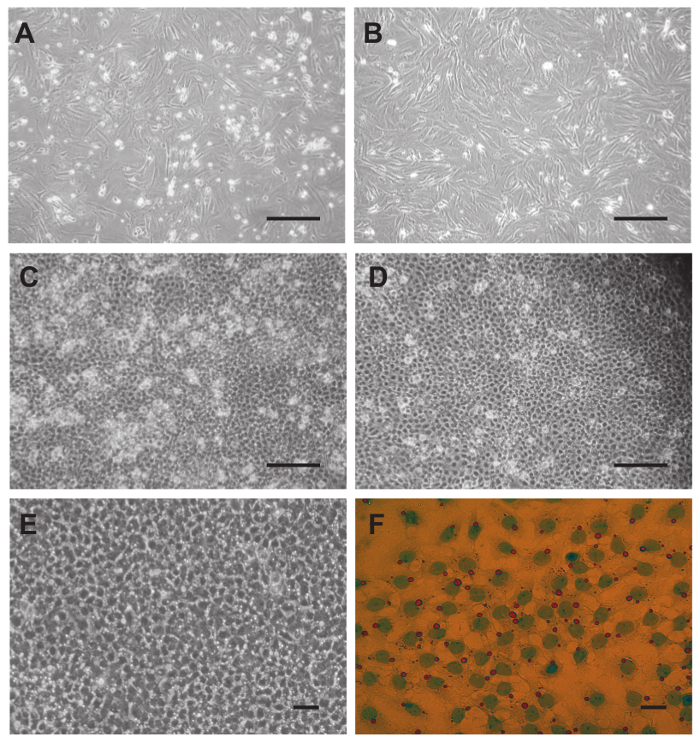
Figure 4. Culture of peritubular cells (A-B) and Sertoli cells (C-F) and removal of contaminating germ cells. (A) Resuspended PTCs from step 4.5 were seeded immediately and photographed the next day. (B) After splitting on day 3 contamination with germ cells is reduced. (C) On day 4 Sertoli cells show a typical cobblestone-like pattern. Floating and adhering germ cells are visible before washing with PBS. (D) The same well after washing 3x with PBS. The number of contaminating germ cells is reduced. Washing thrice with PBS is repeated on day 5 and 6. (E) On day 6 of culture virtually all germ cells have been removed. Sertoli cells have formed a unique looking cell layer, and most cells have acquired a single large vacuole. (F) Oil red O staining shows that these vacuoles are lipid droplets containing largely neutral lipids. Scale bars in (A-D) = 200 µm, in (E) = 50 µm and in (F) = 25 µm. Please click here to view a larger version of this figure.
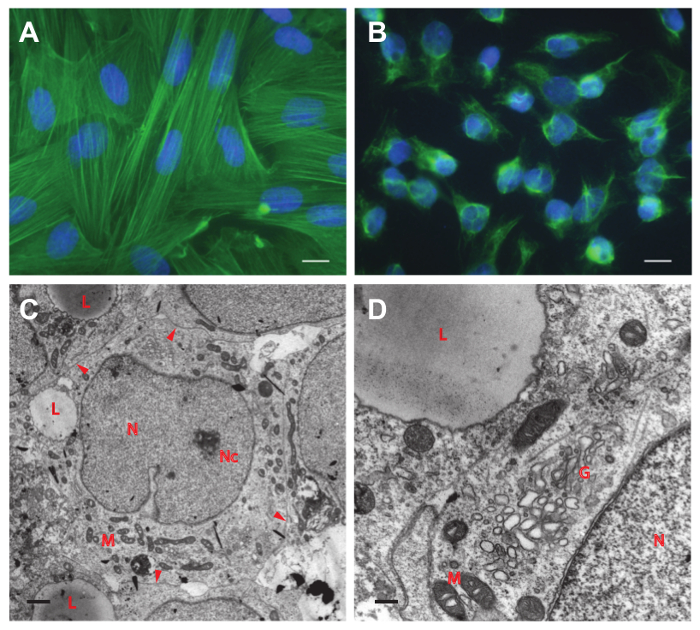
Figure 5. Immunofluorescence staining. Staining of purified primary peritubular cells with actin (smooth muscle) antibody (A) and of Sertoli cells with vimentin antibody (B). No contaminating cells are visible in both fields of view. Scale bar in (A) and (B) = 10 µm. (C) and (D) Electron microscopic pictures of Sertoli cells after 4 days in culture. (C) Note the close contact of adjacent cells (arrowheads) that leads to the cobblestone-like pattern as seen in Figure 4C. A single lipid droplet (L) is observed in the cytoplasm of each cell. (D) Most abundant organelles are mitochondria (M) and the Golgi apparatus (G). The nucleus (N) contains a nucleolus (Nc) and shows the typical fissure-like indentation. Scale bar in (C) = 1 µm and in (D) = 0.25 µm. Please click here to view a larger version of this figure.
