Concurrent Electroencephalography Recording During Transcranial Alternating Current Stimulation (tACS)
Summary
In this article we explain how to set up a concurrent transcranial alternating current stimulation and EEG experiment.
Abstract
Oscillatory brain activities are considered to reflect the basis of rhythmic changes in transmission efficacy across brain networks and are assumed to integrate cognitive neural processes. Transcranial alternating current stimulation (tACS) holds the promise to elucidate the causal link between specific frequencies of oscillatory brain activity and cognitive processes. Simultaneous electroencephalography (EEG) recording during tACS would offer an opportunity to directly explore immediate neurophysiological effects of tACS. However, it is not trivial to measure EEG signals during tACS, as tACS creates a huge artifact in EEG data. Here we explain how to set up concurrent tACS-EEG experiments. Two necessary considerations for successful EEG recording while applying tACS are highlighted. First, bridging of the tACS and EEG electrodes via leaking EEG gel immediately saturates the EEG amplifier. To avoid bridging via gel, the viscosity of the EEG gel is the most important parameter. The EEG gel must be viscous to avoid bridging, but at the same time sufficiently fluid to create contact between the tACS electrode and the scalp. Second, due to the large amplitude of the tACS artifact, it is important to consider using an EEG system with a high resolution analog-to-digital (A/D) converter. In particular, the magnitude of the tACS artifact can exceed 100 mV at the vicinity of a stimulation electrode when 1 mA tACS is applied. The resolution of the A/D converter is of importance to measure good quality EEG data from the vicinity of the stimulation site. By following these guidelines for the procedures and technical considerations, successful concurrent EEG recording during tACS will be realized.
Introduction
Rhythmic dynamics of extracellular electrical currents in the brain have been observed for a century1,2. While for most of this time being considered as non-specific noise in the data, today they are widely considered to play a principal role in information processing in the brain3,4,5,6,7,8,9. Our understanding of the causal link between specific frequencies of oscillatory brain activity and cognitive processes has advanced in the last decade through the development of various intervention approaches for directly modulating oscillatory activity8,10. Transcranial alternating current stimulation (tACS) is one such promising approach to modulate rhythmic activity in the brain10. tACS is a non-invasive brain stimulation method, which applies weak alternating (sinusoidal) currents from the scalp and modulates the excitability of the cerebral cortex in a frequency-specific manner11,12,13,14,15. While being a promising technique for studying the role of rhythmic activity in the brain, the neurophysiological mechanisms of tACS are still elusive. Several studies have reported effects of tACS on perceptual11,13,16,17,18 and motor functions19,20,21,22, as well as effects on higher-order cognitive processes23,24,25,26,27,28. Neurophysiological evidence for entrainment of brain oscillations after stimulation have been presented using EEG13,14,15. There are currently few reports of neurophysiological evidence in humans for an effect of tACS during stimulation12,13,22. As the brain is highly robust to external perturbation, such online evidence is crucial for understanding the immediate neurophysiological effects of tACS.
Electroencephalography (EEG), capturing electrophysiological activity in the brain with high temporal resolution, is an ideal choice for studying endogenous and entrained oscillatory neural activities. Recent studies by Helfrich and colleagues reported online neurophysiological effects of tACS, but at the same time measuring EEG during tACS has proven difficult due to the prominent tACS artifact12,13. For successful concurrent tACS-EEG experiments, recording good quality EEG data is one important aspect, which is the focus of the current article, and at the same time the pre-processing method to remove the tACS artifact is also crucial. In our lab, we have been developing our own pre-processing pipeline allowing for the removal of the tACS artifact from EEG data29. Here we will describe how to successfully record EEG signals from the area of stimulation, and technical considerations important for successful recording.
Protocol
Ethics statement: Procedures involving human subjects were approved by the ethics committee of Canton Bern (KEK-BE 007/14).
Note: Figure 1 illustrates montages, as well as the design of the tACS electrodes (see also Discussion), and EEG cap. We use an EEG cap made of an elastic material (Figure 1D) to hold the tACS electrode attached on the scalp.
1. Montages
Note: The representative results are obtained from the following tACS electrode montages.
- Montage 1: Place both electrodes on the scalp, at the left dorsolateral prefrontal cortex (DLPFC) (F3 electrode) and left posterior parietal cortex (PPC) (P3 electrode) (Figure 1A).
- Montage 2: Place one tACS electrode on the scalp at the left DLPFC (F3 electrode), and place another tACS electrode on the left shoulder (Figure 1B).
