Simple and Efficient Production and Purification of Mouse Myelin Oligodendrocyte Glycoprotein for Experimental Autoimmune Encephalomyelitis Studies
Summary
We describe a simple protocol using only basic lab equipment to generate and purify large quantities of a fusion protein that contains mouse Myelin Oligodendrocyte Glycoprotein. This protein can be used to induce experimental autoimmune encephalomyelitis driven by both T and B cells.
Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS), thought to occur as a result of autoimmune responses targeting myelin. Experimental autoimmune encephalomyelitis (EAE) is the most common animal model of CNS autoimmune disease, and is typically induced via immunization with short peptides representing immunodominant CD4+ T cell epitopes of myelin proteins. However, B cells recognize unprocessed protein directly, and immunization with short peptide does not activate B cells that recognize the native protein. As recent clinical trials of B cell-depleting therapies in MS have suggested a role for B cells in driving disease in humans, there is an urgent need for animal models that incorporate B cell-recognition of autoantigen. To this end, we have generated a new fusion protein containing the extracellular domain of the mouse version of myelin oligodendrocyte glycoprotein (MOG) as well as N-terminal fusions of a His-tag for purification purposes and the thioredoxin protein to improve solubility (MOGtag). A tobacco etch virus (TEV) protease cleavage site was incorporated to allow the removal of all tag sequences, leaving only the pure MOG1-125 extracellular domain. Here, we describe a simple protocol using only standard laboratory equipment to produce large quantities of pure MOGtag or MOG1-125. This protocol consistently generates over 200 mg of MOGtag protein. Immunization with either MOGtag or MOG1-125 generates an autoimmune response that includes pathogenic B cells that recognize the native mouse MOG.
Introduction
MS is a human disease characterized by chronic inflammation and neurodegeneration of the CNS which is thought to be driven by an autoimmune response directed towards myelin. The loss of myelin and axons over time result in the gradual decline of cognitive and motor function1. "Experimental Autoimmune Encephalomyelitis" is an umbrella term for animal models of autoimmune disease directed towards CNS myelin. Like human MS, EAE is typically characterized by immune cell infiltration of the CNS and, in some cases, demyelination2. However, the degree to which any given EAE model resembles human MS in part depends on the species or strain used and on the complexity of the underlying anti-myelin autoimmune response.
Anti-myelin autoimmunity can be experimentally induced in several ways, but the most common method used today is to immunize mice with a short peptide of amino acids mimicking the immunodominant CD4+ T cell epitope of a myelin protein. This represents the minimum requirement to induce a pathogenic response. Perhaps the most common of these is a 21 amino acid peptide derived from myelin oligodendrocyte glycoprotein (MOG35-55), which is used to induce EAE in C57Bl/6 mice3. However, for some experimental purposes it is desirable or even necessary to immunize with larger protein antigens and indeed there are several advantages to this over immunization with short peptide. First, because of MHC restriction, short peptides are usually only effective in a very limited range of strains, while larger protein antigens representing either the whole protein or a specific domain can be processed normally for presentation in multiple inbred mouse strains or even in different species4. Second, a larger protein antigen is capable of inducing a more complex immune response incorporating more types of lymphocytes in antigen recognition, rather than limiting antigen recognition to CD4+ T cells. For example, B cells via their B cell receptor (BCR) interact directly with whole rather than processed protein. We and others have shown that B cells activated by MOG35-55 immunization do not recognize MOG protein5. Since B cells were recently demonstrated to play a pathogenic role in human MS6, EAE models that incorporate B cells in autoimmune pathology are increasingly important.
Despite the advantages of using larger protein antigens to induce EAE, there remain few commercially available sources for such proteins. Indeed, while short peptides like MOG35-55 can be synthesized very quickly and at a relatively low cost, the commercial options for MOG protein are limited and cost substantially more to purchase. Nonetheless, there are several expression vectors available for research groups to generate MOG extracellular domain (MOG1-125) themselves. However, all of the expression systems that we have identified in the literature are based on older technologies that have since been replaced with more efficient expression systems7. Further, most are based on rat or human MOG8. For some investigations of autoimmunity in mice, an antigen based on the mouse MOG autoantigen is preferable. Finally, all MOG-based proteins that we have identified, either commercial or as expression vectors, are fusion proteins containing additional amino acids to the MOG1-125 base. These include a tag for purification and usually other sequences as well, many of which with a function we were unable to identify.
To address these limitations, we generated a novel fusion protein based on the mouse MOG extracellular domain fused to a tag containing thioredoxin to combat the known insolubility of MOG protein5. The tag sequence also contains a 6xHis sequence for purification and a TEV protease cleavage site that allows for the complete removal of all tag sequences, if desired. This is the only method that we are aware of to generate pure MOG1-125 protein. To facilitate production of large amounts of protein, the MOG1-125 sequence was codon-optimized for bacterial expression and the MOGtag fusion protein was inserted into the pET-32 expression system. Here, we describe in detail the protocol to produce and purify MOGtag protein, and pure MOG1-125, using non-specialized equipment available to most immunology laboratories.
Protocol
1. Protein Induction
NOTE: In the following steps, BL21 Escherichia coli bacteria transformed with a pET-32 vector containing the sequence for the MOGtag fusion protein (see reference5 and Figure 1) are grown to high densities and are then induced to express the MOGtag protein. See Figure 2 for overall timeline – note that days are approximate and alternate stop points are noted in the protocol. If starting with purified pET-32 MOGtag vector DNA, it will be necessary to chemically transform it into competent BL21 E. coli bacteria using ampicillin selection, as has been well-described9. Successful transformation can be confirmed by purifying DNA from selected bacteria using a standard commercial kit, followed by digestion with the restriction enzymes Age1 and Sac1 to produce a 424 bp band on an agarose gel10.
- Inoculate 5 ml of sterile Lysogeny broth (LB) broth (0.1 mg ampicillin/ml) with a BL21-MOGtag glycerol stock and incubate it overnight at 37 °C, 200 rpm.
- Place two 1 L Erlenmeyer flasks containing 500 ml of sterile LB broth in a 37 °C incubator in preparation for the next day.
- Add 500 µl of 100 mg/ml ampicillin to each of the flasks containing 500 ml LB from step 1.2 (final concentration 0.1 mg/ml ampicillin). Transfer 1 ml of the overnight culture from step 1.1 to each of the two flasks of LB broth. Incubate at 37 °C and 200 rpm for 5 hr or up to an optical density of 0.6.
- Take one 1 ml aliquot from one of the flasks and transfer it into a separate 1.5 ml centrifuge tube. Pellet the cells at 16,000 x g for 1 min and remove the supernatant. Label the tube as T0 (pre-induction) and then put the bacterial pellet into a -20 °C freezer for future sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis (see section 8 and Figure 3).
- Add 0.5 ml of 1 M Isopropyl β-D-1-thiogalactopyranoside, Isopropyl β-D-thiogalactoside (IPTG) to each culture flask. Incubate the flasks at 37 °C, 200 rpm for 4 hr, then at room temperature at 75 rpm overnight.
2. Harvesting MOGtag Protein
NOTE: At this stage, the bacteria will have produced large quantities of MOGtag protein. To harvest MOGtag, bacteria are first lysed in a Triton X-100 buffer followed by sonication. MOGtag is then released from inclusion bodies and denatured with imidazole and guanidine, resulting in a crude protein solution containing the MOGtag protein.
