In Situ Visualization of the Phase Behavior of Oil Samples Under Refinery Process Conditions
Summary
This article describes a setup and method for the in situ visualization of oil samples under a variety of temperature and pressure conditions that aim to emulate refining and upgrading processes. It is primarily used for studying isotropic and anisotropic media involved in the fouling behavior of petroleum feeds.
Abstract
To help address production issues in refineries caused by the fouling of process units and lines, we have developed a setup as well as a method to visualize the behavior of petroleum samples under process conditions. The experimental setup relies on a custom-built micro-reactor fitted with a sapphire window at the bottom, which is placed over the objective of an inverted microscope equipped with a cross-polarizer module. Using reflection microscopy enables the visualization of opaque samples, such as petroleum vacuum residues, or asphaltenes. The combination of the sapphire window from the micro-reactor with the cross-polarizer module of the microscope on the light path allows high-contrast imaging of isotropic and anisotropic media. While observations are carried out, the micro-reactor can be heated to the temperature range of cracking reactions (up to 450 °C), can be subjected to H2 pressure relevant to hydroconversion reactions (up to 16 MPa), and can stir the sample by magnetic coupling.
Observations are typically carried out by taking snapshots of the sample under cross-polarized light at regular time intervals. Image analyses may not only provide information on the temperature, pressure, and reactive conditions yielding phase separation, but may also give an estimate of the evolution of the chemical (absorption/reflection spectra) and physical (refractive index) properties of the sample before the onset of phase separation.
Introduction
The study of the phase behavior of oil samples in a wide range of temperatures, pressures, and reactive conditions can yield very useful information for the operator of a refinery that processes a variety of feeds. In particular, the fouling of process units and lines by an uncontrolled formation of coke or sediments can severely affect production (loss of throughput) and energy efficiency (increase in heat transfer resistance)1,2,3. Possible plugging caused by the accumulation of fouling material may require a shutdown for clean-up purposes, which would have a highly negative economic impact4. Conducting an assessment of the fouling propensities of feeds can be highly valuable for the optimization of process conditions5 and the blending of refinery streams.
We have developed an in situ analyzer of petroleum stability in our laboratory to allow the visualization of oil samples subject to refinery process conditions. This apparatus relies on a specifically designed reactor made of stainless steel fittings and equipped with a sealed sapphire window at the bottom. The main principle of the device is the illumination of the sample inside the reactor at the desired range of temperature and pressure and the imaging of the resulting cross-polarized reflection. While previous published work relative to this setup focused on thermal cracking processes to emulate visbreaking conditions6,7,8,9 (which do not require high pressure), the reactor design was overhauled to investigate the behavior of samples under hydroconversion (catalytic cracking under high H2 pressure) and aquathermal10 (thermal cracking under high-pressure steam) conditions. Thus, the device was revised in order to operate in the 20-450 °C temperature range and the 0.1-16 MPa pressure range, with the ability to sustain both 450 °C and 16 MPa for reaction times of up to 6 h.
The first level of analysis on the visual information of the samples under a particular range of temperature, pressure, and reactive conditions is to determine whether the sample is single-phase or multiphase. This system is unique in that it allows for the visualization of opaque isotropic material and is not limited to the visualization of anisotropic material described in other work11. While the main indicator of the fouling propensity of samples is the tendency to drop sediments out of the bulk liquid; gas-liquid, liquid-liquid, liquid-solid, and more complex phase behaviors can be observed. However, valuable information can also be extracted from the visual evolution of a liquid as it remains homogeneous (single-phase). In particular, the brightness of the images is related to the refractive index and the extinction coefficient of the sample, while the color of the sample is a subset of its spectral information in the visible light range (380-700 nm), which can be used as a descriptor of its chemistry9.
Protocol
Caution: Use all appropriate safety practices when performing an experiment under high temperature and pressure conditions, including the use of engineering controls (H2 flow limiter, pressure regulators, and rupture disc assembly) and personal protective equipment (safety glasses, temperature-resistant gloves, lab coat, full-length pants, and closed-toe shoes). Consult all relevant material safety data sheets (MSDS) before use. Carry out micro-reactor loading and clean-up in a fume hood, as these steps involve the use of harmful volatile organic solvents (toluene and dichloromethane).
NOTE: Setup description (see supplemental file).
1. Micro-reactor Loading
- Clamp the micro-reactor vertically and upside-down, with the bottom face-seal (thus positioned at the top) open.
NOTE: At this stage, the sapphire window, the custom-machined magnet, the 1/16" ferrule, the brass pad, and the bottom nut should not be assembled yet.- Ensure that the fittings that are used to connect the micro-reactor to the gas lines are closed.
- Load around 0.6 g of sample into the reactor through the open face-seal using a thin spatula.
- If the sample is originally kept in a large container, make a sub-sample before loading the micro-reactor.
- To estimate the amount of sample loaded inside the reactor, weigh the container and the spatula before and after loading, and calculate the mass difference.