- Montage 3: Place one tACS electrode on the scalp at the left PPC (P3 electrode), and place another tACS electrode on the left shoulder (Figure 1C).
2. Preparation of tACS Electrodes
- If a reference tACS electrode will be placed on the shoulder (Montage 2 and 3), do this first.
- Before placing the shoulder electrode, prepare the skin with an abrasive skin preparing gel for EEG and electrocardiography. Use a gauze pad to scrub the skin lightly with the skin preparing gel.
- Apply EEG gel on the tACS electrode and place the electrode on the shoulder.
- Secure the electrode on the shoulder with adhesive tape.
- Put on the EEG cap. Adjust the position of the cap according to the international 10-20 system for electrode positioning30, and fasten the chin strap of the EEG cap.
- Mark spots to indicate where the tACS electrode will be positioned on the scalp. Use a water-based red pen, firstly because insulating effects of the color material of the pen are reduced, and secondly, it can be easily washed away with water.
- If there is a problem with the pen not reaching to the scalp for the marking, due to the holes in the EEG cap for gel insertion being too tight (Figure 1D), use a wooden stick, for example the wooden handle of a cotton swab.
- Paint the tip of the stick thoroughly and use this tip to mark the scalp.
- Remove the EEG cap and check if the marking was successful. If needed, fill in the marking, so that it can easily be spotted later.
- Perform the following steps (2.5.1-2.5.4) depending on the length of the participant's hair. If the participant has short hair (up to about 10 centimeters), skip the following steps (it should also be noted that certain hairstyles, such as dread locks, make the application of tACS electrodes impossible). If the participant has longer hair:
- Place the tACS electrode with its center marked by the red spot on the scalp. Note that no EEG gel should be put on the tACS electrode at this moment.
- Thread out all the hair inside the inner ring of the tACS electrode.
- Bind the threaded out hair with cable binders. Pay attention to that hair around the tACS electrode does not get bound up with the tACS electrode by the cable binders.
- After the hair has been bound, remove the tACS electrode.
- Apply EEG gel to the scalp tACS electrode.
- Before applying the gel, connect the scalp and shoulder tACS electrodes to the stimulator, but do not turn on the stimulator yet. Apply a thin layer of EEG gel onto the tACS electrode. A sparse application of gel is important.
- Carefully place the tACS electrode back on the head.
- If the participant has longer hair, thread the bound hair back through the inner hole of the tACS electrode, without it touching the EEG gel on the tACS electrode.
- While placing the tACS electrode, pay close attention to the red mark on the scalp being kept in the middle of the tACS electrode. Once the tACS electrode has been placed on the scalp, its position may no longer be changed.
- Remove the cable binders from the hair once the tACS electrode has been placed.
- Turn on the stimulator and monitor the impedance. While carefully putting some pressure on the tACS electrode, pay very close attention that the red marking spot is always kept in the middle of the tACS electrode.
- Carefully lift the edges of the tACS electrode and apply some more EEG gel beneath the hair, not between the tACS electrode and hair (Figure 2). This is especially important if the participant has a lot of hair (see discussion).
- Continue putting pressure on the tACS electrode until impedance is stably below 10 kΩ. Monitor the impedance of the tACS electrode by the tACS stimulator.Carefully add additional EEG gel if necessary, but always sparsely.
Note: The impedance of the tACS electrode monitored by the tACS stimulator is measured between the tACS electrodes, which has the drawback of not providing separate impedance value information for each electrode. Depending on the EEG amplifier system, it might also be possible to measure the impedance of the tACS electrodes through this, and then be able to measure the impedance for each electrode separately. - Pay attention to any gel escaping from the tACS electrode, and remove excess EEG gel with a cotton swab.
3. Mounting the EEG Cap
- After the impedance of the tACS electrodes reaches below the threshold of 10 kΩ, mount the EEG cap again. Put on the EEG cap very gently and carefully, particularly if the material of the EEG cap is elastic, since it is otherwise easy to move the position of the scalp tACS electrode during this step.
Note: The shift of the tACS electrode spreads out the EEG gel beneath the tACS electrode and causes the EEG gel to bridge with the EEG electrodes. It is important not to pull down an elastic cap with force, as this may cause it to rebound afterwards, which would also result in moving the tACS electrode. - Fasten the strap of the EEG cap.
4. Preparation of EEG Electrodes
- Apply EEG gel of appropriate viscosity (as discussed in detail in the discussion) to the EEG electrodes to create contact between the scalp and EEG electrodes. Begin with the ground and reference EEG electrodes. Then proceed to the electrodes located in the middle and vicinity of the tACS electrode. Then continue to the remaining electrodes (see Discussion).