- Take one 1 ml aliquot from one of the overnight cultures from step 1.5 and transfer it into a 1.5 ml microcentrifuge tube. Pellet the cells at 16,000 x g for 1 min and remove the supernatant. Label the tube TO/N (post-induction) then put the bacterial pellet into a -20 °C freezer for future SDS page analysis (Figure 3).
- Distribute the cultures evenly amongst 250 ml bottles compatible with high speed centrifugation and keep these on ice from this point forward. Pellet the bacterial cells at 22,000 x g for 15 min at 4 °C.
- Discard the supernatant. Store the bacterial pellets at -20 °C or continue to step 2.4.
- Prepare lysis buffer (0.1 mg/ml hen egg lysozyme, 0.1% Triton-X (v/v) in phosphate buffered saline (PBS)) by adding 60 µl of Triton X-100 and 120 µl of 50 mg/ml hen egg lysozyme stock solution to 60 mL of PBS.
- Resuspend and combine all of the bacterial pellets from step 2.3 in a total of 30 ml of lysis buffer. Transfer this volume to a round bottom 50 ml tube capable of high speed centrifugation. Place this tube in a 30 °C water bath for 30 min. During the incubation time, shake the tube twice to resuspend the cells.
- After the incubation, transfer the tube onto ice and sonicate the solution at 20 kHz, amplitude 70%, pulse on 3 sec, pulse off 3 sec, and five pulses per round. Sonicate the solution for six total rounds and allow the solution to cool on ice in-between rounds.
- Centrifuge the solution at 24,000 x g for 15 min at 4 °C. Discard the supernatant and repeat steps 2.5-2.7 once. Store the pellet at -20 °C or continue to step 2.8.
- Prepare buffer A (500 mM NaCl, 20 mM Tris-HCl, 5 mM imidazole, pH 7.9) by adding 0.8766 g of NaCl, 0.09456 g of Tris-HCl, and 0.01021 g of imidazole to a 100 ml beaker. Add H2O until the volume reaches 28 ml then adjust the pH of the solution to 7.9. Then, transfer the solution to a 100 ml graduated cylinder and add H2O until the volume is 30 ml.
- Resuspend the pellet from step 2.7 in 30 ml of buffer A and incubate this solution at 4 °C for 3 hr. During this time weigh out 17.2 g of guanidine-HCl.
- Sonicate the solution on ice using the same settings as in step 2.6. Then add 17.2 g of guanidine-HCl to the solution. Incubate this on ice for 1 hr to solubilize the MOGtag protein.
- Centrifuge the solution at 24,000 x g for 30 min at 4 °C. Collect the supernatant and store the supernatant at 4 °C until protein purification.
3. Protein Purification
NOTE: In the following steps the MOGtag protein will be purified through 4 rounds of absorption onto charged nickel resin (via the His-tag) and elution.
- Make the Following Buffers:
- Prepare Buffer B (500 mM NaCl, 20 mM Tris-HCl, 5 mM imidazole, 6 M guanidine-HCl, pH 7.9). Add 5.844 g of NaCl, 0.6304 g of Tris-HCl, 0.0681 g of imidazole, and 114.64 g guanidine-HCl to a 500 ml beaker. Then add H2O until it reaches 190 ml and adjust the pH to 7.9. Transfer the solution to a 250 ml graduated cylinder and add H2O until the volume is 200 ml.
- Prepare Elution buffer (500 mM NaCl, 20 mM Tris-HCl, 0.5 M imidazole, 6 M guanidine-HCl, pH 7.9). Add 5.844 g of NaCl, 0.6304 g of Tris-HCl, 6.808 g of imidazole, and 114.64 g guanidine-HCl to a 500 mL beaker. Add H2O until it reaches 190 ml and adjust the pH to 7.9. Transfer the solution to a 250 ml graduated cylinder and add H2O until the volume is 200 ml.
- Prepare Strip buffer (500 mM NaCl, 20 mM Tris-HCl, 100 mM EDTA, pH 7.9). Add 5.844 g of NaCl, 0.6304 g of Tris-HCl, 40 ml 500 mM EDTA to a 500 mL beaker. Add H2O until it reaches 190 mL and adjust the pH to 7.9. Transfer the solution to a 250 ml graduated cylinder and add H2O until the volume is 200 ml.
- Prepare Charge buffer (0.1 M nickel sulfate). Add 5.257 g nickel sulfate to a 500 ml beaker and add H2O until it reaches 190 ml (CAUTION, do not handle nickel sulfate outside of a fume hood until the nickel sulfate has been dissolved in H2O). Once the nickel sulfate has dissolved, transfer the solution to a 250 ml graduated cylinder and add H2O until the volume reaches 200 ml.
- Split 10 ml of nickel resin between two conical 50 ml centrifuge tubes capable of withstanding centrifugal forces over 4,500 x g (5 ml in each tube).
- Charge and equilibrate the nickel resin:
- Wash the resin by adding 40 ml H2O to each tube containing resin. Lay the tubes horizontally onto a rocker and let them agitate for 5 min at 4 °C. Once finished, centrifuge the tubes at 4,500 x g for 8 min at 4 °C.
- Discard the supernatant by pipetting to avoid disturbing the pellet. Add 40 ml of charge buffer to each tube. Transfer the tubes onto a rocker and let them agitate for 15 min at 4 °C. Once finished, centrifuge the tubes at 4,500 x g for 8 min at 4 °C.
- Discard the supernatant then add 40 ml of buffer B to the tubes. Transfer the tubes onto a rocker and let them agitate for 5 min at 4 °C. Once finished, centrifuge the tubes at 4,500 x g for 8 min at 4 °C then discard the supernatants.
- Optional: Take 150 µl of the solubilized protein collected in step 2.11 and transfer it to a 1.5 ml microcentrifuge tube. Label the tube as pre-incubation and freeze this at -20 °C for future SDS page analysis (Figure 3, Crude MOGtag).
- Purify the MOGtag Protein:
- Transfer the entire volume of solubilized protein from step 2.11 (~40 ml, minus the small sample removed in step 3.4) to the first tube (Figure 2, tube 1) containing nickel resin from step 3.3, mix, and place horizontally on a rocker at 4 °C for 1 hr.
- Centrifuge the tube at 4,500 x g for 8 min at 4 °C. Once finished, transfer the supernatant to the second tube (Figure 2, tube 2) of nickel resin and incubate as described in the previous step. In the meantime, continue with steps 3 to 6 below with tube 1.
- Resuspend the nickel resin in tube 1 in 40 ml of elution buffer and place the tube horizontally on a rocker at 4 °C for 5 min. Then, centrifuge the tube at 4,500 x g for 8 min at 4 °C.
- Transfer the supernatant containing eluted MOGtag protein into a 250 ml bottle labelled 'purified MOGtag protein' and keep this bottle at 4 °C. With each elution step, pool the resulting supernatant in this bottle.
- Add 40 ml of strip buffer to the nickel resin and place horizontally on a rocker for 5 min at 4 °C.
- Centrifuge the tube at 4,500 x g for 8 min at 4 °C. Discard the supernatant, then recharge the nickel resin as listed in step 3.3.
- Once finished, move forward with the second tube as listed in step 3.5. A total of 4 rounds of absorption of the solubilized protein from step 2.11 onto the charged nickel resin and elution will recover most of the protein although, if desired, additional protein could be recovered in additional rounds of absorption and elution.
