Sediment Core Extrusion Method at Millimeter Resolution Using a Calibrated, Threaded-rod
Summary
An extrusion method using a calibrated threaded-rod is presented, which allows for mm scale subsampling of aquatic sediment cores. Millimeter-scale sampling is necessary to fully characterize recent event stratigraphy in sediment records.
Abstract
Aquatic sediment core subsampling is commonly performed at cm or half-cm resolution. Depending on the sedimentation rate and depositional environment, this resolution provides records at the annual to decadal scale, at best. An extrusion method, using a calibrated, threaded-rod is presented here, which allows for millimeter-scale subsampling of aquatic sediment cores of varying diameters. Millimeter scale subsampling allows for sub-annual to monthly analysis of the sedimentary record, an order of magnitude higher than typical sampling schemes. The extruder consists of a 2 m aluminum frame and base, two core tube clamps, a threaded-rod, and a 1 m piston. The sediment core is placed above the piston and clamped to the frame. An acrylic sampling collar is affixed to the upper 5 cm of the core tube and provides a platform from which to extract sub-samples. The piston is rotated around the threaded-rod at calibrated intervals and gently pushes the sediment out the top of the core tube. The sediment is then isolated into the sampling collar and placed into an appropriate sampling vessel (e.g., jar or bag). This method also preserves the unconsolidated samples (i.e., high pore water content) at the surface, providing a consistent sampling volume. This mm scale extrusion method was applied to cores collected in the northern Gulf of Mexico following the Deepwater Horizon submarine oil release. Evidence suggests that it is necessary to sample at the mm scale to fully characterize events that occur on the monthly time-scale for continental slope sediments.
Introduction
Sediment core samples from lacustrine, estuarine, and marine (continental shelf and slope) environments have provided records of salinity, temperature, organic and inorganic pollutants and many other environmental parameters on decadal to millennial time-scales1-3,6,8,13,17. In most cases, the standard practices are to section these cores at half-centimeter or centimeter intervals5,15. This resolution is appropriate for multi-year, decadal or higher scale resolution in most cases. The need for increased extrusion resolution has recently been demonstrated in some reports which detected variability of sedimentary biomarkers/proxies on a fine scale along the vertical profile of the sediment core11,16. In the case of recent sedimentation that occurs on time-scales of months to one year, it is then necessary to use finer resolution subsampling methods (e.g., mm scale). This is often challenging with aquatic sediments due to the unconsolidated nature of the surface sediments.
We present a sediment core extrusion method that provides mm scale sediment sub-samples. We then apply this extrusion method to the sediments of the northern Gulf of Mexico following the Deepwater Horizon (DWH) event. This application demonstrates the efficacy of millimeter-scale subsampling in characterizing sub-annual event stratigraphy related to anthropogenically influenced depositional systems.
Monthly or sub-annual scale resolution in sedimentary records is particularly advantageous when characterizing short-term event stratigraphy. Environmental assessments using sub-annual resolution are able to fully characterize anthropogenically induced sedimentation events.
Sediments in the northern Gulf of Mexico that were affected by the Deepwater Horizon oil event provide an example of event stratigraphy fully characterized using millimeter (sub-annual) scale resolution sampling. Following the Deepwater Horizon (DWH) event in 2010, continental slope sediments of the northeastern Gulf of Mexico (nGoM) came in contact with hydrocarbons through an order of magnitude increase in flocculent hydrocarbon deposition4,9,10,12,14,18. The increase in sedimentation was caused by a Marine Oil Snow Sedimentation and Flocculent Accumulation (MOSSFA) event4,9,10,12,14,18. This resulted in approximately 6-10 mm of sediment accumulation in a 6-12 month period from mid-2010 to early 20114. It was necessary to sub-sample these sediment cores at millimeter-scale to fully characterize the inputs, rates of sedimentation, and post-depositional processes.
Protocol
1. Collect Sediment Cores
- Collect aquatic sediment core using multi-core, box core, piston core, etc.4,7,12,14. Ensure that the core section is 1 m or less.
- Insert polycarbonate or acrylic puck into the bottom of the core. Ensure that the puck is consistent with the inner diameter of the core tube. Insert a rubber gasket on the outermost diameter of the puck to retain the entirety of the sediment core.
- Upon retrieval of the core, extrude immediately or package for transport and storage (see steps 1.4 through 1.6 for storage and transport).
- Insert a foam or acrylic puck into the surface of the core tube and gently press down until the foam or acrylic is just above the sediment interface to maintain the integrity of the sediment-water interface during transport and storage.