- Slide the custom-machined magnet onto the thermocouple.
- Slide the 1/16" front ferrule so that the larger circle is facing up.
- Ensure that the sealing surface (i.e., the fitting groove where the sealing ring sits) of the bottom face-seal fitting is clean and dry.
NOTE: Given the highly viscous nature of most heavy oil samples, it is very likely that the sealing surface got accidently smeared by the sample during the loading process.- Dip the tip of a cotton swab in toluene and apply it to the sealing surfaces to clean them. Be careful not to drip toluene inside the reactor cavity, which would contaminate the sample.
- If cleaning with toluene is required, make sure the sealing surfaces are dry before proceeding to the next step.
- Ensure that the sapphire window is clean and dry.
- If the sapphire window is dirty, use a cotton swab soaked in a suitable solvent, and then perform a final wash using acetone to clean the window surfaces; let it air dry.
- Place the sealing ring on the sealing surface, then the sapphire window on the top of the sealing ring, and then the brass pad on the top of the sapphire window; it is preferable to apply tiny, pinhead-sized drops of lubricant on the brass pad.
- Thread the bottom nut on the bottom face-seal fitting while encapsulating the brass pad and the sapphire window. Adjust the bottom nut until it reaches finger-tight position.
- While holding the reactor upside-down, transfer it to a vice. Use a wrench to tighten the bottom nut by 90° from finger-tight position.
NOTE: After this step, the reactor does not need to be held upside-down any longer. - Check the micro-reactor for potential defects in the seal.
NOTE: The window may show some chips or cracks, or a faulty seal can be identified if the compressed surface of the seal on the window does not make a continuous circle.- In case of a defect, open the micro-reactor for inspection.
- After taking remedying action, use a brand-new sealing ring when attempting to reseal the reactor.
2. Micro-reactor Installation
- Once the micro-reactor is loaded and sealed, connect the reactor to the gas lines and perform tests for leaks.
- Always begin leak testing by using N2 at a maximum pressure of 5 MPa.
NOTE: The preferred method for leak testing is the pressure-decay test, where the setup is pressurized and then isolated from the cylinder (closing valves V2 and V3). If the pressure remains stable for an extended amount of time (more than 30 min), no leak is observed. - Carry out additional leak tests at higher pressures if the target pressure for the upcoming experiment is higher than 5 MPa.
NOTE: These additional leak tests can be performed with maximum pressure increments of 6 MPa until the desired pressure condition for the experiment is matched. Consider 16 MPa as the upper limit of pressure for both leak testing and setup operation.
NOTE: If the gas used to pressurize the setup in the upcoming experiment is not inert (such as flammable gases), carry out another series of leak tests using the target gas contingent on a successful series of leak tests with N2.
- Always begin leak testing by using N2 at a maximum pressure of 5 MPa.
- After successful leak tests, depressurize the setup before undertaking the next installation steps.
- Place the micro-reactor in the stainless steel heating block, which is itself inserted in the coil heater. Position the assembly on the platform located above the microscope objective.
- Encase the reactor, the heater, and the heating block with the two halves of a casing filled with ceramic wool. Clamp the two halves of the casing together using a hose clip.
- Fine-tune the position of the reactor over the microscope objective.
- Turn the microscope on using cross-polarized light. Adjust the vertical position of the objective using the lowest magnification so as to focus on the inside surface of the sapphire window.
- Position the reactor so that the field of view at the lowest magnification (typically 50X) covers a radial portion of the window surface where the inner boundary comprises the edge of the 1/16" front ferrule, as described in Figure 1.
NOTE: Actual micrographs acquired by the software should be centered subsets of this field of view, which would avoid showing the ferrule itself.
- Connect the thermocouple of the micro-reactor (TT1) to the temperature controller (TIC1).
- Turn on the motor driving the external magnet to a speed of 120 rpm.
- Pressurize the setup to the desired set-point.
NOTE: Atmospheric pressure runs are performed by opening all outlet valves to the vent. Batch experiments can be performed by closing valve V4. Experiments under a constant head of pressure (preferable for high-pressure conditions) can be carried out by using the back-pressure regulator PV2.
3. Regular Procedure for the Visualization of Cracking Reactions
- Throughout the entire experiment, place the microscope objective under the reactor only when visualizing the sample or taking a snapshot. Avoid leaving the microscope objective under the reactor when it is not needed.
NOTE: Leaving the microscope objective under the reactor at high temperatures will cause an artificial brightening of the images, resulting in poor data, and may lead to a deterioration of the objective. - Turn the temperature controller on and apply a temperature set-point of 200 °C. Once the sample temperature reaches 200 °C, perform a round of verifications.