- Once four (or more) rounds of absorption and elution are complete, store the pooled purified protein at 4 °C overnight. Nickel resin can be stored in 40 ml of a 20% ethanol solution at 4 °C until it is needed again.
4. Measuring Protein Concentration
NOTE: Before proceeding further it is necessary to quantify the amount of purified MOGtag protein generated in section 3. This value will be used to determine the final volume to concentrate the protein to at the end of the protocol. We describe a standard Bradford Assay here. The concentration of purified MOGtag protein is determined by comparing the spectral absorbance of serially diluted MOGtag protein to a standard curve of bovine serum albumin (BSA) at a known concentration.
- Make 10x acetate buffer by adding 23 ml glacial acetic acid to 8.2 g sodium acetate in a 3 L beaker and then add 1.9 L of H2O to dissolve the sodium acetate. Transfer this solution to a 2 L graduated cylinder and add H2O until the volume reaches 2 L. Then store it in a 2 L bottle at room temperature. Make 1 ml of 1x acetate buffer by adding 100 µl of 10x acetate buffer to 900 µl of H2O.
- Set up a 96-well plate for dilutions by adding 30 µl of 1x acetate buffer to wells G2-8 and 60 µl to G9. Add 48 µl of 1x acetate buffer to H2 and H3.
- Set up the initial serial dilution of the BSA standard in row G as follows: Add 216 µl of 1x acetate buffer and 54 µl of commercially available 2 mg/ml BSA standard in well G1 and mix thoroughly. Add 210 µl from well G1 to well G2, mix thoroughly, and then add 180 µl of well G2 to well G3. Add 150 µL of well G3 to well G4, mix thoroughly, and continue this trend of adding 30 µl fewer to each subsequent well until well G8. (See Table 1 for lists of equivalent dilution factors).
- Set up triplicate samples of each dilution by transferring 10 µl of well G1 to wells A1, B1, and C1, and 10 µl of well G2 to wells A2, B2, and C2, and so on. Use a multichannel pipette.
- To set up dilutions of MOGtag, add 60 µl of purified MOGtag protein from step 3.6 to well H1. Add 12 µl from well H1 to well H2, mix thoroughly, then add 12 µl of well H2 to well H3, mix thoroughly (this produces a 1x dilution in H1, 1/5 dilution in H2, and 1/25 dilution in H3).
- Set up triplicate samples for the MOGtag dilutions by transferring 10 µl of wells H1-H3 to rows D, E, and F, as described in step 4.4.
- Mix 2 ml of protein assay dye reagent concentrate with 8 ml of H2O. Add 200 µl of this mixture to all preloaded wells in rows A, B, C, D, E, and F, using a multichannel pipette, if available. Pop any bubbles in the wells using a needle before reading the protein concentration.
- Within 10 min of adding the dye reagent, measure the 595 nm absorbance of all the wells in rows A, B, C, D, E, and F.
- Use Rows A, B, and C to set up a standard curve where positions 1-9 correspond to 0.4, 0.35, 0.3, 0.25, 0.2, 0.15, 0.1, 0.05, and 0 mg/ml BSA. Based upon this curve, calculate the concentration of MOGtag protein using the dilution of MOGtag that best fits the standard curve. Usually, there will be 200 to 250 mg of MOGtag protein from 1 L of bacterial culture using the protocol described here.
NOTE: To make MOG1-125 proceed from here to the optional section 7 listed at the end of the protocol. Otherwise, continue to section 5.
5. Dialysis
NOTE: Dialysis is performed to gradually remove guanidine from the solution containing purified, denatured MOGtag to allow the protein to refold. Care must be taken during this step as MOG itself is very insoluble, and while this is improved by the presence of the thioredoxin tag, it is still prone to come out of solution. Therefore, refolding should be performed gradually and at a relatively low MOGtag concentration.
- Before starting dialysis, dilute the purified MOGtag protein with buffer B (buffer B can be made as listed in step 3.1) until the concentration is 0.5 mg/ml or less, based on the quantity of MOGtag calculated in section 4.
- Cut approximately 30 cm of snakeskin dialysis tubing and secure one end with a locking hemostat by folding the end of the snake skin over three times and clamping the folded end with the hemostat. Fill the snakeskin with diluted MOGtag protein (between 60 to 90 ml of MOGtag protein per tube) then remove air bubbles from the snakeskin by forcing them out of the open end. Finally, seal the other end of the tube using a second locking hemostat.
- Repeat step 5.2 to fill additional sections of dialysis tubing until all MOGtag protein has been transferred into snakeskin dialysis tubing. Ensure there are no leaks.
- Make 1x acetate with 4 M guanidine by weighing out 382.12 g of guanidine-HCl and adding 100 ml of 10x acetate buffer made in step 4.1 to a 2 L beaker. Add 0.5 L of H2O to the beaker and allow the guanidine-HCl to dissolve. Once dissolved, transfer the solution to a 1 L graduated cylinder and add H2O until the volume reaches 1 L. Repeat this recipe for every 2 snakeskins that are required.
- Perform dialysis as follows: fill a large bucket (minimum 10 L) with 1 L of 1x acetate buffer with 4 M guanidine from step 5.4. Place up to 2 sections of dialysis tubing containing MOGtag into the bucket, leaving room for a magnetic stir bar to spin unhindered in the bottom. Put the bucket in a 4 °C room on a magnetic stir plate and turn it on to a slow rotation rate (i.e. not high, just enough to move the fluid):
NOTE: Perform the following steps to gradually reduce the amount of guanidine in the buffer. Stir times are given as minimums, but can be left overnight. Dialysis will take a minimum of 3 days, but this can be extended if desired. Regularly check to make sure that the tubing is intact and that the ends are securely closed.- Let the bucket sit and stir for 4-5 hr.
- Add 1 L of 1x acetate buffer (100 ml 10x acetate buffer and 900 ml of H2O) to each bucket.
- Repeat steps 5.5.1 and 5.5.2 a total of 3 times for a total of 4 L of buffer in the bucket.
- Discard half of the buffer in the bucket and refill with 1 L of 1x acetate buffer (100 ml 10x acetate buffer and 900 ml of H2O, no guanidine) and set up the tubing and stir bar.
- Repeat steps 5.5.1 and 5.5.2 once (i.e. until there is a total of 4 L of buffer in the bucket).
- Finally, replace the entire 4 L volume in the bucket with 4 L of fresh 1x acetate buffer and let stir for 4-5 hr. For best results, do this step on the day of protein concentration.
- Carefully remove the dialysis tubing with refolded MOGtag and discard bucket buffer.
6. Concentrating MOGtag Protein
NOTE: In the final step, refolded MOGtag protein is concentrated to the working dilution for storage. As MOGtag is very insoluble, it should not exceed 5 mg/ml. This concentration is approximately equimolar with 0.4 mg/ml MOG35-55 peptide, which is commonly used to induce EAE in mice (mixed 1:1 with complete Freund's adjuvant (CFA)). During the concentration process it is not uncommon for a small amount of protein to come out of solution in the form of white precipitate. Excessive precipitation is a problem, however.
- Calculate the final desired volume to achieve a MOGtag concentration of 5 mg/ml, based on the value calculated in section 4.
- Line a pan with aluminum foil and cover the aluminum foil with polyethylene glycol (PEG) 3350 and PEG 8,000 at a 1:1 ratio. The PEG 3350 is incorporated into this mixture as it can help prevent protein aggregation during concentration and is an effective cyropreservative11.