- Place cap on top of the core tube and seal with electrical tape. Place cap on bottom of core tube and seal with electrical tape. Label the top cap with necessary project and sample identifiers.
- Store cores at desired temperature based on desired analysis.
NOTE: For example, cores used for organic chemical analysis or biological analysis can be frozen (-20 °C), while short-lived radioisotope cores can be stored at ambient temperature (~20-25 °C).
2. Prepare Sub-sample Vessels and Tools
- Label sub-sample vessels (e.g., jars, bags or beakers) with project name, core site and increment (e.g., PROJECT NAME_CORE SITE_0 – 2 mm), along with any other pertinent identification information (e.g., date, core type).
- Assemble and sterilize (methanol) necessary cutting implements (e.g., acrylic paddles, putty knives, etc.) and personal protective equipment (e.g., gloves, lab coats, etc.).
Note: These implements and their sterilization procedures will depend on the type of analysis to be done on each sub-sample. For example, the use of metal and acrylic implements (as opposed to plastic) is essential for organic chemical analysis, whereas acrylic and plastic implements (as opposed to metal) must be used for inorganic trace element analyses.
3. Prepare Sediment Core for Extrusion
- If the core has been stored or preserved, remove the bottom cap first. To do this cut the bottom cap off with a razor blade and allow for the top cap to maintain a vacuum, which holds the sediment in the tube while transferring to the extruder (the extrusion puck must already be inserted in the bottom of the preserved cores) (Figure 1).
- When extruding immediately upon collection, insert the extrusion puck into the bottom of the core. Then gently set the core tube onto the piston and fasten the core to the extruder using the clamps.
- Ensure that there is at least 5 cm of core tube remaining above the uppermost clamp for the sampling collar.
- Remove top cap.
- Place the sampling collar on top of the core tube. Make sure the collar is sitting flush with the uppermost extent of the core tube to avoid any sample loss.
- Sample (or discard if not needed) the water above the sediment at this point using a syringe or siphon.
- After extracting the water, begin turning the piston to align the surface-most sediment with the surface of the sampling collar.
4. Extrusion
- Turn the piston to the desired sampling resolution (typically 1-2 mm, 1 full rotation = 2 mm sub-sample)(Figure 1).
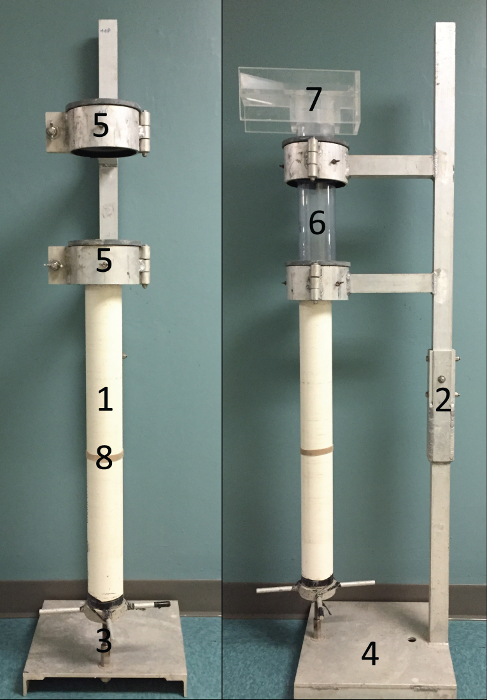
Figure 1: Photographs of Extruder. Photographs of the extruder defining the piston (1), coupling (2), threaded-rod (3), extruder base (4), clamps (5), core tube (6), sampling collar (7), and rubber band (8). Please click here to view a larger version of this figure.
- Use the acrylic plate (cut to the inner diameter of the sampling collar) to make the initial sample cut. Subsequently move the sub-sample towards the edge of the sampling collar slowly while positioning the appropriate sampling vessel below the mouth of the sampling collar.
- Begin to push the sample into the sampling vessel. After the majority of the sample is in the vessel, use the smaller implements (e.g., putty knife) to move any remaining sample in the sampling collar to the sampling vessel.
- Use the smaller implement to clean the remaining amount of sample from the acrylic plate and any other sampling surface into the sampling vessel.
- Once the sample is completely transferred from the sampling collar to the vessel, clean the sampling tools with deionized water, laboratory wipes and/or other sterilizing fluid (e.g., methanol). Clean the sampling collar accordingly with laboratory wipes, deionized water and other sterilizing solution.