NOTE: A round of verifications entails verifying the pressure, temperature, reactor position, focal distance of the microscope objectives, and stirring. As the temperature changes, the platform supporting the reactor and the heating assembly deforms slightly, so the vertical position of the microscope objective must be adjusted for the sapphire/sample interface to remain in focus. Stirring can be detected by the motion of the 1/16" ferrule or of small heterogeneity in the sample (such as small mineral solids). - If everything is in order as the sample reaches 200 °C, perform a set-point change to 300 °C. Once the sample temperature reaches 300 °C, perform another round of verifications.
- Repeat the previous step, with 350 °C as the new temperature set-point.
NOTE: 350 °C can generally be considered as the higher temperature limit where cracking reactions are not significant (in the time scale of min). - Change the set-point temperature to the desired reaction temperature, generally in the 400-450 °C range.
- After making the final temperature set-point change, begin monitoring the reaction and recording data at regular time intervals, preferably every min.
- Carry out each step of data recording as follows: rotate the nosepiece of the microscope to place the objective under the reactor. Adjust the focus. Take a snapshot. Rotate the nosepiece to move the objective away from underneath the reactor. Note the temperature.
NOTE: For future quantitative image analyses, snapshots should be taken with consistent settings throughout the experiment, namely in terms of magnification, lighting conditions, and camera acquisition settings (photosensitivity response and exposure time). As guidelines, the micrographs presented in this manuscript were taken with 100X magnification, maximum lighting conditions (using a halogen lightbulb), linear sensitivity response of the camera, and exposure times ranging from 200-400 ms. - Perform the data recording steps repeatedly for as long as needed.
NOTE: Generally, the duration of the observation is guided by visual changes in the sample (color, brightness, and heterogeneity) or by an estimate of reaction conversion.
NOTE: Preferably, avoid continuing the experiment after the formation of large amounts of mesophase coke (which makes the reactor more difficult to clean).
- Carry out each step of data recording as follows: rotate the nosepiece of the microscope to place the objective under the reactor. Adjust the focus. Take a snapshot. Rotate the nosepiece to move the objective away from underneath the reactor. Note the temperature.
4. Shutdown and Clean-up
- Terminate the experiment by turning the temperature controller and the stirrer off and depressurizing the setup. Let the reactor cool.
NOTE: Cooling the reactor can be facilitated by removing the micro-reactor from the casing and out of the heating assembly. Applying a cool air flow to the micro-reactor can also make this process faster and easier.- Once the micro-reactor is cooled to room temperature, disconnect it from the gas lines of the setup, place it in a vice to loosen the bottom nut, and unseal the micro-reactor.
- In a fume hood, take the micro-reactor apart by removing the bottom nut, the brass pad, the sapphire window, the 1/16" ferrule, and the magnet. Remove the sealing ring.
NOTE: Coke may have formed during the experiment, which may cause the 1/16" ferrule and the magnet to be stuck to the thermocouple.- Use tweezers to pull the 1/16" ferrule and the magnets out. Use a spatula to lever the sealing ring out of the sealing groove. However, take care not to scratch the sealing groove in the process.
- To remove the bulk of the material stuck to the micro-reactor walls, scrub the inside cavity of the micro-reactor with solvent-soaked (toluene or dichloromethane) pieces of paper towels. Repeat the process with pieces of emery cloth, preferably coarse grit (#100).
NOTE: During this process, do not scratch the sealing surfaces. At the end of this step, the metallic shine of stainless steel inside the micro-reactor cavity should be apparent. - Remove the material stuck to the flat surfaces of the custom-machined magnet using a piece of emery cloth, preferably coarse grit (#100).
- Use a solvent-soaked 1/16" wire to remove the material stuck inside the hole of the custom-machined magnet.
- Use solvent-soaked (toluene, dichloromethane, or acetone) cotton swabs to remove the material stuck to the sapphire window.
- To remove the remainder of the material stuck to the reactor walls, including the sealing surfaces, use solvent-soaked (toluene or dichloromethane) cotton swabs.
NOTE: The cleanup process is finished when, after scrubbing with a solvent-soaked cotton swab, the cotton swab comes out with negligible traces on it.
NOTE: However tedious this process might be, this step is important to avoid cross-contamination between experiments. - Let the micro-reactor air-dry.
5. Image Analysis9
- Extract information from micrographs pertaining to the mean values of the red, green, and blue (RGB) channels, as well as the corresponding information in the hue, saturation, and intensity (HSI) color space.
NOTE: The HSI color space is described by cylindrical coordinates, where hue, saturation and intensity correspond to the angular, radial, and vertical coordinates, respectively. The relationships between the RGB values of a pixel and the corresponding HSI values are given by the following equations12,13, where m is the minimum of RGB values, while α and β are the pair of chromaticity coordinates:
 Eq. 1
Eq. 1
 Eq. 2
Eq. 2
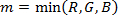 Eq. 3
Eq. 3
 Eq. 4
Eq. 4
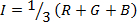 Eq. 5
Eq. 5
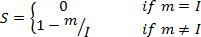 Eq. 6
Eq. 6
Representative Results
The visual evolution of Athabasca Vacuum Residue is representative of the behavior of asphaltenic heavy crude oil samples and asphaltenic vacuum residue samples under thermal cracking conditions. However, using different samples and/or different temperature, pressure, or reaction conditions can give rise to a wide variety of phase behaviors. Micrographs corresponding to the thermal cracking experiment on an Athabasca Vacuum Residue sample at final set-point conditions of 435 °C and Patm (N2) are given in Figure 3, while Figure 4 shows the evolution of temperature during the experiment.