- Put the snakeskin tubing (with MOGtag protein inside) on top of the aluminum foil and cover with PEG 8000. Let this sit at room temperature and check the volume regularly until the volume is equal to or below the estimated final volume (calculated in step 6.1), the actual volume will be measured in the next step. If the pan becomes oversaturated with water during the concentration process, set up a fresh pan with aluminum foil and PEG 3350 and PEG 8000.
- Wash the outside of the snakeskins with H2O and transfer the MOGtag protein to a separate glass bottle using a serological pipette. Keep track of the volume as the protein is transferred to ensure the volume is correct.
- If the volume has been reduced below the estimated final volume, add 1x acetate buffer until the desired volume is reached. If the volume is above the estimated final volume, transfer the MOGtag protein back into the dialysis tubing and continue the concentration (steps 6.2-6.3).
- Distribute the MOGtag protein amongst 1.5 ml tubes (0.5 ml per tube), store the tubes at -80 °C until needed. To induce EAE, mix this volume 1:1 with CFA.
- Run an SDS PAGE gel to confirm the expression of the MOGtag protein using the samples taken in steps 1.4, 2.1, 3.4, and the purified MOG protein from step 6.4.
7. Generating MOG1-125 from MOGtag Using TEV Protease (Optional)
NOTE: This optional step continues from the end of step 4. If MOG1-125 without any extra tag sequences is required, the tag sequences can be removed using TEV protease (Figure 4). As far as we are aware, there is no other expression system capable of generating pure MOG1-125. However, it should be noted that without the thioredoxin tag, MOG1-125 is highly insoluble and this may cause problems during purification and handling, and for this reason remove the tag if absolutely necessary for experimental reasons. Several steps are required to generate pure MOG1-125. MOGtag is first dialyzed into TEV protease cleavage buffer. Following digestion with TEV protease, the volume is reduced to aid with later purification steps, then dialyzed into buffer B, and then the His-tag containing tag sequence is removed using nickel resin. Finally, protein is quantified and pure MOG1-125 is concentrated to the final concentration.
- Make 1 L of 10x TEV cleavage buffer (500 mM Tris-HCl, 5 mM EDTA) by adding 1.861 g EDTA, 44.4 g Tris-HCl, and 26.5 g Tris to a 2 L beaker. Add H2O until the volume reaches 990 ml then adjust the pH to 8 to dissolve the EDTA. Transfer the solution to a 1 L graduated cylinder and bring the volume up to 1 L using H2O.
- Dilute MOGtag protein from step 3.6 to 0.5 mg/ml using buffer B (the same buffer described in step 3.1), based on the quantity of protein calculated in section 4. Transfer 120 ml of diluted MOGtag protein to snakeskin dialysis tubing as described in steps 5.2 to 5.3. The volume can be scaled up however the amount of TEV protease required to cleave all of the MOGtag protein will be substantial.
- Follow the dialysis protocol listed in step 5.5, except replace the 10x acetate buffer with 10x TEV cleavage buffer made in step 7.1.
- Transfer the MOGtag, now in TEV cleavage buffer, to a new 250 ml glass bottle and monitor the increased volume from dialysis. Add 1 µl of β-mercapto-ethanol per every 2.85 ml of MOGtag protein added to the bottle. Add at least 1 mg of TEV protease (stock TEV solution at 5 mg/ml) for every 50 mg of MOGtag protein (TEV protease can be added up to a 1:10 TEV:MOG protein ratio) and incubate at room temperature, protected from light for at least 72 hr.
NOTE: At the end of the TEV protease incubation, white precipitates should be seen at the bottom of the glass bottle. - Before transferring the protein to dialysis tubing, add guanidine-HCl to the solution until the proteins dissolve to prevent the protein precipitates from sticking to the glass bottle. Transfer 150 µl of this solution to a 1.5 ml microcentrifuge tube and label the tube as "MOG with TEV". Store the solution at -20 °C for future SDS-PAGE analysis.
- Transfer all of the fluid from step 7.5 into dialysis tubing as listed in steps 5.2 to 5.3. Fill a large bucket with 2 L of H2O and place the dialysis tube into the bucket. Incubate this at 4 °C with light stirring overnight. This step is important to dilute out the guanidine to allow the protein concentration to occur quickly and to begin removing the EDTA from the solution that will interfere with protein purification.
- Concentrate the protein in the dialysis tubing as listed in steps 6.2 to 6.3 (except only use PEG 8000) such that the final volume of the solution is ~40 ml. Wash the dialysis tubing with H2O and remove any residual PEG 8000 still attached to the tubing. This reduction in volume makes it possible to fit all of the digested protein into a 50 ml tube containing nickel resin, as described in step 3.5.
- Dialyze the Digested Protein to Buffer B:
- Fill a large bucket with 1 L of buffer B (29.22 g NaCl, 3.152 g Tris-HCl, 0.3405 g Imidazole, 573.18 g of guanidine-HCl, pH 7.9).
- Place the snakeskins containing digested protein from step 7.7 in the bucket along with a stir magnet (as described in greater detail in step 5.5). Put the bucket into a 4 °C cold room on a stir plate set to a slow stir and allow the snakeskins to dialyze for 5 or more hr.
- Empty the bucket and refill it with another 1 L of buffer B and let this sit in the 4 °C cold room on a stir plate set to a slow stir and allow the snakeskins to dialyze for 5 or more h.
- Perform purification of the MOG1-125 protein as detailed in section 3, with the important difference that the cleaved tag sequences will be bound by the nickel resin, leaving MOG1-125 in solution. Again, 4 rounds of purification will be performed on the same volume, but in this case the goal is to improve purity, rather than to recover as much protein as possible. See Figure 4.
- Make the protein purification buffers listed in step 3.1
- Charge both tubes of nickel resin as described in step 3.3.
- Purify MOG1-125 by the protocol detailed in step 3.5, with the essential exception that the eluate from the nickel resin containing tag is discarded (keep 150 µl of the eluate in a 1.5 ml microcentrifuge tube for future SDS-PAGE analysis), while the solution containing MOG1-125 is retained as the final product.
- Measure the Concentration of MOG1-125 Protein as Described in Section 4:
- Set up the BSA standard curve dilutions as described in steps 4.2 and 4.4.
- Set up the dilutions of MOG1-125 as described in steps 4.5 and 4.6. Alternatively, it may be valuable to generate a greater range of dilutions (1x, 1/2, 1/4, and 1/8, for example). Adjust volumes of 1x acetate buffer and MOG1-125 accordingly.
- Perform the Bradford Assay as described in steps 4.7 to 4.9 and calculate the quantity of MOG1-125 generated. The expected yield is approximately 4 mg or more protein.
- Refold MOG1-125 by gradual removal of guanidine via dialysis, as described in section 5.
- Concentrate MOG1-125 protein as described in section 6. The final concentration should be 2.24 mg/ml, which is equimolar to 5 mg/mL MOGtag.
8. SDS-PAGE Gel to Confirm MOGtag Production and Purity
NOTE: Samples taken from steps 1.4, 2.1, 3.4, and 6.4 are analyzed by standard SDS-PAGE to confirm MOGtag production and purity. This step should be performed after the final purification of either MOGtag or MOG1-125.
- Make a 2x SDS-PAGE loading buffer by adding 1.576 g of Tris-HCl to 2 ml of H2O and set the pH to 6.8. Add 0.4 g SDS into the Tris-HCl solution and then add H2O until the volume reaches 7 ml.