- For EEG electrodes surrounding the tACS electrode, inject gel with the needle tip pointing in a direction away from the tACS electrode. Gently push down the EEG electrodes while applying gel, so that the gel does not escape from beneath the electrodes.
- Use a wooden stick to increase the contact between the EEG electrodes and scalp, as illustrated in Figure 3. Don't use the needle tip for this purpose, as it will scrape the participant's scalp, and is furthermore not as effective for this purpose.
- Push down the gel with the stick towards the scalp, and very gently rub the scalp with the top of the stick with a rotating motion. Try to keep the angle of the stick orthogonally to the scalp for electrodes located in a close vicinity of the tACS electrode, as sideway movements of the stick will spread out the gel under the electrode. If needed, apply some more EEG gel, and then use the wooden stick to further improve the impedance.
- To avoid bridging via leaking gel (Figure 4), be thrifty with applying the gel for lowering the impedance of the EEG electrodes in the immediate vicinity of the tACS electrode. Instead, try to lower the impedance as much as possible using only the wooden stick, before considering adding more gel.
- Once good impedance has been achieved with the wooden stick, carefully insert and bring down the needle until the tip of the needle touches the scalp, then gently apply gel while pulling the needle out, thereby helping to stabilize the contact between the EEG electrode and the scalp.
- Aim for EEG electrode impedances below 5 kΩ for optimal data, as this reduces noise interference and signal distortion.
- Once the impedances have been lowered to the appropriate level, test whether any bridge between the tACS electrode and surrounding EEG electrodes due to leaking gel has been created.
- Apply brief sinusoidal stimulation, with an intensity of experimental interests (e.g., 1 mA peak-to-peak).
Note: Due to limitations of some systems (see table of materials), it is not possible to check for bridging online, but only through applying stimulation and then checking whether any channel of the EEG amplifier becomes saturated. - See whether any channel is saturated while stimulating.
Note: As evident from the representative results, bridging via leaking gel between the tACS and EEG electrodes will result in saturating this channel of the EEG amplifier and rule out recording data from these electrodes. It is not possible to undo a bridging via leaking gel once it has been established. The only option is to interrupt the experiment.
- Apply brief sinusoidal stimulation, with an intensity of experimental interests (e.g., 1 mA peak-to-peak).
- Check impedances once more. Then begin recording.
Representative Results
Examples are shown of unsuccessful and successful concurrent tACS-EEG measurements obtained from two different recordings (Figure 5). Two tACS electrodes were placed on the scalp (F3 and P3 electrodes) and the intensity of tACS was 0.9 mA (peak-to-peak). In the first example, the F3 EEG electrode was bridged with the frontal tACS electrode via gel (note that when mentioning "bridging" throughout the discussion below, we denote the formation of a direct connection by EEG gel creating a contact between the tACS and EEG electrodes). The bridging immediately saturates the F3 channel and EEG signals during tACS could not be recorded (Figure 5A). In the second example, EEG signals were successfully recorded while applying tACS (Figure 5B).
To evaluate the spatial distribution of the magnitude of the tACS artifact, the magnitude of the tACS artifact was calculated during successful recording obtained from three subjects. tACS was applied to either the DLPFC (F3 electrode) or PPC (P3 electrode). The intensity of tACS was 0.9 mA (peak-to-peak).It was observed that the peak-to-peak magnitude of the tACS artifact was inversely correlated with the distance between the EEG and tACS electrode (Figure 6A and 6B). In addition, the position of the EEG reference electrode in relation to the tACS electrode also influenced the spatial distribution of the magnitude of the tACS artifact across the EEG channels (Figure 6A and 6B). The magnitude of the tACS artifact ranges from 10 mV at EEG electrodes more distant from the site of stimulation, while the magnitude can reach up to 100 mV at the EEG electrode in the middle of the tACS electrode. The relationship between the current intensity of tACS and the magnitude of artifacts at the vicinity of the tACS electrode was also examined (Figure 7). It exhibited linear relationships and saturated the voltage range of recording when tACS current intensity was more than 1.6 mA.

Figure 1. Illustration of montage. (A) Montage with two tACS electrodes placed on the scalp (F3 and P3). (B) Montage with one tACS electrode placed on the scalp (F3) and one reference tACS electrode placed on the ipsilateral shoulder. (C) Montage with one tACS electrode placed on the scalp (P3) and one reference tACS electrode placed on the ipsilateral shoulder. (D) An elastic EEG cap holds the scalp tACS electrode in place under the cap. Please click here to view a larger version of this figure.