At room temperature, this sample is a pasty solid; thus, the sapphire window is mostly not wetted by the sample and is in contact with gas (in this case, N2). An air/sapphire interface yields a much brighter reflection than an oil/sapphire interface, so the appropriate illumination and exposure settings to image a liquid sample will always yield white regions if the sapphire surface is in contact with gas. At a higher temperature (> 150 °C), the sample becomes fluid enough to flow and wet the window surface. Small mineral solids inside the sample, which can be identified by small bright elements (Figure 3 A), can serve as an indicator of the stirring efficiency. As the sample is heated to higher temperatures, the images brighten correspondingly, with no color change as long as no significant reaction is taking place. Thermal cracking reactions in asphaltenic vacuum residues cause color and brightness changes that correspond to the chemical transformation of the sample. At extended reaction times, the formation of domains of anisotropic carbonaceous phase (mesophase) can be detected as stationary heterogeneities on the window (Figure 3 D).
An image analysis of the series of micrographs is shown in Figures 5 and 6, which show the evolution of brightness intensity and color with reaction time, respectively. At very early reaction times, the increase in image brightness follows the evolution of the temperature inside the reactor. As the temperature inside the reactor approaches the 435 °C set-point, thermal cracking reactions in the Athabasca Vacuum Residue become prevalent. Thermal cracking reactions in Athabasca Vacuum Residue induce a brightness change in the sample that follows a decreasing exponential trend. In the same period, the color of the sample remains stable in the first part of the reaction before undergoing a shift towards a blue color. The formation of mesophase has the effect of increasing the overall brightness intensity and enhancing the blue color shift9.
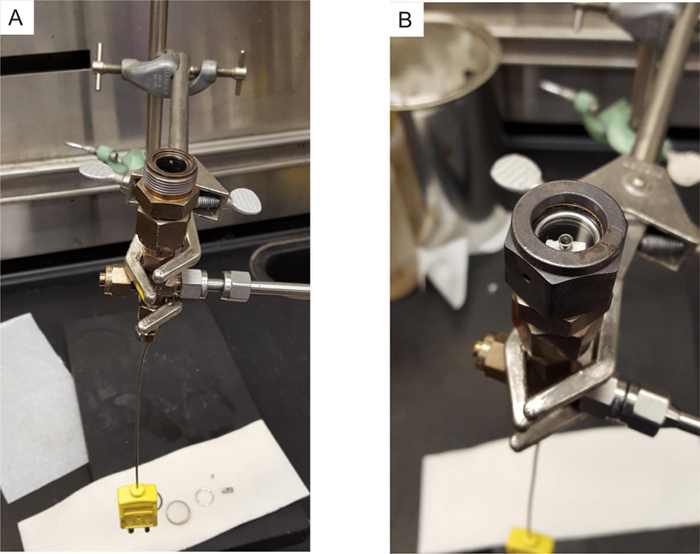
Figure 1: Photographs of the micro-reactor, held upside-down by a clamp. Pre-loading arrangement, with the bottom face opened (A). The loaded and sealed micro-reactor (B). Please click here to view a larger version of this figure.

Figure 2: Examples of preferable fields-of-view, as outlined by red rectangles, with respect to the inner surface of the sapphire window. Please click here to view a larger version of this figure.
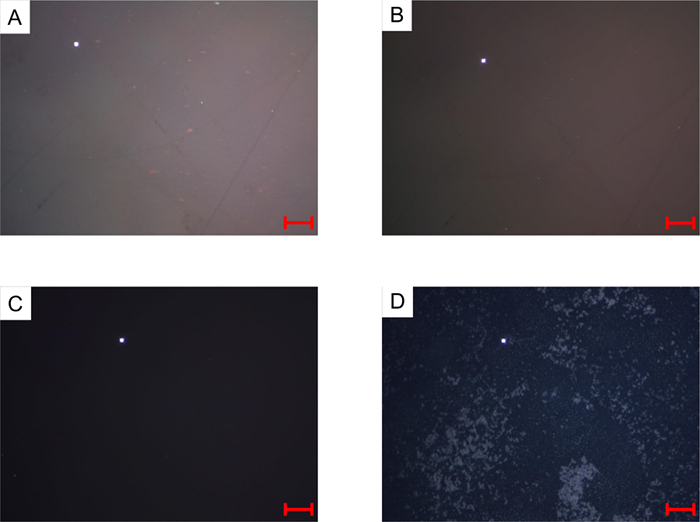
Figure 3: Micrographs taken during a thermal cracking experiment on Athabasca Vacuum Residue with a condition set-point of 435 °C and Patm (N2) after 0 min (A), 25 min (B), 50 min (C), and 80 min (D). Scale bar = 100 µm. Please click here to view a larger version of this figure.