- Add 0.2 g of bromophenol blue to 10 ml of H2O then add 1 ml of this to the solution made in step 8.1. Finally, add 2 ml of glycerol to finish the 2x SDS-PAGE loading buffer. When storing this solution, keep at 4 °C and protect it from light for up to 3 months.
- Thaw the frozen aliquots of bacteria from steps 1.4 and 2.1 as well as the solubilized MOGtag protein from steps 3.4 and 6.4 at room temperature.
- Remove the salts from the solutions collected in steps 3.4 and 6.4 by adding 60 µl of each to protein desalting columns. Purify the proteins following manufacturer's instructions.
- Add 20 µL of β-mercapto-ethanol to 1 ml of the solution made in step 8.2. Add 40 µl of this to the two bacterial pellets from step 8.3 and 60 µl to the desalted proteins from step 8.4 and incubate these at 95 °C for 10 min.
- Make 1x SDS page running buffer by weighing out 5 g of Tris, 28.8 g glycine, and 1 g SDS. Add these to a 1 L beaker and add 500 mL H2O to dissolve everything. Once dissolved, transfer the solution to a 1 L graduated cylinder and fill with H2O until the volume reaches 1 L.
- Set up a 12% polyacrylamide gel in a gel apparatus then add the 1x SDS-PAGE running buffer to the gel apparatus such that the running buffer is just above the top of the wells in the gel. Wash the wells of the gel by pipetting 50 µl of running buffer into the wells five times each.
- Load 10 µl of protein ladder into the first well and add 10 µl of the boiled solutions from step 8.5 to separate wells. Run the gel at 115 V for 60 min.
- Remove the gel from the apparatus and transfer it to a small container. Fill the container with 100 ml of pure H2O and transfer the container to a slowly rotating platform for 8 min. After the 8 min, dump the water and wash the gel twice more with H2O.
- Dump the H2O from the container and add 100 ml of rapid stain reagent to the container. Allow this to sit on a slowly rotating platform overnight, cover with aluminum foil.
- Dump the rapid stain reagent and add approximately 100 ml of H2O to the container. Allow this to sit on a rotating platform for 8 min. Dump the H2O and repeat the H2O wash twice.
- Image the gel using an imager for coomassie blue stains. Look for a band around 31.86 kDa (MOGtag protein) and a fainter band at 63.72 kDa (MOGtag dimers).
Representative Results
Once the purification is complete, samples collected in steps 1.4, 2.1, 3.4, and the final product from step 6.4 should be run on a protein gel (Figure 3A). MOGtag should first appear as a 31.86 kDa band in the TO/N sample, but not T0, and should be the only band in the final pure product. To test whether the MOGtag protein has correctly folded, the MOGtag protein can be used to label MOG-protein specific B cells by FACS. By labeling mouse lymphocytes with a 1:1,000 dilution of MOGtag along with a secondary antibody directed against the His-tag and a fluorescently-labelled tertiary anti-IgG1 antibody, MOG-specific B cells can be identified (Figure 3B). Alternatively, MOGtag can be directly conjugated to fluorophores to reduce background staining (resulting from B cells binding to the secondary and tertiary antibodies) as described in reference5 to identify MOG-specific B cells. This is necessary when trying to identify MOG-binding B cells in wild type mice, as these cells are normally very rare5. IgHMOG mice have a heavy chain knockin that, when paired with an appropriate kappa light chain, confers specificity for MOG. As the binding of MOGtag is enhanced amongst the IgHMOG B cells, this confirms that this protocol generates a properly folded antigen. Importantly, IgHMOG B cells contribute to autoimmune pathology in models of MOG-directed EAE12, confirming that MOGtag induces both T and B cell autoimmunity.
Before starting dialysis to refold the protein as described in section 5, it is necessary to measure the protein concentration using a Bradford assay, as described in section 4. Representative results of a Bradford assay are shown in Table 1 and summarized in Figure 5.
The generation of pure MOG1-125 is accomplished by the addition of TEV protease to MOGtag protein ultimately resulting in the cleavage of the MOGtag protein into MOG1-125 and tag sequence as shown in Figure 6. Subsequent nickel resin purification removes MOGtag, tag sequence, and TEV protease impurities ultimately resulting in pure MOG1-125 as shown in Figure 6.
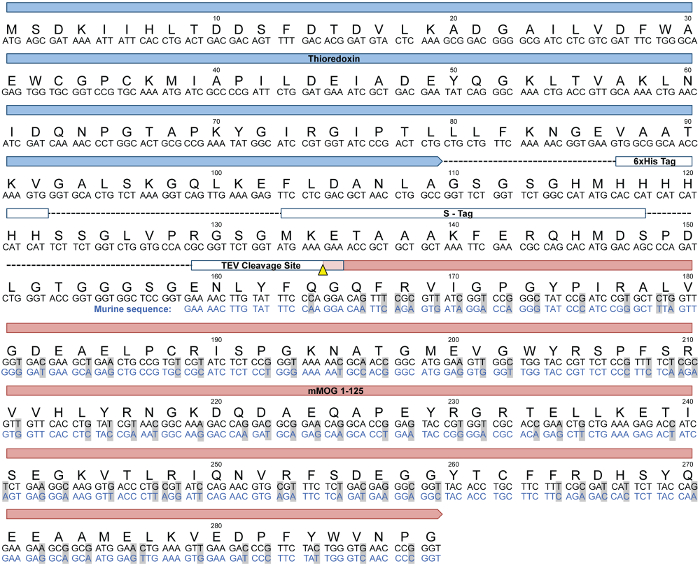
Figure 1: MOGtag protein. (Duplicated here with permission from Dang et. al.5). Linear structure, and amino acid and DNA sequences of the MOGtag fusion protein. The DNA sequence for the extracellular domain of mouse MOG (MOG1-125, lower sequence in blue) was codon-optimized for expression in bacteria (black). This sequence was synthesized and inserted into a vector to create an N-terminal fusion to a tag containing thioredoxin and an S-Tag to counteract the known insolubility of the MOG protein13,14, as well as a 6x His Tag for purification15. A TEV protease cleavage site separates the MOG1-125 from the tag sequences. TEV-mediated cleavage between glutamine-164 and glycine-165 using an alternative consensus TEV cleavage site16 results in removal of all non-MOG amino acids. Please click here to view a larger version of this figure.