Discussion
The seminiferous tubules are bounded by a circular layer of peritubular cells and a basal lamina on the luminal side. Sertoli cells are resting on the basal lamina, establish the blood testis barrier through formation of occluding junctions between adjacent cells and provide the structural framework for the organisation of the seminiferous epithelium. Sertoli cells and adjacent spermatogenic cells maintain intimate contacts throughout germ cell development providing physical contact and communication alike. Therefore, when Sertoli cells are isolated using the procedure described here they get deprived of their intimate contacts to neighboring PTCs and spermatogenic cells. Nevertheless they appear to retain many properties that are characteristic for them in vivo like hormone responsiveness and expression of marker genes although in a time-dependent manner and on a lower level.
Primary Sertoli cells develop large neutral lipid droplets after six days of culture. One of the vital functions of Sertoli cells is phagocytosis of degenerating and apoptotic germ cells and of germ cell material like the so called residual bodies29. It is known that residual bodies and cytoplasts from elongated spermatids adhere to isolated Sertoli cells and get phagocytosed30. As it is impossible to remove all germ cells during the isolation procedure we therefore hypothesize that contaminating germ cells can also adhere to Sertoli cells after plating and get phagocytosed. Since residual bodies contain small lipid droplets it is easily conceivable that Sertoli cells acquire large lipid droplets after extensive phagocytosis of adhering and damaged germ cells. There is also evidence that phagocytotic clearance of apoptic cells actively downregulates inflammatory and immune responses31 which could be important for establishing and maintaining the testis as an immune priviliged organ.
When isolated Sertoli cells are plated on top of a layer of primary peritubular cells both cell types survive longer and do form a basal lamina between them. Morphologically they are able to form structures reminiscent of seminiferous tubules indicating that both cell types have retained their capacity to properly interact7. Like freshly isolated primary Sertoli cells the frequently used mouse Sertoli cell line TM4 does respond to FSH with increased production of cAMP18, but contrary to primary cells it is not able to produce androgen binding protein32. Moreover it has been shown that the mouse Sertoli cell line 1 lacks the immunoprotective properties associated with primary Sertoli cells12.
Similarly the rat Sertoli cell line SCIT-C8 expresses the epithelial markers transferrin, clusterin and stem cell factor but has lost androgen receptor expression and androgen responsiveness whereas it has aquired expression of some mesenchymal markers like fibronectin and entactin-133. Therefore, primary Sertoli cells, although expensive and tedious to isolate, appear to retain significantly more qualitative in vivo properties during cell culture than cell lines do.
Future applications of primary Sertoli cells will focus on immuno-protection of co-cultured or co-transplanted cells, on experiments studying cell-cell interactions, and on investigating the immune privilege of testis.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Guido Verhoeven and Ludo Deboel, Leuven, who were extremely helpful in establishing the isolation of primary Sertoli cells in the Meinhardt lab in Giessen. Monika Fijak, Gießen, is acknowledged for her help with figure 1A and advice. Studies were supported by the Deutsche Forschungsgemeinschaft through grant BH 93/1-1 (S.B.) and funding of the International Research Graduate College JLU Gießen (Germany)/Monash University (Melbourne, Australia) GRK 1871 (S.B.). Support was also obtained from a grant of the State of Hessen within the program "Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz" (LOEWE) called "Männliche Infertilität bei Infektion & Entzündung" (MIBIE).