Figure 4: The temperature inside the reactor during a thermal cracking experiment on Athabasca Vacuum Residue with a set-point of 435 °C and Patm (N2). Please click here to view a larger version of this figure.
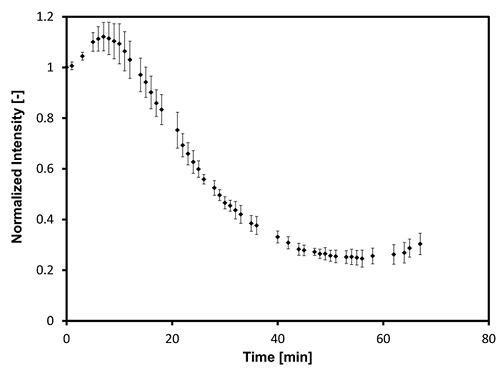
Figure 5: Evolution of the brightness intensity (I) of the micrographs taken during a thermal cracking experiment on Athabasca Vacuum Residue under 435 °C and Patm (N2), normalized by the brightness of the micrograph taken at 350 °C. Please click here to view a larger version of this figure.
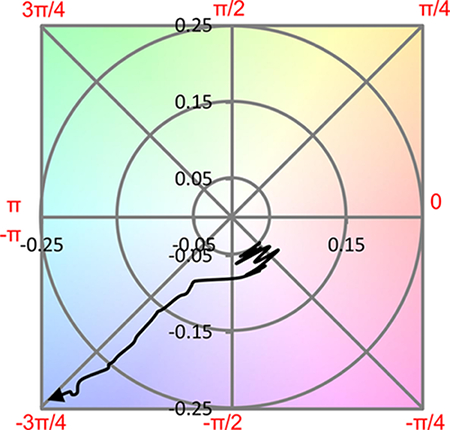
Figure 6: Evolution of the hue and saturation (H and S in polar coordinates) of the micrographs taken during a thermal cracking experiment on Athabasca Vacuum Residue under 435 °C and Patm (N2). Please click here to view a larger version of this figure.
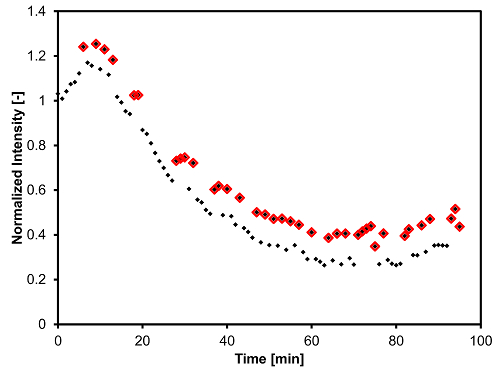
Figure 7: Evolution of the brightness intensity (I) of the micrographs taken during a thermal cracking experiment on Cold Lake bitumen under 435 °C and Patm (N2), normalized by the brightness of the micrograph taken at 350 °C. The data points outlined in red correspond to pictures taken with an overheated objective. Please click here to view a larger version of this figure.
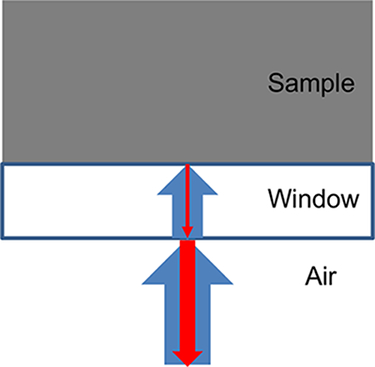
Figure 8: The main incident rays (blue arrows) and reflected rays (red arrows) involved in the illumination of a sample through a window. Please click here to view a larger version of this figure.
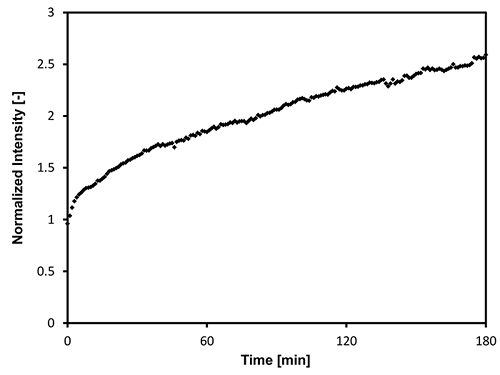
Figure 12: Evolution of the brightness intensity (I) of the micrographs taken during a hydroconversion experiment, normalized by the brightness of the micrograph taken at 350 °C. The hydroconversion experiment was carried out on a heavy vacuum gasoil sample under 420 °C and 15 MPa (H2), with 12.3 wt.% Ni/Mo catalyst. Please click here to view a larger version of this figure.