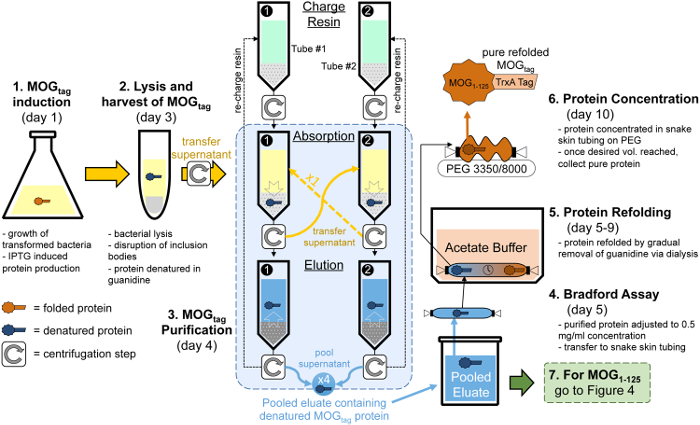
Figure 2: Overview of the steps required to produce pure MOGtag protein. To generate MOGtag protein, bacteria expressing the MOGtag protein are grown to high densities then induced to express MOGtag using IPTG as listed in section 1. After an overnight culture, the bacteria are lysed and through a series of pelleting steps the protein fraction containing inclusion bodies, which contains MOGtag, is extracted as listed in section 2. MOGtag is then purified from the crude protein fraction through four cycles of absorption onto charged nickel resin and elution of the MOGtag protein as listed in section 3. A portion of the pooled eluate is then taken for a Bradford assay to determine the yield of MOGtag protein and the rest of the eluate is dialyzed into acetate buffer over the course of several days as listed in sections 4 and 5. Lastly, the protein is concentrated using PEG 3350 and PEG 8000 to a final concentration of 5 mg/ml based upon the yield of MOGtag determined in the Bradford assay as listed in section 6. The entire process can take a minimum of 10 days, with the start day for each step shown in brackets. However, alternative stop and start points are listed in the protocol, and steps can be spread over a greater amount of time if desired. Please click here to view a larger version of this figure.
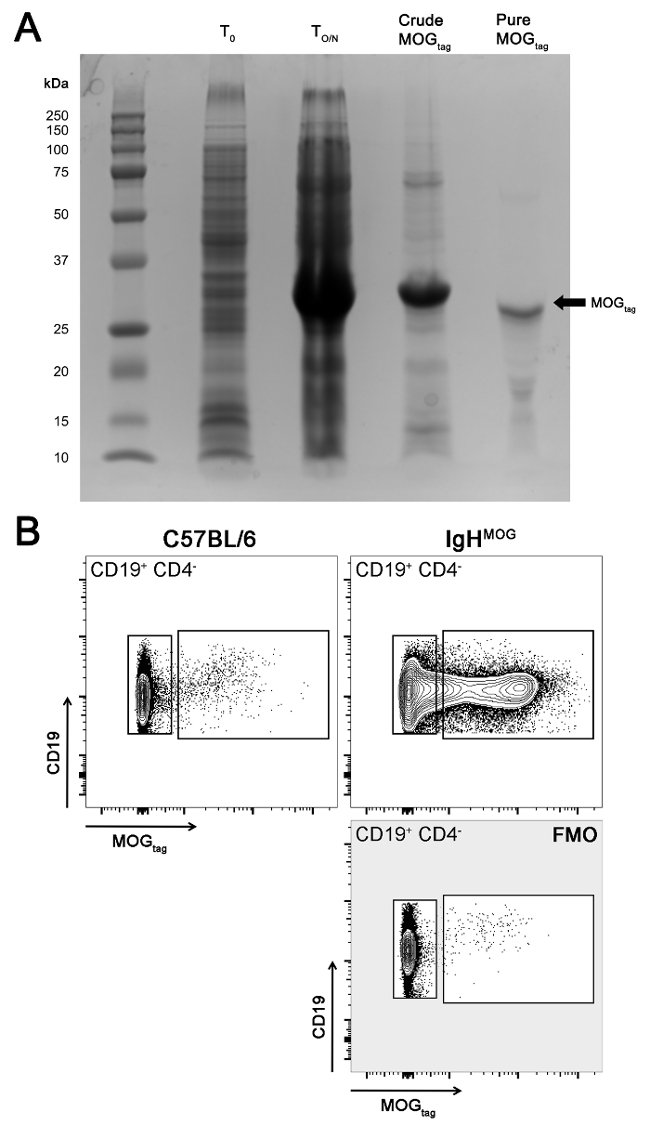
Figure 3: Purification of the MOGtag protein and assessment of its activity. (A) Shown are protein samples that were collected from various points across the protein purification procedure and run on a SDS-PAGE gel. T0= BL21 bacteria prior to protein induction (collected in step 1.4), TO/N= BL21 bacteria post-induction of protein expression (collected in step 2.1), Crude MOGtag= Solubilized MOGtag protein prior to protein purification (collected in step 3.4), Pure MOGtag= MOGtag protein after purification (collected in step 6.4). (B) Binding of the MOGtag protein to CD19pos CD4neg naive B cells from lymph nodes from either wild type C57Bl/6 mice or IgHMOG mice that express an immunoglobulin heavy chain specific for MOG protein7,17 was assessed using flow cytometry. MOGtag-specific B cells were identified by staining lymph node cells with MOGtag followed by a secondary anti-his tag antibody and a fluorescent tertiary anti-IgG1 antibody. Staining of cells from C57BL/6 or IgHMOG mice is shown along with a MOGtag fluorescence minus one (FMO) control stain of IgHMOG cells. Please click here to view a larger version of this figure.
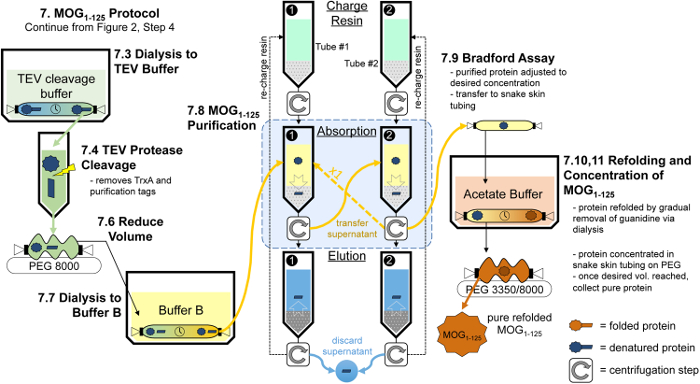
Figure 4: Overview of the steps to generate MOG1-125 from MOGtag protein. After collecting the MOGtag eluate as described in step 3.5 of the protocol, the MOGtag protein is dialyzed into TEV protease cleavage buffer. Once the dialysis is complete, TEV protease is added to the MOGtag solution resulting in the cleavage of MOGtag into the MOG1-125 protein. The volume of the cleavage solution is then reduced and dialyzed into buffer B prior to protein purification. Impurities from the cleavage solution are extracted through four successive rounds of absorption onto charged nickel resin and elution of the impurities ultimately resulting in a solution of pure MOG1-125. The concentration of the MOG1-125 protein is determined through a Bradford assay and the protein is folded over the course of several days through dialysis. Once dialysis is complete, the MOG1-125 protein is concentrated to 2.24 mg/ml using PEG 3350 and PEG 8000. This protocol is discussed in detail in section 7. Please click here to view a larger version of this figure.
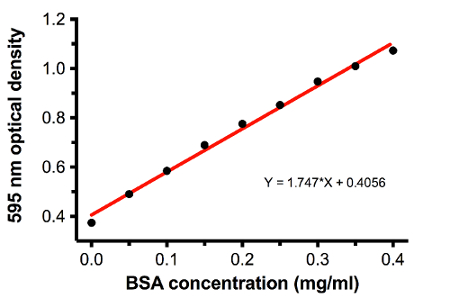
Figure 5: Example of a Bradford assay standard curve for determining the concentration of MOGtag protein. BSA standard readings taken from Table 1 were plotted to obtain a linear regression formula for calculating the MOGtag concentration based upon the optical density at 595 nm. Please click here to view a larger version of this figure.