- Seal the sample vessel and prepare the next sample vessel for extrusion. Repeat steps 4.1 through 4.5 for each subsample.
5. Resetting the Extruder
- Reset the extruder manually. Use a drill and rubber band to expedite the process of resetting the piston to the bottom of the threaded-rod.
- Place the rubber band around the piston near the base for the most stability.
- Stretch the rubber band around the head of the drill and set the direction of the drill to rotate the piston downward.
- Rotate the piston using a low speed on the drill until it reaches the desired height above the base of the extruder.
Note: This height is based on the length of the core to be extruded and desired sampling height.
Representative Results
Cores from site DSH08 were collected in December 2010 (29° 7.25' N, 87° 51.93' W, 1,143 m depth) using an Ocean Instruments MC-800 multicorer. These cores were extruded at 2 mm for the surficial 15 cm (or more) using the protocol above. The pre-DWH (before 2010) and post-DWH (2010) intervals of the core were determined using a paired short-lived radioisotope (234Th and 210Pb) geochronology4. Several other analyses were performed to constrain the sedimentary inputs, deposition rates, and post-depositional processes at this site following the Deepwater Horizon event. In addition to short-lived radioisotope analysis, total aliphatic concentration12, redox sensitive metals (manganese, rhenium)7, and total benthic foraminiferal density14 were quantified. A comparison of each of these parameters at the mm scale and cm scale was performed (Tables 2 and 3, Figure 2). Centimeter scale data was composed of integrated, mean mm scale data.
| Top Depth (mm) | Excess Pb-210 (dpm/g) |
Excess Th-234 (dpm/g) |
Th-234 and Pb-210 Merged Age model (year) |
Total Foraminiferal Density (indiv./cm3) |
[Re] (ng/g) |
[Mn] (mg/g) |
Total Aliphatics (ng/g) |
| 0 | 71.81 | 6.19 | 2010.9 | 1 | 336922.6 | ||
| 2 | 71.81 | 5.14 | 2010.9 | 3 | 0.69 | 10.2 | 53701.4 |
| 4 | 69.91 | 2.72 | 2010.8 | 2 | 0.53 | 15.9 | 77081.2 |
| 6 | 70.32 | 1.57 | 2010.8 | 6 | 0.57 | 12.1 | 48057.4 |
| 8 | 69.67 | 1.15 | 2010.7 | 10 | 0.61 | 11.3 | 42888.0 |
| 10 | 61.39 | 0.29 | 2009.6 | 10 | 0.73 | 8.30 | 50786.4 |
| 12 | 56.50 | 0.64 | 2008.5 | 12 | 0.75 | 7.1 | 51582.9 |
| 14 | 63.31 | 0.00 | 2007.5 | 11 | 52126.8 | ||
| 16 | 51.55 | 0.00 | 2006.5 | 11 | 0.79 | 6.9 | 59046.6 |
| 18 | 51.69 | 0.00 | 2005.6 | 10 | 0.77 | 7.1 | 48384.8 |
| 26 | 44.26 | 2000.7 | 9 | 31774.7 | |||
| 32 | 38.25 | 1997.2 | 9 | 0.83 | 8.3 | 37128.4 | |
| 34 | 41.57 | 1996.0 | 12 | 25849.4 | |||
| 38 | 39.11 | 1993.1 | 29901.6 | ||||
| 42 | 35.18 | 1990.1 | 10 | 0.89 | 8.0 | 25730.4 | |
| 46 | 38.80 | 1987.0 | 12 | 23159.6 | |||
| 48 | 32.58 | 1985.3 | 21387.0 | ||||
| 50 | 26.71 | 1983.3 | 9 | 0.94 | 5.3 | 15331.0 | |
| 70 | 17.32 | 1965.8 | 11 | 1.33 | 2.2 | ||
| 90 | 10.32 | 1945.9 | 2.04 | 1.3 | |||
| 110 | 5.36 | 1923.3 | 2.12 | 1.2 | |||
| 130 | 2.21 | 1899.1 | |||||
| 140 | 1.71 | 1888.5 |
Table 1: Millimeter-scale Resolution Data from Core Site DSH08. Short-lived radioisotope activities, geochronology, benthic foraminiferal density, solid-phase redox sensitive metal concentrations (Mn, Re), and total aliphatic concentration records for core site DSH08 collected in December 2010, subsampled at two millimeter increments4,7,12,14.
