Alternative Method of Removing Otoliths from Sturgeon
Summary
The goal of the protocol is to show an effective method to extract the otoliths from sturgeon carcasses.
Abstract
Extracting the otoliths (ear bones) from fish that have very thick skulls can be difficult and very time consuming. The common practice of making a transverse vertical incision on the top of the skull with a hand or electrical saw may damage the otolith if not performed correctly. Sturgeons (Acipenseridae) are one family in particular that have a very large and thick skull. A new laboratory method entering the brain cavity from the ventral side of the fish to expose the otoliths was easier than other otolith extraction methods found in the literature. Methods reviewed in the literature are designed for the field and are more efficient at processing large quantities of fish quickly. However, this new technique was designed to be more suited for a laboratory setting when time is not pressing and successful extraction from each specimen is critical. The success of finding and removing otoliths using this technique is very high and does not compromise the structure in any manner. This alternative technique is applicable to other similar fish species for extracting the otoliths.
Introduction
Sturgeon (Acipenseridae) populations throughout the world have been declining for over a century due to impacts including habitat loss, population fragmentation, and overfishing such that many populations are protected by state and federal laws1,2. Management agencies throughout the USA and the world have identified sturgeon as a target species for restoration and recovery1. Population characteristics (growth, recruitment, diet) of most sturgeon populations have been studied to better understand basic biology and life history characteristics1. Due to the protected status of most sturgeon populations, age evaluation is difficult in small threatened populations where no sacrifice or harm to individuals is warranted.
The extraction of bony structures for the purpose of age estimation and growth analysis has been a common technique3.The use of fin ray sections to determine age of sturgeon has become a practical nonlethal method, but verification of the technique has been limited and age can be very difficult to decipher in older individuals (> 15 years)4. Researchers evaluating the use of multiple bony structures in sturgeon for their use in validating the age of Gulf Sturgeon (Acipenser oxyrinchus desoti)5, Atlantic Sturgeon (A. o. oxyrinchus)6, White Sturgeon (A. transmontanus)7, and Lake Sturgeon (A. fluvescens)8 concluded that the first marginal pectoral fin ray provided the most accurate age but concluded this invasive procedure to be deleterious to larger individuals. Currently, there is the development of using otolith shape metrics to distinguish between North American sturgeon species with a very high rate of success9. Details on the process of removing the otolith with a dorsal transverse incision at the base of the skull is a common otolith extraction method found throughout the literature and clearly detailed by Secor et al. (1991)10. However, this method is difficult to implement on sturgeon that have very thick skulls with otoliths that are small and delicate and would tend to break. A further review of the literature shows no other detailed methodology of the otolith removal process for sturgeon. The objective of this paper was to detail an alternative method of extracting otoliths from sturgeon by entering the brain cavity from the ventral side.
Protocol
Important Note: Due to the protected status of all species of sturgeon in the United States, permits to acquire and handle fish were obtained prior to laboratory procedure. All specimens were acquired from hatchery sources post mortem due to natural causes and disposed of following standard procedure.
1. Specimen Preparation
- Before any incisions lay sturgeon carcass on its side and using a measuring tape to measure total length from the snout to the tip of the tail to the nearest 1 mm. (Figure 1A)
- Weigh sturgeon carcass on a tared scale to the nearest 1 g. Evaluate carcass for any external tags or marks and record.
2. Otolith Extraction
- Make a vertical incision with a large fillet knife between the base of the skull and the first dorsal scute through the body to separate the head from the body (Figure 1B). After removing the head, turn the head so the mouth is facing up (Figure 1C).
- Remove soft tissue of the mouth and gills by using the fillet knife to cut along the ventral side of the skull (Figure 1D). Make an incision with an electric bone saw from the tip of the snout to the base of the skull to completely bisect the head (Figure 1E). Separate the two halves by hand to expose the brain cavity (Figure 2A).
- Remove and discard brain matter (Red box left) with forceps to view semicircular canals (Red box right) within the brain cavity (Figure 2B).
- Remove semicircular canal cartilage from brain cavity with forceps (Figure 2C). Carefully puncture semicircular canal cartilage with the tip of the forceps and remove otolith (Figure 2D). Remove the otolith (hard bony structure inside the cartilage) with forceps from the semicircular canal cartilage (Figure 2E).
- Repeat steps 2.3 – 2.4 to remove otolith from the other half of the skull.