Discussion
Critical Steps within the Protocol
The first critical step in the protocol is ensuring the integrity of the metal-to-sapphire seal, especially if the experiment is to be carried out under pressure. Thus, the parallelism, the smoothness, and the cleanliness of the sealing surfaces should be carefully inspected, and the leak tests should be thorough. Since the modulus of rupture of sapphire is a decreasing function of temperature14, thicker sapphire windows should be used for work at high pressure and high temperature. As a guideline, 8 mm-thick sapphire windows are used in our experiments aiming to emulate hydroconversion conditions (400-450 °C and 16 MPa H2).
The second critical step relates to obtaining high-quality images, which require the bright illumination of the sample; a clean train of optics; adapted microscope settings (wide iris aperture and long working-distance objectives); and the proper alignment between the microscope objective, the reactor window, and the supporting platform.
For the quantitative analysis of image information, it is critical not to overheat the microscope objective while performing the observations. The method described in step 3.6.1 of the Protocol prevents such overheating. If the operator omits to remove the objective from underneath the reactor between two snapshots taken one min apart, the second picture will appear noticeably brighter as a result. To illustrate this issue, the data points outlined in red on Figure 7 correspond to pictures where the operator had left the objective underneath the reactor in the previous min.
Lastly, it is important to clean the reactor thoroughly between experiments in order to avoid cross-contamination.
Modifications and Troubleshooting
Poor data quality generally results from a poorly controlled operating variable (temperature, pressure, or stirring), or a problem in the train of optics. Possible problems in the train of optics include: poor illumination; a small iris aperture; misaligned cross-polarizers; dirty mirrors, filters beam splitters, or objectives; a misaligned reactor or supporting platform above the objective; a dirty or scratched sapphire window; a maladjusted field of view; overheated objectives; and out-of-focus objectives.
Limitations of the Technique
For present configuration of the experimental setup, the main limitation of this technique is the lack of ability to reproduce the same level of image brightness across different experiments. In addition to the cleanliness and the alignment of the train of optics, image brightness was found to be very sensitive to the positioning and tilt of the reactor over the objective, which currently are not tightly controlled parameters. However, normalizing the image brightness of a series of micrographs in a given experiment by the image brightness of a micrograph taken at a reference temperature within the same series provides a satisfying workaround, as it yields reproducible data.
Significance of the Technique with Respect to Existing/Alternative Methods
The combination of cross-polarizers in the train of optics of an inverted microscope with a reactor window made of sapphire allows the observation of high-contrast images of the sample in situ. When shining light on an opaque sample through a window, two main reflections are involved, as shown in Figure 8: the reflection of the light on the outside surface of the window in contact with air, and the reflection of the light on the inside surface of the window in contact with the sample. The intensity of the reflection at each interface is given by the following equation15:
 Eq. 7
Eq. 7
where indices 1 and 2 refer to the media located before and beyond the interface, respectively; n describe refractive indices; and κ is the extinction coefficient. In air/sapphire and sapphire/oil reflections, the contribution of the extinction coefficient to the reflection can be neglected. Considering the refractive index of sapphire in the C-axis direction (extraordinary ray) as 1.765 (average in the 380-700 nm range)16, the intensity of the first reflection at the air/sapphire interface is about 7.7% of the incident light. Since most oil samples have refractive indices ranging from 1.45 to 1.617, the intensity of the second reflection at the sapphire/oil interface can be considered as less than 0.9% of the incident light. On first approximation, the air/sapphire reflection is at least more than 9 times brighter than the sapphire/oil reflection. Hence, when observations are made under bright-field settings (using unpolarized light), visuals of the sample are outshined by the air/sapphire reflection. To illustrate this issue, micrographs taken under bright-field settings during the thermal cracking experiment on an Athabasca Vacuum Residue sample at final set-point conditions of 435 °C and Patm (N2) are presented in Figure 9 (the microscope lamp voltage was reduced to 10 V and the camera exposure was reduced to 25 ms to avoid blow-outs).

Figure 9: Micrographs taken during a thermal cracking experiment on Athabasca Vacuum Residue with a condition set-point of 435 °C and Patm (N2) after 0 min (A), 25 min (B), 50 min (C), and 80 min (D), taken using bright-field microscope settings instead of cross-polarizers. Scale bar = 100 µm. Please click here to view a larger version of this figure.
As can be seen by comparing Figure 9 with Figure 3, the presented method for observing the sample using cross-polarized light and a sapphire window has the advantage of yielding high-contrast images that are able to describe isotropic media.