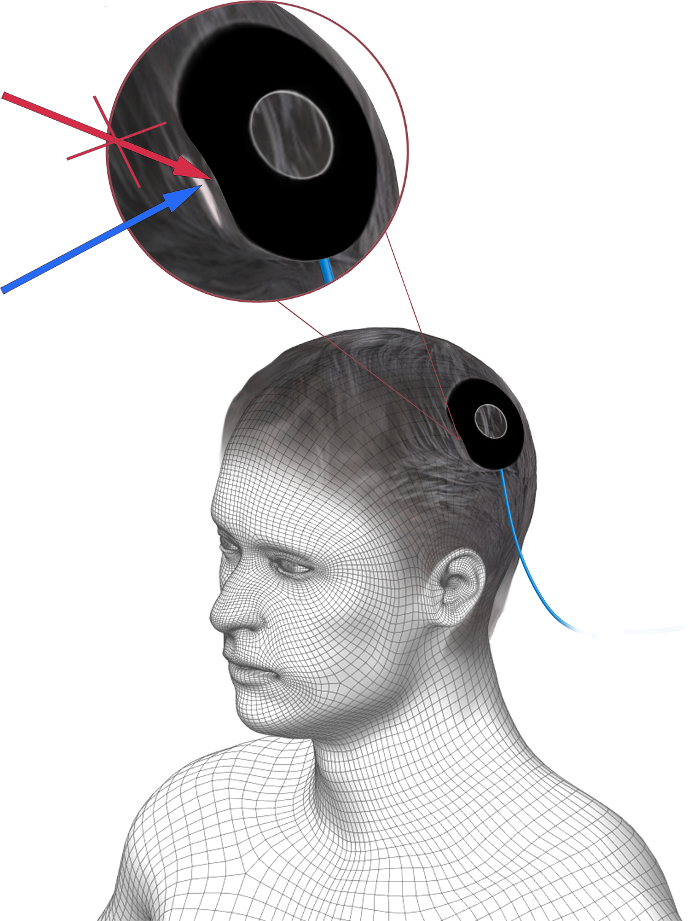
Figure 2. Correct application of additional EEG gel underneath a tACS electrode. Additional EEG gel is to be applied under the tACS electrode to improve the homogeneity of the connection to the scalp. The additional gel should be applied between the hair and the scalp (blue arrow), and not between the tACS electrode and hair, to improve the contact. Please click here to view a larger version of this figure.

Figure 3. Improving the connection of the EEG electrodes to the scalp. (A) Apply EEG gel to the EEG electrodes using a syringe. Use the tip of the needle to brush away hair below the EEG electrode, then carefully insert and bring down the needle until the tip of the needle touches the scalp. Apply gel while pulling the needle out, to create a connection between the scalp and the EEG electrode. (B) Use a wooden stick (e.g., the wooden handle of a cotton swab or similar) to further improve the contact between the EEG electrodes and the scalp. Push down the gel with the stick towards the scalp, and very gently rub the scalp with the top of the stick with a rotating motion. Try to keep the angle of the stick orthogonally to the scalp for electrodes located in a close vicinity of the tACS electrode, as sideway movements of the stick will spread out the gel under the electrode. If needed, apply some more EEG gel, and then use the wooden stick to further improve the impedance. For electrodes located in a close vicinity of the tACS electrode it is also important to be cautious with applying more gel for the purpose of improving contact. Rather try to improve contact as much as possible using the wooden stick. Finally, once good impedance has been achieved with the wooden stick, add some additional gel to stabilize the contact between the EEG electrode and the scalp. Please click here to view a larger version of this figure.

Figure 4. Example of leaking EEG gel creating direct contact between tACS and EEG electrodes. Leaking EEG gel, which creates direct contact between the tACS and the EEG electrode, is observed. Bridging such as this between the tACS and EEG electrodes can be created e.g., by adding an excess amounts of EEG gel under the tACS electrode or the EEG electrode in the vicinity of the tACS electrode, or by the tACS electrode being moved. Please click here to view a larger version of this figure.