| Top Depth (mm) | Excess Pb-210 (dpm/g) |
Excess Th-234 (dpm/g) |
Th-234 and Pb-210 Merged Age model (year) |
Total Foraminiferal Density (indiv./cm3) |
[Re] (ng/g) |
[Mn] (mg/g) |
Total Aliphatics (ng/g) |
| 0 | 70.70 | N/A | 2010 | 4 | 0.60 | 12.4 | 111730.1 |
| 1 | 56.89 | 2006.2 | 11 | 0.76 | 7.3 | 52385.5 | |
| 2 | 44.26 | 2000.5 | 9 | 0.00 | 31774.7 | ||
| 3 | 39.65 | 1995.5 | 12 | 0.83 | 8.3 | 30959.8 | |
| 4 | 35.52 | 1989.7 | 11 | 0.89 | 8.0 | 22273.3 | |
| 5 | 26.71 | 1981.9 | 9 | 0.94 | 5.3 | 15331.0 | |
| 6 | |||||||
| 7 | 17.32 | 1967.1 | 11 | 1.33 | 2.2 | ||
| 8 | |||||||
| 9 | 10.32 | 1945.2 | 2.04 | 1.3 | |||
| 10 | |||||||
| 11 | 5.36 | 1917.6 | 2.12 | 1.2 | |||
| 12 | |||||||
| 13 | 2.21 | ||||||
| 14 | 1.71 |
Table 2: Centimeter-scale Resolution Data from Core Site DSH08. Short-lived radioisotope activities, geochronology, benthic foraminiferal density, solid-phase redox sensitive metal concentrations (Mn, Re), and total aliphatic concentration records for core site DSH08 collected in December 2010, integrated at one centimeter increments4,7,12,14.
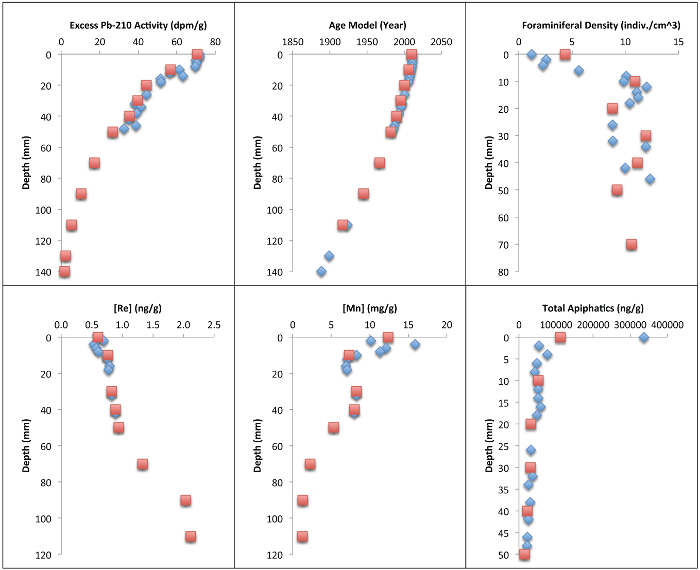
Figure 2: Graphical Representation of Millimeter and Centimeter-scale Resolution Data. Short-lived radioisotope activities, age model, benthic foraminiferal density, solid-phase redox sensitive metal concentrations (Mn, Re), and total aliphatic concentration records for core site DSH08 collected in December 2010, subsampled at two millimeter increments (blue diamonds) and one-centimeter increments (red squares)4,7,11,13. Please click here to view a larger version of this figure.
Millimeter-scale subsampling (and sedimentary conditions, see4) allowed for 234Th to be used as a chronometer on the sub-annual scale (n = 7). At the cm scale, this data would not be viable for producing a geochronology because the surface centimeter would be reduced to one measurement (n = 1). Total aliphatic concentrations increased from 36,322.3 ng/g dw (pre-DWH) to 336,922.6 ng/g dw (post-DWH) according to the mm scale records, whereas the post-DWH increase according to the centimeter-scale integrated mean was 111,730.1 ng/g dw. Total benthic foraminiferal density decreased from pre-DWH (mean = 11 indiv./cm3) to post-DWH (mean = 1 indiv./cm3) at the mm scale (n = 17) and from pre-DWH (mean = 10 indiv./cm3) to post-DWH (mean = 4 indiv./cm3) at the cm scale (n = 7). The subtle increase in rhenium in the surficial 2 mm, which is indicative of reducing conditions, would also not be resolved at centimeter resolution.