- Dip the otoliths in 92% Ethanol for 5 sec to remove any cartilage. Place otoliths in an open 25 ml scintillation vial to dry for 24 hr.
Representative Results
The adult size of sturgeon across the world varies greatly but the location of the otoliths is consistent. The use of a sharp fillet knife was found to remove the soft tissue of the mouth easily shown in Figure 1D. An electric bone saw is the preferred tool for the lateral incision to expose the brain cavity in Figure 1E. However, attention to bisecting the midline of the skull is needed otherwise the otoliths may be crushed during this process. If this process is performed correctly then the brain cavity will be exposed evenly with no damage to the otoliths as shown in Figure 2A. The brain matter is easily removed with the use of forceps as in Figure 2B and finding the semicircular canal cartilage which contains the otolith is easily found at the base of the brain cavity as shown in Figure 2C. The cartilage found in the semicircular canal is easily removed from the otolith with a pair of forceps as viewed in Figure 2D.
This procedure successfully shows the removal of the sturgeon otolith and is a preliminary step in a more quantitative analysis of the fish's life history. Outcome success can be gauged by the removal of the otolith without breaking the structure. Age determination and growth rates are two metrics that can be evaluated with the use of the removed otoliths. Shape analysis for species differentiation is another measurable metric that can be developed from the otoliths.
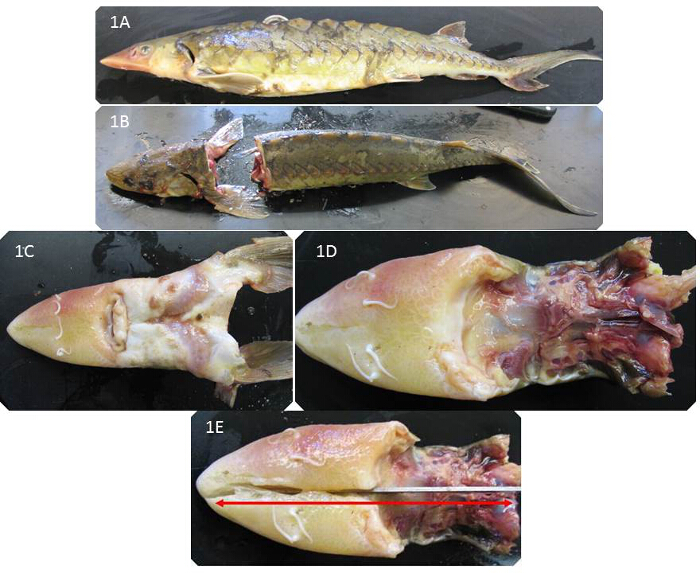
Figure 1. Placement of Sturgeon Carcass for Otolith Removal. (A) Sturgeon Carcass Lateral View for Measurement and Weighing, (B) Vertical Incision to Separate Sturgeon Head from Body, (C) Flip Head to View Mouth and Ventral Side of Head, (D) Soft Tissue Removed to Expose Ventral Side of Skull, (E) Lateral Incision from Tip of Snout to Base of Skull Please click here to view a larger version of this figure.
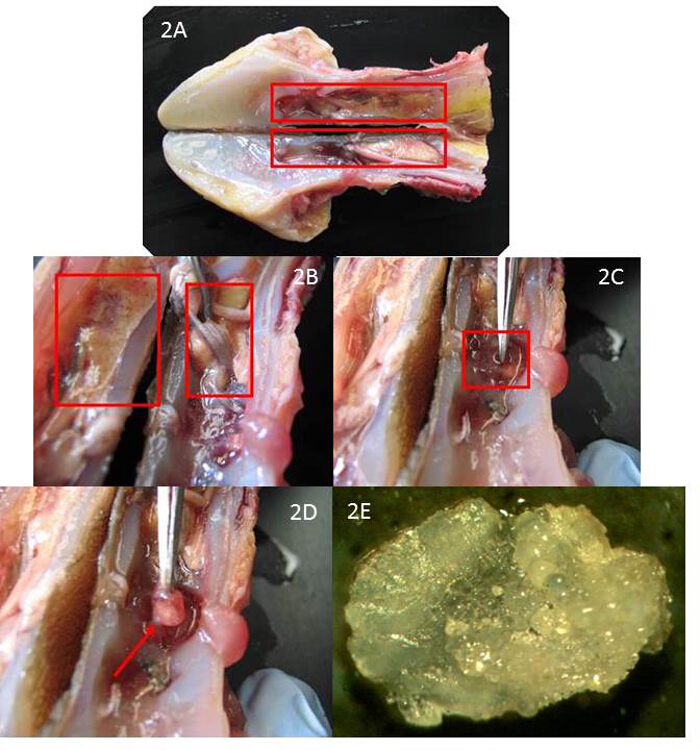
Figure 2. Internal Examination of the Brain Cavity to Remove Otoliths. (A) Brain Cavity Exposed after Head is Separated, (B) Brain Matter Removed to Expose Semicircular Canals, (C) Semicircular Canal Cartilage Removed, (D) Otolith Viewed and Removed from Semicircular Canal Cartilage, (E) Shortnose Sturgeon Otolith Please click here to view a larger version of this figure.