As the light is reflected at the air/sapphire interface, its polarization plane does not change. Thus, the cross-polarizer setting cancels this reflection before it hits the CCD camera. As light travels through sapphire, however, its polarization plane rotates because of the sapphire birefringence. This phenomenon ultimately allows sample imaging, even if the oil sample itself is isotropic and the polarization plane of the light does not change upon the sapphire/oil reflection. If the cross-polarizer setting is used in combination with an optically isotropic window (such as fused silica or yttrium-aluminum-garnet, YAG), then only an anisotropic medium (changing the polarization plane of the light at the window/sample interface) and depolarized fluorescence can be viewed. Figure 10 presents micrographs taken during a thermal cracking experiment on an Athabasca Vacuum Residue sample at final set-point conditions of 435 °C and Patm (N2) using the cross-polarizer setting and a 4 mm-thick YAG window.
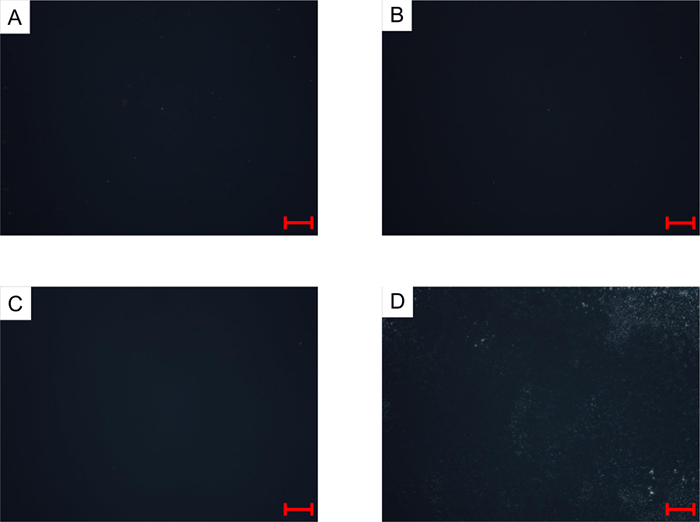
Figure 10: Micrographs taken during a thermal cracking experiment on Athabasca Vacuum Residue with a condition set-point of 435 °C and Patm (N2) after 0 min (A), 25 min (B), 50 min (C), and 80 min (D), taken using a YAG window instead of a sapphire window. Scale bar = 100 µm. Please click here to view a larger version of this figure.
In comparison to the presented technique, the top-down, hot-stage configuration used in other works11,18 has the disadvantage of featuring a gap of gas between the inner surface of the reactor window and the liquid sample. In such a configuration, using a sapphire window would produce images dominated by the brightness of the sapphire/gas reflection, very similar to the use of bright field with an inverted microscope. Thus, the operators of the top-down hot stage used a reactor window made of YAG, which only allows for the observation of anisotropic material, as explained previously.
The optical properties of a sample may evolve as it undergoes a change in temperature, pressure, or reaction time. The formation of a multiphase system can be characterized by the formation of heterogeneity on the window surface. Figure 11 shows examples of gas-liquid-anisotropic solid, liquid-isotropic solid, liquid-anisotropic semi-solid, and liquid-liquid crystal multiphase systems.

Figure 11: Examples of varied phase behaviors observed during thermal cracking (A, B, and C) and coal liquefaction (D) experiments. Gas-liquid-anisotropic solid (A), liquid-isotropic solid (B), liquid-anisotropic semi-solid (C), and liquid-liquid crystal (D) multiphase systems. Scale bar = 100 µm. Please click here to view a larger version of this figure.
For homogeneous, single-phases systems, changes in the brightness and color of the sample can be related to physical and chemical properties. Following Equation 7, changes in sample brightness are attributed to changes in the refractive indices. In particular, the greater the difference in refractive indices between the sample and the sapphire, the brighter the reflection. For instance, as a heavy oil sample is heated to temperatures below 300 °C, the refractive index of the oil decreases while the refractive index of the sapphire slightly increases, yielding brighter images. During isothermal cracking reactions of vacuum residue samples, images undergo an exponential decrease in brightness; this is attributed to an increase in refractive index due to an increase in aromaticity and density. Conversely, hydroconversion reactions at constant temperature produce a gradual increase in sample brightness, which corresponds to a decrease in refractive index following a decrease in the density of the sample.
Color changes follow the evolution of the spectral properties of the sample, which correspond to its chemistry. Most notably, vacuum residue samples have exhibited a red-to-blue color shift when subjected to thermal cracking reactions for an extended amount of time prior to the formation of sediments. Given enough thermal cracking reaction time, such samples undergo an increase in aromaticity and begin to form oligomers. The formation of more conjugated species leads to a change in spectral properties, where the predominant light absorption of the sample shifts from shorter wavelengths to longer wavelengths. Since reflection spectra are the counterpart of absorption spectra, the corresponding spectral shift in the reflected light goes from longer wavelengths to shorter wavelengths, matching the color change from red to blue9.