Figure 5. tACS saturates the EEG amplifier through bridging via gel. Raw data from two different recordings, referenced to CPz, during montage with scalp tACS electrodes placed at the DLPFC (F3 electrode) and PCC (P3 electrode). (A) The signal recorded at electrode F3 is saturated due to bridging via leaking EEG gel between the F3 EEG electrode and the tACS electrode. (B) Signals are successfully recorded from all electrodes. The magnitude of the tACS artifact at the F3 electrode exceeds more than 50 mV. Please click here to view a larger version of this figure.

Figure 6. The magnitude of tACS artifacts across EEG channels. Peak-to-peak magnitudes of tACS artifacts averaged across three subjects (mV). The data is raw data, referenced to CPz. (A) The magnitude of the tACS artifact during the montage with one scalp tACS electrode placed at the left DLPFC (F3 electrode) and the other tACS electrode placed on the left shoulder (Montage 2, Figure 1B). (B) The magnitude of the tACS artifact during the montage with one tACS electrode placed on the left PPC (P3 electrode) and the other tACS electrode placed on the left shoulder (Montage 3, Figure 1C). (C) EEG channel locations. Red: channel under stimulation site, blue: channels in close vicinity of stimulation site, Ref (bold black): reference electrode (CPz). Please click here to view a larger version of this figure.

Figure 7. The magnitude of the tACS artifact linearly correlates with intensity of stimulation. Peak-to-peak magnitude of the tACS artifact (mV) from one subject at channel F3. Intensities of 0.5 to 2 mA were applied in steps of 0.1 mA. The data is raw data, referenced to CPz. Montage with one scalp tACS electrode placed at the left DLPFC (F3 electrode) and the other tACS electrode placed on the left shoulder (Montage 2, Figure 1B). The data shows a perfect linear relationship between intensity of stimulation applied and magnitude of tACS artifact, in the intensity range of 0.5 to 1.6 mA. The voltage resolution was set to 150 mV, but the actual maximal acquisition range was 161.6 mV beyond which the signal was saturated. The dashed line marks the maximal range of the voltage. With stimulation intensities of 1.7 mA and higher, when resulting artifact magnitudes were more than 161.6 mV, the F3 channel was saturated. Please click here to view a larger version of this figure.
Discussion
The procedures to set up concurrent tACS-EEG experiments are described here. We now turn to discuss considerations for the setup of the tACS-EEG recordings, of which the two first considerations are vital for successful concurrent tACS-EEG recordings.
Avoiding tACS-EEG electrode bridging via gel
It is crucial to avoid bridging between EEG and tACS electrodes through leaking EEG gel, as bridging immediately saturates the respective channel of an EEG amplifier. For this reason the viscosity of the EEG gel is a crucial parameter for successful tACS-EEG recording. Never use a fluid EEG gel, as a fluid EEG gel risks escaping out from the tACS electrode and bridge with adjacent EEG electrodes. At the same time, a very viscous EEG gel has a disadvantage in penetrating the hair and lubricating the skin to reduce the impedance. For the EEG electrodes in close proximity of the tACS electrode, a more viscous gel can be used, as one can use a wooden stick to lower the impedance. For the tACS and remaining EEG electrodes, use a slightly less viscous (though still not fluid) EEG gel. This type of gel requires less effort to lower impedances. As it is difficult to scrape under the tACS electrode, it is better to use a slightly less viscous gel here.
Dealing with tACS artifact magnitudes
The second issue is to handle the large magnitude of the tACS artifact, ranging from 10 mV at EEG electrodes distant from the area of stimulation, to more than 100 mV at the site of stimulation during the present stimulation intensity of 0.9 mA (Figure 6). Figure 7 illustrates the linear relationship between stimulation intensities (0.5 to 2.0 mA peak-to-peak) and the resulting magnitude of the artifact at the site of stimulation (channel F3). A first measure is to keep a low impedance of both EEG and tACS electrodes. Insufficient contact between the tACS electrode and the scalp creates larger amplitudes of the tACS artifact in the EEG data, and in addition applied electronic current would tend to be inhomogeneous. Second, one needs to consider the resolution level of the A/D converter of the EEG system. A 24 bits A/D converter can theoretically cover a range of 1.68 V with a 0.1 µV/bit resolution. In contrast, a 16 bits A/D converter with a 0.1 µV/bit resolution would cover a voltage range of 6.5 mV – too low to cover the range of the tACS artifact (Figure 6). Hence the voltage recording resolution needs to be lowered. In order to cover artifact magnitudes of up to 100 mV at the site of stimulation with a 16 bits system, the voltage recording resolution would theoretically need to be lowered to above 1.53 µV/bit. In fact recent concurrent tACS-EEG studies with a 16 bits system could not record the EEG signals from the vicinity of the stimulation site due to saturation of the amplifier even when the resolution was lowered to 0.5 µV/bit12,13.