Materials
| Actin (smooth muscle) antibody clone 1A4 | Dako | M0851 | Monoclonal mouse anti human antibody. Use 1:100 dilution for immunofluorescence. |
| Albumin bovine fraction V Standard grade, lyophilized |
Serva | 11930.04 | Filter (0.45 µm) BSA solutions used for immunofluorescence. |
| Corning™ Cell Strainers 70 µm | Corning | 431751 | White color |
| Collagenase A from Clostridium histolyticum | Roche | 10103586001 | |
| DAPI mountant ProLong® Gold Antifade | Life technologies | P-36931 | |
| D-glucose | Sigma | G8644 | 100 g/l |
| Dulbecco´s PBS without Ca2+/Mg2+ | Gibco | 14190-094 | |
| DNase I | Roche | 10104159001 | |
| Electron microscope | Zeiss | EM 109S | |
| Fluorescence microscope | Zeiss | Axioplan 2 | |
| Hyaluronidase from bovine testis | Sigma | H3506 | |
| Inverted light microscope | Olympus | CKX41 | Routine cell culture microscope |
| Mouse IgG / IgM (H+L) polyclonal secondary Antibody |
Life technologies | A-10684 | Alexa Fluor® 488 conjugate |
| Oil Red O | Sigma | O0625 | Has replaced Sudan III and Sudan IV because of brighter color. |
| Paraformaldehyde | Sigma | P6148 | |
| Penicillin (5,000 U/ml)/ Streptomycin (5,000 µg/ml) |
Gibco | 15070-063 | 100x solution |
| RPMI-1640 | Gibco | 21875-034 | Contains 300 mg/L L-glutamine |
| Trypsin from porcine pancreas | Sigma | T5266 | |
| Trypan Blue Stain (0.4%) | Gibco | 15250-061 | |
| Trypsin inhibitor from soybean | Sigma | T6522 | The Sigma product is considerably cheaper than the previously used BPTI (Aprotinin) from Roche. |
| Vimentin antibody (V9) | Sigma | V6630 | Monoclonal mouse anti pig vimentin antibody. Use 1:100 dilution for immunofluorescence. |
| Wistar WU rats | Charles River | N/A | Should be 19 days old on day of experiment. |
References
- Nieschlag, E., Behre, H., Nieschlag, S. . Andrology. Male Reproductive Health and Dysfunction. , (2010).
- Esaki, S. Über Kulturen des Hodengewebes der Säugetiere und über die Natur des interstitiellen Hodengewebes und der Zwischenzellen. Z. Mikrosk. Anat. Forsch. 15, 368-404 (1928).
- Hall, P. F., Irby, D. C., De Kretser, D. M. Conversion of cholesterol to androgens by rat testes: comparison of interstitial cells and seminiferous tubules. Endocrinology. 84, 488-496 (1969).
- Welsh, M. J., Wiebe, J. P. Rat sertoli cells: a rapid method for obtaining viable cells. Endocrinology. 96, 618-624 (1975).
- Vitale, R., Fawcett, D. W., Dym, M. The normal development of the blood-testis barrier and the effects of clomiphene and estrogen treatment. Anat. Rec. 176, 331-344 (1973).
- Dorrington, J. H., Roller, N. F., Fritz, I. B. Effects of follicle-stimulating hormone on cultures of Sertoli cell preparations. Mol. Cell. Endocrinol. 3, 57-70 (1975).
- Tung, P. S., Skinner, M. K., Fritz, I. B. Fibronectin synthesis is a marker for peritubular cell contaminants in Sertoli cell-enriched cultures. Biol. Reprod. 30, 199-211 (1984).
- Verhoeven, G., Cailleau, J. Testicular peritubular cells secrete a protein under androgen control that inhibits induction of aromatase activity in Sertoli cells. Endocrinology. 123, 2100-2110 (1988).
- Tung, P. S., Dorrington, J. H., Fritz, I. B. Structural changes induced by follicle-stimulating hormone or dibutyryl cyclic AMP on presumptive Sertoli cells in culture. Proc. Natl. Acad. Sci. U. S. A. 72, 1838-1842 (1975).