Discussion
The extruder can be modified to accommodate multiple diameters of core tube. If the core diameter is changed, then the piston, puck, and clamp diameters must be adjusted accordingly. This modification allows for broad applications in lacustrine and marine sediment collection. The sediment cores can also be extruded in the field or in the lab. A common modification to ease shipment of this extrusion system is to build it in two sections; a lower section (base and piston) can then be coupled to the upper section (clamps).
There are some limitations to this extrusion method. The first of these is that each core, or core section, must be cut to one-meter length or less. As with any extrusion method, there is also inevitably some compaction. However, compaction caused by this method is minimal. The reproducibility of multiple records extruded in this fashion is within 2-4 mm. This reproducibility is estimated on comparisons between various records (trace metal, organic geochemistry, benthic foraminifera, sedimentology) collected on the same deployment of an eight core multicore system. This extrusion method is also best suited for sediments that are primarily (>50%) silt and clay sized particles. Sediment predominantly (>50%) consisting of sand size particles tends to bind, causing additional compaction, due to a higher friction coefficient. The final limitation associated with this method is the amount of sediment available from each increment at millimeter-scale resolution. This method provides approximately 15-20 g of wet mass and 3-10 g of dry mass at 2 mm resolution, which may be restrictive for some analytical protocols.
The sedimentary records of the Deepwater Horizon event in the northern Gulf of Mexico demonstrate the efficacy of millimeter-scale subsampling. First of all, 234Th dating would not have been possible without millimeter-scale subsampling. This dating method can only be applied under certain circumstances, which are further discussed4. The pulse of oiled-flocculent material following the Deepwater Horizon event satisfied these conditions, depositing up to 8 mm of material at certain sites in the northern Gulf of Mexico within 6-12 months. Without mm scale sampling, the geochronology of this event would not have been resolved on the sub-annual scale (Tables 2 and 3). In addition to the 234Th records, redox-sensitive trace metals, benthic foraminiferal density, and the organic geochemistry records of this event would have been limited to one data point in the surface centimeter (Table 3). Instead, using millimeter-scale sub sampling provided a detailed and robust (5-10 data point) record of the MOSSFA event. Specifically, a 4-fold increase (n = 18) above pre-Deepwater Horizon values in total aliphatics using mm scale subsampling would have been reduced to a 2-fold increase, using cm scale subsampling (n = 6). Accordingly, a decrease in benthic foraminiferal density of 90% using mm scale subsampling would have been reduced to a decrease of 60% using cm scale subsampling. Without this high resolution sampling, discrete double peaks of Mn oxide, as well as changes in sedimentary Re concentrations associated with non steady-state redox changes would not be resolved. Overall, this extrusion system provides the ability to subsample sediment cores at the mm scale, retaining the entire volume of the sample and can be modified for broad applications in aquatic sediment sampling. Future applications of this method may include assessment of past oil spills, due to the mm scale event stratigraphy associated with subsurface oil releases. Other applications may include lacustrine records of mm scale climate variability. Millimeter scale sub-sampling has been proven effective in characterizing event stratigraphy in the context of anthropogenically influenced systems.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was made possible in part by a grant from BP/The Gulf of Mexico Research Initiative, C-IMAGE, DEEP-C and in part by the British Petroleum/Florida Institute of Oceanography (BP/FIO)-Gulf Oil Spill Prevention, Response, and Recovery Grants Program. The authors thank Nico Zenzola for his input in development of this procedure. The authors also thank the crew of the R/V Weatherbird II for their help during the field program.
Data can be accessed at the GRIIDC website: https://data.gulfresearchinitiative.org/ (data/R1.x135.119:0004/), (data/Y1.x031.000:0003/), (data/Y1.x031.000:0006/), (R1.x135.120:0004).
Materials
| Extruder | Custom Fabrication | Aluminum base and clamps, steel threaded rod | |
| Piston | Custom Fabrication | PVC tubing with acrylic cap | |
| Polycarbonate Core Tube | SABIC Poymershapes | 68374192 | |
| Acrylic puck/Rubber Gasket | Custom Fabrication | ||
| Acrylic sampling collar | Custom Fabrication | ||
| Acrylic plate | Custom Fabrication | One edge bevelled at 45 degree angle | |
| Putty knife | Fisher Scientific | 19-166-432 | |
| Steel/Acrylic Plates | Custom Fabrication | ||
| Electrical tape | McMaster Carr | 76455A28 | |
| Siphon or Syringe | Fisher Scientific | 14-176-227, 14-823-2A | |
| Razor blade | Fisher Scientific | 12-640 | |
| Drill | Ryobi | P-882 | |
| Thick rubber band | Staples | 831636 | 2-3 cm in width, larger diameter than piston |
| Personal protection equipment | Fisher Scientific | Gloves-19-058-801C, lab coat- 17-100-850, Goggles-19-181-501 |
e.g. gloves, lab coat, goggles |
| Sample labels | Fisher Scientific | 15920 | |
| Sample vessels | Fisher Scientific | Whirlpak- 01-812-3, Jar- 02-911-791 |
e.g.whirlpak bags, jars, etc. |
| Laboratory wipes | Fisher Scientific | 06-666-11 | e.g. kim wipes |
| Methanol | Fisher Scientific | BP1105-1 | |
| Deionized water |
References
- Abrahim, G. M. S., Parker, R. J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamacki Estuary, Auchland, New Zealand. Env. Mon. and Assess. 136, 227-238 (2008).