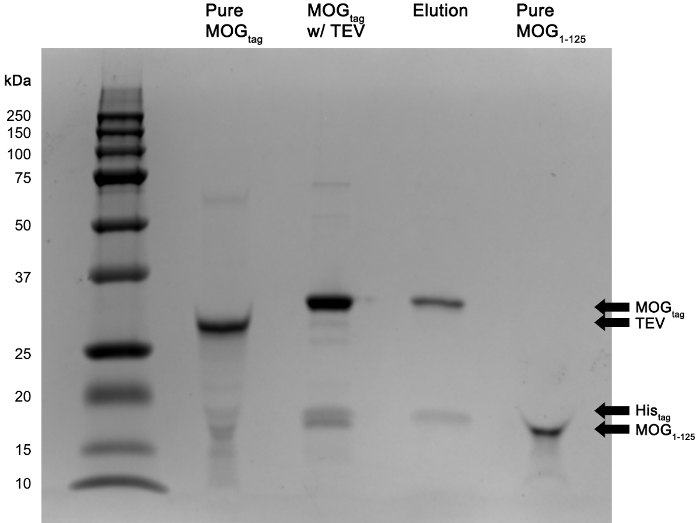
Figure 6: TEV cleavage of MOGtag protein and subsequent purification of MOG1-125. Shown are protein samples run on an SDS-PAGE gel demonstrating purification of MOG1-125. Pure MOGtag = MOGtag protein prior to TEV cleavage (collected in step 6.4), MOGtag w/ TEV = Protein fraction that was collected after 72 hr of incubation of MOGtag with TEV protease (collected in step 7.5), Elution = Protein fraction that remained bound to the nickel resin during the MOG1-125 purification protocol (collected in step 7.9), Pure MOG1-125 = MOG1-125 protein after purification (collected in step 7.12). Please click here to view a larger version of this figure.
| BSA (mg/ml) | 0.4 | 0.35 | 0.3 | 0.25 | 0.2 | 0.15 | 0.1 | 0.05 | 0 |
| 1.079 | 0.998 | 0.948 | 0.853 | 0.769 | 0.699 | 0.583 | 0.493 | 0.373 | |
| 1.071 | 1.014 | 0.95 | 0.854 | 0.777 | 0.681 | 0.579 | 0.484 | 0.375 | |
| 1.069 | 1.017 | 0.944 | 0.848 | 0.781 | 0.687 | 0.592 | 0.494 | 0.374 | |
| MOGtag dilution | 1 | 1/5 | 1/25 | ||||||
| 1.327 | 1.013 | 0.493 | |||||||
| 1.332 | 1.063 | 0.491 | |||||||
| 1.367 | 1.088 | 0.488 |
Table 1: Representative values from a Bradford assay for determining the concentration of MOGtag protein. BSA dilutions at known concentrations are used for determining the standard curve shown in Figure 5. The dilutions of MOGtag protein post purification are used to determine the final MOGtag protein concentration. Rows contain the optical density measured at 595nm and each row represents one replicate of a total of 3 replicates at each indicated concentration.
Discussion
Here, we have described a protocol for the production of MOGtag protein and how to generate pure MOG1-125 from the MOGtag protein. This protocol is based both on standard His-tag based protein purification methods, as well as a previously described protocol for the generation of an older MOG-based protein15. Although it is not described here, the primary usage of the MOGtag protein is to induce EAE through immunization with protein antigen. A protocol describing how EAE is induced in mice, which is compatible with the MOGtag protein, can be found in reference3. We have previously demonstrated that immunization with MOGtag or MOG1-125 derived from MOGtag not only induces CNS autoimmune disease with greater spinal cord inflammation and demyelination compared to the standard MOG35-55 peptide, but also that pathogenic IgHMOG B cells that recognize MOG protein are activated to produce a germinal center response in response to MOGtag or MOG1-125, but not to MOG35-555. Therefore, immunization with MOGtag does indeed induce an appropriate anti-MOG B cell response.
MOGtag protein is purified through absorption onto charged nickel resin (via the His-tag) and elution. Because of the large quantity of protein generated in the previous steps, multiple rounds of absorption and elution are required to collect most of the protein. We have found that at least 4 rounds are required to isolate the majority of protein if using an appropriate volume of resin for 50 ml tubes. If desired, the protocol could be scaled up to use more or larger tubes and more nickel resin to reduce the number of rounds of absorption. Alternatively, high performance liquid chromatography (HPLC) with nickel columns can effectively purify his-tagged proteins18 and indeed we have found that HPLC can efficiently purify MOGtag protein (unpublished observations). As HPLC is not accessible to many standard immunology labs, the protocol listed here is designed to be performed using common lab equipment. In addition to his-tag purification, the MOGtag protein does contain an S-tag that is compatible with S-tag purification protocols if preferred14.
Recombinant proteins based on MOG (mostly human or rat) have been described previously8. These were based on older expression systems that have since been replaced by systems driven by stronger transcriptional promoters capable of producing larger quantities of protein in Escherichia coli bacteria. Our MOGtag expression system uses the efficient T7 promoter in the pET-32a(+) vector, which is significantly more efficient than systems available for in-house production of MOG protein19. However, it should be noted that the MOGtag protein described here is based on mouse MOG. Immunization with human MOG protein has been shown to induce EAE in mice that has different features compared to disease induced using murine MOG8. Further, rat MOG may be more immunogenic than mouse MOG in mice in some cases20. Therefore, for some experimental purposes mouse-based MOGtag may not be ideal. We are in the process of generating several different version of the MOGtag protein than may suit some purposes better, and the purification protocol described here will work for all of these. In the meantime, it is possible that the purification protocol described here may work for other MOG expression systems, or even other proteins, as long as they incorporate a 6x His Tag for absorption to nickel resin. However, without the thioredoxin tag, solubility of MOG protein at higher concentrations may be an issue, and of course it will not be possible to generate pure MOG1-125 as this is unique to the MOGtag system.
Measuring the MOGtag protein concentration prior to protein refolding via dialysis is essential to the success of this purification protocol. If the concentration is too high, the protein may aggregate and fall out of solution instead of folding into individual proteins. This can be seen during the dialysis protocol as white precipitate forming at the bottom of the dialysis tubing. If this is occurring, dissolve the MOGtag protein again with 6 M guanidine and measure the protein concentration. Dilute the protein to 0.5 mg/ml then start dialysis again as no precipitates should form at 0.5 mg/ml. For MOG1-125, precipitates can be expected to form as the protein is highly insoluble. We have found that this will not affect the folding of MOG1-125 provided that the protein was adequately diluted prior to dialysis.
For the TEV protease to effectively cleave the MOGtag protein into pure MOG1-125 it is important to use a sufficient amount of TEV protease. Older versions of TEV proteases are inactivated through self-cleavage21 however more modern versions suffer from insolubility issues22 thus it is important to add enough TEV protease to achieve sufficient cleavage of the MOGtag protein before TEV protease inactivation. Since commercially available TEV protease can be costly, we recommend producing and purifying TEV protease as described in reference22. When using pure mouse MOG1-125 protein for experiments, it is important to note that the protein is highly insoluble and may precipitate out of solution, and therefore must be mixed extensively prior to use.
In summary, here we have described a simple protocol for producing and purifying large quantities of MOGtag protein. Furthermore, the addition of a TEV protease cleavage site to our MOGtag protein provides the opportunity to generate pure MOG1-125 if needed. MOGtag protein is recognized and bound by anti-myelin autoimmune B cells and MOGtag-induced EAE incorporates B cell-mediated pathology5. Therefore, MOGtag protein overcomes the major hurdles limiting the wide-spread use of protein antigen to induce EAE.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the Multiple Sclerosis Society of Canada. RWJ is the recipient of the Waugh Family MS Society of Canada Doctoral Studentship Award.