- Teng, Y., et al. Isolation and culture of adult Sertoli cells and their effects on the function of co-cultured allogeneic islets in vitro. Chin. Med. J. (Engl.). 118, 1857-1862 (2005).
- Kohno, S., Ziparo, E., Marek, L. F., Tung, K. S. Murine Sertoli cells: major histocompatibility antigens and glycoconjugates. J. Reprod. Immunol. 5, 339-350 (1983).
- Dufour, J. M., et al. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 17, 525-534 (2008).
- Majumdar, S. S., Tsuruta, J., Griswold, M. D., Bartke, A. Isolation and culture of Sertoli cells from the testes of adult Siberian hamsters: analysis of proteins synthesized and secreted by Sertoli cells cultured from hamsters raised in a long or a short photoperiod. Biol. Reprod. 52, 658-666 (1995).
- Zhang, H., Liu, B., Qiu, Y., Fan, J., Yu, S. Pure cultures and characterization of yak Sertoli cells. Tissue Cell. 45, 414-420 (2013).
- Ziparo, E., Geremia, R., Russo, M. A., Stefanini, M. Surface interaction in vitro between Sertoli cells and germ cells at different stages of spermatogenesis. Am. J. Anat. 159, 385-388 (1980).
- Anway, M. D., Folmer, J., Wright, W. W., Zirkin, B. R. Isolation of Sertoli cells from adult rat testes: an approach to ex vivo studies of Sertoli cell function. Biol. Reprod. 68, 996-1002 (2003).
- Scarpino, S., et al. A rapid method of Sertoli cell isolation by DSA lectin, allowing mitotic analyses. Mol. Cell. Endocrinol. 146, 121-127 (1998).
- Mather, J. P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 23, 243-252 (1980).
- Korbutt, G. S., Elliott, J. F., Rajotte, R. V. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 46, 317-322 (1997).
- Fritz, I. B. Somatic cell-germ cell relationships in mammalian testes during development and spermatogenesis. Ciba Found. Symp. 182, 271-281 (1994).
- Whaley, P. D., Chaudhary, J., Cupp, A., Skinner, M. K. Role of specific response elements of the c-fos promoter and involvement of intermediate transcription factor(s) in the induction of Sertoli cell differentiation (transferrin promoter activation) by the testicular paracrine factor PModS. Endocrinology. 136, 3046-3053 (1995).
- Bhushan, S., et al. Uropathogenic Escherichia coli block MyD88-dependent and activate MyD88-independent signaling pathways in rat testicular cells. J. Immunol. 180, 5537-5547 (2008).
- Meinhardt, A., Hedger, M. P. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol. Cell. Endocrinol. 335, 60-68 (2011).
- Fijak, M., et al. Influence of Testosterone on Inflammatory Response in Testicular Cells and Expression of Transcription Factor Foxp3 in T Cells. Am. J. Reprod. Immunol. , 12-25 (2015).
- Mamidi, M. K., et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J. Transl. Med. 10, 229 (2012).
- Ito, S., Karnovsky, M. J. Formaldehyde-glutaraldehyde fixatives containing trinitro compounds. J. Cell. Biol. 39, 168-169 (1968).
- Galdieri, M., Ziparo, E., Palombi, F., Russo, M. A., Stefanini, M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J. Androl. 5, 249-254 (1981).
- Fijak, M., Meinhardt, A. The testis in immune privilege. Immunol. Rev. 213, 66-81 (2006).
- Jegou, B. The Sertoli cell in vivo and in vitro. Cell Biol. Toxicol. 8, 49-54 (1992).
- Pineau, C., Le Magueresse, B., Courtens, J. L., Jegou, B. Study in vitro the phagocytic function of Sertoli cells in the rat. Cell Tissue Res. 264, 589-598 (1991).
- Ren, Y., Savill, J. Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563-568 (1998).
- Ducray, A., et al. Establishment of a mouse Sertoli cell line producing rat androgen-binding protein (ABP). Steroids. 63, 285-287 (1998).
- Konrad, L., et al. Rat Sertoli cells express epithelial but also mesenchymal genes after immortalization with SV40. Biochim. Biophys. Acta. 1722, 6-14 (2005).