Considerations for reducing electrode impedance
The reason to first begin working on the impedances of the EEG electrodes located in the middle or vicinity of the tACS electrode, is that these EEG electrodes require some patient and careful work to avoid bridging. By starting with these electrodes, there is time to wait until the applied gel has had some time to lubricate the scalp, before considering applying more EEG gel if necessary. Additional gel should be applied under the tACS electrode once it has been placed on the scalp, in particular if the participant has a lot of hair. The reason is not just to reduce impedance – good impedance can be achieved without this step – but to achieve a uniform connection with the scalp throughout the surface of the tACS electrode.
Design and montage considerations
Figure 1 illustrates the montage of the tACS electrodes. The doughnut-shaped design of the scalp tACS electrode/electrodes and the rectangular shoulder tACS electrode are depicted. The shape of the scalp tACS electrode allows for an EEG electrode to be placed in the middle of the stimulated area. One advantage of the doughnut shaped design is that it allows for recording signal from the stimulated area. Secondly, it also makes it easy to keep the position of the tACS electrode unchanged. Depending on the site of stimulation, some other shape of the tACS electrode would be more suitable. A rectangular tACS electrode shape is better suited when recording from a site in between EEG electrodes.
It should be cautioned that the shape and position of the tACS electrode is not the same as the area actually being stimulated, but might be slightly shifted31. When deciding the position of the tACS electrodes, modeling of the current flow to estimate the best position of the electrodes for targeting the region of interest is always strongly advised.
The current setup is suitable for the modulation of rhythmic activity in large-scale networks. More focal stimulation can be achieved in several ways13,32,33,34. First, reduce the size of the tACS electrode. Nitsche and colleagues have shown that a 3.5 cm2 electrode can modulate the excitability of the motor cortex with tDCS32. A second approach is to exploit a high-definition configuration13,33,34, where one stimulation electrode is surrounded by four reference electrodes. Another advantage of the high definition configuration is that the density of EEG electrodes can be increased, since conventional rubber electrodes limit the space to place EEG electrodes and sixty four EEG electrodes is not feasible to implement in the current setup. While these modifications for higher spatial specificity require different setup procedures, the technical considerations described here still apply.
In this protocol we place the tACS electrodes according to the international 10-20 system for EEG electrode positioning30. Whileindividual optimization of a stimulation location would be the alternative, it might constitute a problem for comparison when varying the stimulation location among individuals in the experiment, as the stimulation site varies in relation to the EEG recording sites. The recently demonstrated combined use of magnetoencephalography (MEG) and tACS, by Neuling and colleagues35, might overcome this problem and tACS artifact-related problems, as spatial filtering methods with MEG beamforming allows to estimate brain activity independent of a tACS site.
Concerning the montage, two monopolar montages are described here, i.e., with extracephalic location of the reference electrode (Figure 1B and 1C), and one unipolar montage, i.e., with both electrodes located on the scalp (Figure 1A) (see further classifications of electrode montages by Nasseri et al.36). The advantage of using a monopolar montage is the avoidance of additional cephalic stimulation of no interest for the study. The primary concern when choosing a monopolar montage is current flow though subcortical structures including the brainstem, with the potential risk of modulating vital brainstem functions. Both extracephalic and ipsilateral shoulder placement of the reference electrode has been confirmed not to modulate brainstem functions for 1 mA intensity of tDCS37,38 (e.g., heart rate variability, respiratory rate and blood pressure). As a monopolar montage can have clear advantages depending on the experimental design, there is a need for comprehensively testing the effect on vital brainstem functions during higher stimulation intensities and different monopolar montages, as well as for comparing the influence between tDCS and tACS.
Note that high-definition configuration is another solution for avoiding the problem of the bipolar montage of additional cephalic stimulation of no interest. The high-definition configuration with one stimulation electrode surrounded by four reference electrodes leads to high current density under the center electrode and low current density under the four surrounding electrodes. As the effect of stimulation depends on the density of the current, this means a unidirectional modulation under the center electrode for the high-definition configuration, in contrast to the bi-directional modulation of a two electrode configuration39.
Visual flicker perception induced by tACS is a critical limiting factor for the stimulation intensity when placing the tACS electrode on the frontal lobe, due to retinal stimulation by tACS. In particular, tACS at beta-band frequency induces visual flickering even at low intensity of tACS11. In our experience 0.9 mA (peak-to-peak) stimulation over the DLPFC (F3 electrode) at 6 Hz is a suitable intensity level to minimize the sensation of visual flickering.