Materials
| BL21 E.coli– pet32-MOGtag | Kerfoot lab | These bacteria are required to make the MOGtag protein. Glycerol stocks of these bacteria are available upon request. | |
| LB broth miller | Bioshop | LBL407.1 | |
| Ampicillin | bio basic | AB0028 | Reconsititute the powder into 50% ethanol/ 50% H2O at 100 mg/ml. Store at -20 °C. |
| IPTG | Bioshop | IPT002.5 | Reconsititute the powder into H2O at 1M and store at -20 °C. |
| Chicken-egg lysozyme | Bioshop | LYS702.10 | Reconstitute in H2O at 50 mg/ml and store at -80 °C. |
| Triton-X100 | Sigma | T-8532 | |
| Phosphate buffered saline | life technologies | 20012-027 | Commercial phosphate buffered saline is not required, any standard lab made phosphate buffered saline is sufficient. |
| Sodium chloride | Bioshop | SOD004.1 | |
| Tris-HCl | Bioshop | TRS002.1 | |
| Imidazole | Bioshop | IMD508.100 | |
| Guanidine-HCl | Sigma | G3272 | The quality must be greater than 98% purity. |
| 0.5M EDTA | bioshop | EDT111.500 | |
| Nickel (II) sulfate | Bioshop | NIC700.500 | |
| His bind resin | EMD Millipore | 69670-3 | Store in 20% ethanol 80% H2O at 4 °C |
| Anhydrous ethanol | Commercial Alcohols | P016EAAN | Dilute with water as needed. |
| Glacial acetic acid | Bioshop | ACE222.1 | |
| Sodium acetate trihydrate | Bioshop | SAA305.500 | |
| bovine serum albumin standard | bio-rad | 500-0206 | |
| Bio-rad protein assay dye reagent concentrate | bio-rad | 500-0006 | |
| Ethylenediamine tetraacetic acid, disodium salt dihydrate | Fisher scientific | BP120-500 | |
| Tris-base | Bioshop | TRS001.1 | |
| 7000 MW Snakeskin dialysis tubing | Thermoscientific | 68700 | |
| 2-mercaptoethanol | Sigma | M3148-25ml | This reagent should not be handled outside of a fume hood. |
| AcTEV protease | lifetechnologies | 12575-015 | Producing your own TEV protease can be accomplished using (https://www.addgene.org/8827/) and purified as in reference 17 |
| Polyethyleneglycol 3350 | Bioshop | PEG335.1 | |
| polyethyleneglycol 8000 | Bioshop | PEG800.1 | |
| Nunc MaxiSorp flat-bottom 96 well plate | ebioscience | 44-2404-21 | |
| Sonicator | Fisher scientific | FB-120-110 | |
| Eon microplate spectrometer | Biotek | 11-120-611 | This equipment uses the Gen5 data analysis software. |
| Gen5 data analysis software | BioTek | ||
| sodium dodecyl sulphate | Bioshop | SDS001 | |
| bromophenol blue | Bioshop | BRO777 | |
| Glycerol | Bioshop | GLY001 | |
| Protein desalting columns | Thermoscientific | 89849 | |
| Glycine | Bioshop | GLN001 | |
| precast 12% polyacrylamide gel | bio-rad | 456-1045 | |
| Rapid stain reagent | EMD Millipore | 553215 | |
| Gel dock EZ imager | bio-rad | 1708270 | |
| White Light Sample Tray | bio-rad | 1708272 | Used along with gel dock EZ imager for coomassie blue stains |
| Protein ladder | bio-rad | 1610375 |
References
- Compston, A., Coles, A. Multiple sclerosis. Lancet. 372 (9648), 1502-1517 (2008).
- Baxter, A. G. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 7 (11), 904-912 (2007).
- Bittner, S., Afzali, A. M., Wiendl, H., Meuth, S. G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. (86), (2014).
- Shetty, A., et al. Immunodominant T-cell epitopes of MOG reside in its transmembrane and cytoplasmic domains in EAE. Neurol Neuroimmunol Neuroinflamm. 1 (2), 22 (2014).
- Dang, A. K., Jain, R. W., Craig, H. C., Kerfoot, S. M. B cell recognition of myelin oligodendrocyte glycoprotein autoantigen depends on immunization with protein rather than short peptide, while B cell invasion of the CNS in autoimmunity does not. J Neuroimmunol. 278, 73-84 (2015).
- Barun, B., Bar-Or, A. Treatment of multiple sclerosis with anti-CD20 antibodies. Clin Immunol. 142 (1), 31-37 (2012).
- Bettadapura, J., Menon, K. K., Moritz, S., Liu, J., Bernard, C. C. Expression, purification, and encephalitogenicity of recombinant human myelin oligodendrocyte glycoprotein. J Neurochem. 70 (4), 1593-1599 (1998).
- Oliver, A. R., Lyon, G. M., Ruddle, N. H. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 171 (1), 462-468 (2003).
- . JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. Molecular Cloning. J Vis Exp. , (2016).
- . JoVE Science Education Database. Basic Methods in Cellular and Molecular Biology. Restriction Enzyme Digests. J Vis Exp. , (2016).
- Hamada, H., Arakawa, T., Shiraki, K. Effect of additives on protein aggregation. Curr Pharm Biotechnol. 10 (4), 400-407 (2009).
- Dang, A. K., Tesfagiorgis, Y., Jain, R. W., Craig, H. C., Kerfoot, S. M. Meningeal Infiltration of the Spinal Cord by Non-Classically Activated B Cells is Associated with Chronic Disease Course in a Spontaneous B Cell-Dependent Model of CNS Autoimmune Disease. Front Immunol. 6, 470 (2015).
- LaVallie, E. R., et al. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology (N Y). 11 (2), 187-193 (1993).
- Raines, R. T., McCormick, M., Van Oosbree, T. R., Mierendorf, R. C. The S.Tag fusion system for protein purification. Methods Enzymol. 326, 362-376 (2000).
- Amor, S., et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 153 (10), 4349-4356 (1994).
- Kostallas, G., Lofdahl, P. A., Samuelson, P. Substrate profiling of tobacco etch virus protease using a novel fluorescence-assisted whole-cell assay. PLoS ONE. 6 (1), e16136 (2011).
- Litzenburger, T., et al. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. 188 (1), 169-180 (1998).
- Zhang, H. Y., et al. Separation and purification of Escherichia coli-expressed human thymosin-alpha1 using affinity chromatography and high-performance liquid chromatography. Protein Expr Purif. 77 (2), 140-145 (2011).
- Tegel, H., Ottosson, J., Hober, S. Enhancing the protein production levels in Escherichia coli with a strong promoter. FEBS J. 278 (5), 729-739 (2011).
- Akirav, E. M., Bergman, C. M., Hill, M., Ruddle, N. H. Depletion of CD4(+)CD25(+) T cells exacerbates experimental autoimmune encephalomyelitis induced by mouse, but not rat, antigens. J Neurosci Res. 87 (15), 3511-3519 (2009).
- Kapust, R. B., et al. Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 14 (12), 993-1000 (2001).
- Blommel, P. G., Fox, B. G. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr Purif. 55 (1), 53-68 (2007).