Discussion
The overview of an alternative method of extracting otoliths from sturgeon carcasses has been detailed. It is important to note that special attention is needed to the lateral incision placement on the midline of skull in order to bisect the skull evenly to ensure no harm to the otoliths during dissection. If the incision is not deep enough to completely bisect the skull it will be very difficult to expose the brain cavity where the location of the otoliths is clearly visible. With experience, the overall technique is relatively simple and leads to a high percentage of the otoliths being found intact. This new method gives researches an alternative way to extract otoliths for species other than sturgeon that may be difficult using standard otolith extraction methods.
Limitations to this method are the applicability to a laboratory setting not for field use. The time needed for carcass preparation is not conducive to samples collected in the field in a timely manner. Another concern is the use on sturgeon species that are typically protected under state or federal law due to population declines.
It is very difficult to obtain permits to conduct this type of evaluation at the expense of sacrificing individuals that are needed to keep populations alive. However, when dead specimens are available through natural causes this technique provides a useful way to collect more information than would be available otherwise.
It is important to note the significance of this technique to successfully remove otoliths from sturgeon using this alternative method. Existing techniques removing otoliths from the dorsal region of the head are still useful and beneficial but not applicable for sturgeon. Using this new technique to expose the brain cavity also gives access and applicability to other areas neural function and brain morphology. This new technique may be beneficial to use on other species of fish with very thick skull walls or where there is a limited amount of fish to use for otolith extraction.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Special thanks to the St. Regis Mohawk Environmental Division, Welaka National Fish Hatchery, US Fish and Wildlife Service Research Unit, Southern Illinois University, US Fish and Wildlife Service Northeast Fisheries Center, Sterling Caviar, University of California-Davis, Garrison Dam National Fish Hatchery, Bears Bluff National Fish Hatchery, and US Fish and Wildlife Service Panama City Fisheries Resource Office for their help supplying sturgeon for this project. This article is Contribution 2013 of the USGS Great Lakes Science Center.
Materials
| Measuring Board | Metric meter stick is preferred | ||
| Weighing scale | |||
| Filleting Knife | |||
| Scalpel | |||
| Forceps | |||
| Sample vials | 25 ml scintillation vials work well |
References
- Auer, N. A., LeBreton, G. T. O., Beamish, F. W. H., McKinley, R. S. Conservation. Sturgeons and Paddlefish of North America. 27, 252-276 (2004).
- Campana, S. E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods). Journal of Fish Biology. 59, 197-242 (2001).
- Bruch, R. M., Campana, S. E., Davis-Foust, S. L., Hansen, M. J., Janssen, J. Lake Sturgeon age validation using bomb radiocarbon and known-age fish. Transactions of the American Fisheries Society. 138, 361-372 (2009).
- Baremore, I. E., Rosati, J. D. A validated, minimally deleterious method for ageing sturgeon. Fishery Bulletin. 112, 274-282 (2014).
- Stevensen, J. T., Secor, D. H. Age determination and growth of Hudson River Atlantic sturgeon, Acipenser oxyrhynchus. Fishery Bulletin. 97, 153-166 (1999).
- Brennan, J. S., Cailliet, G. M. Comparative age-determination techniques for white sturgeon in California. Transactions of the American Fishery Society. 118, 296-310 (1989).
- Rossiter, A., Noakes, D. L. G., Beamish, F. W. H. Validation of age estimation for the lake sturgeon. Transactions of the American Fisheries Society. 124, 777-781 (1995).
- Chalupnicki, M. A., Dittman, D. E. North American Sturgeon Otolith Morphology. Copeia. , 260-266 (2016).
- Secor, D. H., Dean, J. M., Laban, E. H. . Manual for otolith removal and preparation for microstructural examination. , (1991).