Depending on the design of the experiment, it might be necessary to control the stimulator with an external device (if this function is available for the stimulator used). We use a waveform analog output board to control the stimulator and send triggers to the EEG amplifier (see further hardware and software specifications in the table of materials). In case of the stimulator that used here (see Table of Materials), the noise level of current output with the remote control is higher than that with the embedded stimulator interface. Hence the option to remote-control the stimulator should be chosen only if required by the experimental design.
Troubleshooting saturation of EEG channels
We have shown that bridging between the tACS and EEG electrodes via leaking EEG gel results in saturating the respective channel of the EEG amplifier and rules out recording data from these electrodes (Figure 5A). There are other reasons for the saturation of an EEG channel. One reason can be that the gain of the amplifier is too narrow, and the voltage recording resolution has not been adjusted accordingly. In this case the voltage recording resolution needs to be lowered to cover the range of the magnitude of the tACS artifact. Another reason is that the recording site is too close to the stimulation site. In this case, even a very coarse voltage recording resolution might still not cover the range of the artifact. Recording should be located further away from the stimulation site.
The current protocol comprehensively depicts the settings and technical considerations for concurrent tACS-EEG experiments. With methods to remove the tACS artifact and protocols for good quality recording during tACS, tACS will truly be a promising method tofurther our understanding of the most prominent feature of brain activity, rhythmic dynamics.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This project has been supported by the Japan Science and Technology Agency (JST) PRESTO program.
Materials
| Stimulator for tACS: Eldith DC-Stimulator plus | NeuroConn GmbH, Germany | For remote input, be sure to order a model with this feature enabled | |
| Analog Output board for sending triggers: Static and Waveform Analog Output board, model NI PCI-6723 | National Instruments, USA | 13-bit, 32 channels. | |
| Matlab and data acquisition toolbox | The MathWorks, Inc., USA | The 'Data acquisition toolbox' available for MATLAB provides functions to control data acquisition hardware such as an analog output board, produced by several manufacturers. | |
| EEG system: eegosports, with a 32 channel waveguard EEG cap | ANT neuro, Netherlands | ||
| tACS electrodes | NeuroConn GmbH, Germany | 305090-05 305050 | Materials: conductive-rubber electrodes. Dimensions of scalp electrodes: Outer Ø: 60 mm, Inner Ø:25 mm (Part# 305090-05) Cut from the original size Ø 75mm Dimensions of shoulder electrode: 50 x 50 mm (Part# 305050) |
| EEG gel | Inselspital, Bern, Switzerland | Electrode paste, containing abrasives (i.e. pumice) which scrub the skin, improving the electrode-to-skin contact. | |
| Abrasive skin preparing gel for EEG and electrocardiography: Nuprep | Weaver and Company, USA | ||
| Cotton swabs, wooden handle | Salzmann MEDICO, Switzerland | Dimensions: 150 x 1.5 mm; wooden handle Ø 2.2 mm |
|
| Adhesive tape: Leukofix | BNS medical GmbH, Germany | 04.107.12 |
References
- Berger, P. D. H. On the electroencephalogram of humans. Arch Psychiatr Nervenkr. 87 (1), 527-570 (1929).
- Finger, S. . Origins of Neuroscience: A History of Explorations Into Brain Function. , (2001).
- Engel, A. K., Fries, P., Singer, W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2 (10), 704-716 (2001).
- Varela, F., Lachaux, J. P., Rodriguez, E., Martinerie, J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2 (4), 229-239 (2001).
- Fries, P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 9 (10), 474-480 (2005).
- Canolty, R. T., Knight, R. T. The functional role of cross-frequency coupling. Trends Cogn Sci. 14 (11), 506-515 (2010).
- Fell, J., Axmacher, N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 12 (2), 105-118 (2011).
- Thut, G., Miniussi, C., Gross, J. The functional importance of rhythmic activity in the brain. Curr Biol. 22 (16), R658-R663 (2012).
- Buzsáki, G., Draguhn, A. Neuronal oscillations in cortical networks. Science. 304 (5679), 1926-1929 (2004).
- Paulus, W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 21 (5), 602-617 (2011).
- Kanai, R., Chaieb, L., Antal, A., Walsh, V., Paulus, W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. 18 (23), 1839-1843 (2008).