- Binford, M. W., Kahl, J. S., Norton, S. A. Interpretation of 210Pb profiles and verification of the CRS dating model in PIRLA project lake sediment cores. J. Paleolimnology. 9, 275-296 (1993).
- Brenner, M., Schelske, C. L., Keenan, L. W. Historical rates of sediment and nutrient accumulation in marshes of the Upper St. Johns River Basin, Florida. J. Paleolimnology. 26, 241-257 (2001).
- Brooks, G. R., et al. Sediment Pulse in the NE Gulf of Mexico Following the 2010 DWH Blowout. PLoS ONE. 10 (7), 0132341 (2015).
- Engstrom, D. R. A lightweight extruder for accurate sectioning of soft-bottom lake sediment cores in the field. Limno. and Oceano. 38 (8), 1796-1802 (1993).
- Gordon, E., Goñi, M. Controls on the distribution and accumulation of terrigenous organic matter in sediments from the Mississippi and Atchafalaya river margin. Mar. Chem. 92, 331-352 (2004).
- Hastings, D. W., et al. Changes in sediment redox conditions following the BP DWH Blowout event. Deep-Sea Res. II. , (2014).
- Jones, P. D., et al. High-resolution palaeoclimatology of the last millennium a review of current status and future prospects. The Holocene. 1, 3-49 (2009).
- Paris, C. B., et al. Evolution of the Macondo Well Blowout: Simulating the Effects of the Circulation and Synthetic Dispersants on the Subsea Oil Transport. Env. Sci. & Tech. 121203084426001, (2012).
- Passow, U., Ziervogel, K., Aper, V., Diercks, A. Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Env. Res. Letters. 7, 035301 (2012).
- Radović, J. R., Silva, R. C., Snowdon, R., Larter, S. R., Oldenburg, T. B. P. Rapid screening of glycerol ether lipid biomarkers in recent marine sediment using APPI-P FTICR-MS. Anal. Chem. 88 (2), 1128-1137 (2016).
- Romero, I. C., et al. Hydrocarbons in Deep Sea Sediments Following the 2010 Deepwater Horizon Blowout in the Northeast Gulf of Mexico. PLoS ONE. 10 (5), e0128371 (2015).
- Santschi, P. H., Rowe, G. T. Radiocarbon-derived sedimentation rates in the Gulf of Mexico. Deep-Sea Res. II. 55, 2572-2576 (2008).
- Schwing, P. T., Romero, I. C., Brooks, G. R., Hastings, D. W., Larson, R. A., Hollander, D. J. A Decline in Deep-Sea Benthic Foraminifera Following the Deepwater Horizon Event in the Northeastern Gulf of Mexico. PLOSone. 10 (3), 0120565 (2015).
- Valsangkar, A. B. A device for finer-scale sub-sectioning of aqueous sediments. Current Science. 92 (4), 5-8 (2007).
- Wörmer, L., Elvert, M., Fuchser, J., Lipp, J. S., Buttigieg, P. L., Zabel, M., Hinrichs, K. -. U. Ultra-high-resolution paleoenvironmental records via direct laser-based analysis of lipid biomarkers in sediment core samples. NAS Proceedings. 111 (44), 15669-15674 (2014).
- Yeager, K. M., Santschi, P. H., Rowe, G. T. Sediment accumulation and radionuclide inventories (239, 240 Pu , 210 Pb and 234 Th ) in the northern Gulf of Mexico, as influenced by organic matter and macrofaunal density. Marine Chemistry. 91, 1-14 (2004).
- Ziervogel, K., et al. Microbial activities and dissolved organic matter dynamics in oil-contaminated surface seawater from the Deepwater Horizon oil spill site. PLoS One. 7 (4), e34816 (2012).