- Helfrich, R. F., Schneider, T. R., Rach, S., Trautmann-Lengsfeld, S. A., Engel, A. K., Herrmann, C. S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 24 (3), 333-339 (2014).
- Helfrich, R. F., et al. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol. 12 (12), e1002031 (2014).
- Zaehle, T., Rach, S., Herrmann, C. S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PloS One. 5 (11), e13766 (2010).
- Neuling, T., Rach, S., Herrmann, C. S. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front Hum Neurosci. 7, 161 (2013).
- Feurra, M., Paulus, W., Walsh, V., Kanai, R. Frequency specific modulation of human somatosensory cortex. Front Psychol. 2, 13 (2011).
- Laczò, B., Antal, A., Niebergall, R., Treue, S., Paulus, W. Transcranial alternating stimulation in a high gamma frequency range applied over V1 improves contrast perception but does not modulate spatial attention. Brain Stimul. 5 (4), 484-491 (2012).
- Strüber, D., Rach, S., Trautmann-Lengsfeld, S. A., Engel, A. K., Herrmann, C. S. Antiphasic 40 Hz oscillatory current stimulation affects bistable motion perception. Brain Topogr. 27 (1), 158-171 (2014).
- Joundi, R. A., Jenkinson, N., Brittain, J. -. S., Aziz, T. Z., Brown, P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol. 22 (5), 403-407 (2012).
- Wach, C., Krause, V., Moliadze, V., Paulus, W., Schnitzler, A., Pollok, B. Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) on motor functions and motor cortical excitability. Behav Brain Res. 241, 1-6 (2013).
- Wach, C., Krause, V., Moliadze, V., Paulus, W., Schnitzler, A., Pollok, B. The effect of 10 Hz transcranial alternating current stimulation (tACS) on corticomuscular coherence. Front Hum Neurosci. 7, 511 (2013).
- Pogosyan, A., Gaynor, L. D., Eusebio, A., Brown, P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 19 (19), 1637-1641 (2009).
- Santarnecchi, E., et al. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol. 23 (15), 1449-1453 (2013).
- Polanìa, R., Nitsche, M. A., Korman, C., Batsikadze, G., Paulus, W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol. 22 (14), 1314-1318 (2012).
- Jaušovec, N., Jaušovec, K. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Biol Psychol. 96, 42-47 (2014).
- Jaušovec, N., Jaušovec, K., Pahor, A. The influence of theta transcranial alternating current stimulation (tACS) on working memory storage and processing functions. Acta Psychol (Amst). 146, 1-6 (2014).
- Sela, T., Kilim, A., Lavidor, M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci. 6, 22 (2012).
- Voss, U., et al. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci. 17 (6), 810-812 (2014).
- Morishima, Y., Fehér, K. D. A method for removing tACS artifacts from EEG data. Program No. 303.05. Neuroscience 2014 Abstracts. , (2014).
- Jasper, H. H. The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol. 10, 371-375 (1958).
- Dmochowski, J. P., Datta, A., Bikson, M., Su, Y., Parra, L. C. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 8 (4), 046011 (2011).
- Nitsche, M. A., et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 97 (4), 3109-3117 (2007).
- Villamar, M. F., Volz, M. S., Bikson, M., Datta, A., Dasilva, A. F., Fregni, F. Technique and considerations in the use of 4×1 ring high-definition transcranial direct current stimulation (HD-tDCS). J Vis Exp. (77), e50309 (2013).
- Datta, A., Bansal, V., Diaz, J., Patel, J., Reato, D., Bikson, M. Gyri -precise head model of transcranial DC stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2 (4), 201-207 (2009).
- Neuling, T., Ruhnau, P., Fuscà, M., Demarchi, G., Herrmann, C. S., Weisz, N. Friends, not foes: Magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. Neuroimage. 118, 406-413 (2015).
- Nasseri, P., Nitsche, M. A., Ekhtiari, H. A framework for categorizing electrode montages in transcranial direct current stimulation. Front Hum Neurosci. 9, 54 (2015).
- Vandermeeren, Y., Jamart, J., Ossemann, M. Effect of tDCS with an extracephalic reference electrode on cardio-respiratory and autonomic functions. BMC Neurosci. 11, 38 (2010).
- Santarnecchi, E., et al. Time Course of Corticospinal Excitability and Autonomic Function Interplay during and Following Monopolar tDCS. Front Psychiatry. 5, 86 (2014).
- Datta, A., Elwassif, M., Battaglia, F., Bikson, M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng. 5 (2), 163-174 (2008).