Future Applications or Directions after Mastering This Technique
While our studies involving the use of this setup have been primarily related to phase separation phenomena during visbreaking and hydroconversion of heavy petroleum samples in downstream operation, the technique could be applied to the investigation of other phase separation mechanisms occurring in oil processing units and lines (wax crystallization, demulsification, etc.). More generally, this technique could be applied to any system where tracking the optical properties of a sample in situ is of great importance.
Our current research efforts are focused on establishing more relationships between the spectral properties and the physical properties (solubility in particular) of petroleum samples. At present, the spectral information contained in images is limited, since it is expressed in three color channels (RGB). Therefore, the most promising development of this technique lies in the implementation of hyperspectral characterization.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Daniel Palys for supplying Figure 12 and for his assistance in managing laboratory supplies.
Materials
| Sapphire window, C-plane, 3mm thick – 20 mm diam., Scratch/Dig: 80/50 | Guild Optical Associates | ||
| C-seal | American Seal & Engineering | 31005 | |
| Type-K thermocouple | Omega | KMQXL-062U-9 | |
| Ferrule (1/16") | Swagelok | SS-103-1 | Inserted for creating a clearance gap between the magnet and the window surface |
| Coil Heater | OEM Heaters | K002441 | |
| Temperature controller | Omron | E5CK | |
| Inverted microscope | Zeiss | Axio Observer.D1m | Require cross-polarizer module |
| Toluene, 99.9% HPLC Grade | Fisher | Catalog # T290-4 | Harmful, to be handled in fume hood |
| Methylene chloride, 99.9% HPLC Grade | Fisher | Catalog # D143-4 | Harmful, to be handled in fume hood |
| Acetone, 99.7 Certified ACS Grade | Fisher | Catalog # A18P-4 |
References
- Gray, M. R. . Upgrading Petroleum Residues and Heavy Oils. , (1994).
- Wiehe, I. A. . Process Chemistry of Petroleum Macromolecules. , (2008).
- Rahimi, P. M., Teclemariam, A., Taylor, E., deBruijn, T., Wiehe, I. A. Thermal Processing Limits of Athabasca Bitumen during Visbreaking Using Solubility Parameters. Heavy Hydrocarbon Resources, ACS Symposium Series, Volume 895. , (2005).
- Wiehe, I. A., Kennedy, R. J. Application of the Oil Compatibility Model to Refinery Streams. Energy Fuels. 14 (1), 60-63 (2000).
- Rahimi, P., Gentzis, T., Cotté, E. Investigation of the Thermal Behavior and Interaction of Venezuelan Heavy Oil Fractions Obtained by Ion-Exchange Chromatography. Energy Fuels. 13 (3), 694-701 (1999).
- Bagheri, S. R., Gray, M. R., McCaffrey, W. C. Influence of Depressurization and Cooling on the Formation and Development of Mesophase. Energy Fuels. 25 (12), 5541-5548 (2011).
- Bagheri, S. R., Gray, M. R., Shaw, J., McCaffrey, W. C. In Situ Observation of Mesophase Formation and Coalescence in Catalytic Hydroconversion of Vacuum Residue Using a Stirred Hot-Stage Reactor. Energy Fuels. 26 (6), 3167-3178 (2012).
- Bagheri, S. R., Gray, M. R., McCaffrey, W. C. Depolarized Light Scattering for Study of Heavy Oil and Mesophase Formation Mechanisms. Energy Fuels. 26 (9), 5408-5420 (2012).
- Laborde-Boutet, C., Dinh, D., Bender, F., Medina, M., McCaffrey, W. C. In Situ Observation of Fouling Behavior under Thermal Cracking Conditions: Hue, Saturation and Intensity Image Analyses. Energy Fuels. 30, 3666-3675 (2016).
- Dinh, D. . In-Situ Observation of Heavy-Oil Cracking using Backscattering Optical Techniques. MSc Thesis. , (2015).
- Rahimi, P., et al. Investigation of Coking Propensity of Narrow Cut Fractions from Athabasca Bitumen Using Hot-Stage Microscopy. Energy Fuels. 12 (5), 1020-1030 (1998).
- Hanbury, A. Constructing cylindrical coordinate colour spaces. Pattern Recognition Letters. 29 (4), 494-500 (2008).
- Gonzalez, R. C., Woods, R. E. . Digital Image Processing, Third Edition. , (2008).
- Wachtman, J. B., Maxwell, L. H. Strength of Synthetic Single Crystal Sapphire and Ruby as a Function of Temperature and Orientation. J. Am. Ceram. Soc. 42 (9), 432-433 (1959).
- Kaye, G. W. C., Laby, T. H. . Tables of physical and chemical constants / originally compiled by G.W.C. Kaye and T.H. Laby ; now prepared under the direction of an editorial committee. , (1995).
- Malitson, I. H., Dodge, M. J. Refractive Index and Birefringence of Synthetic Sapphire. J. Opt. Soc. Am. 62 (11), 1405 (1972).
- Buckley, J. S., Hirasaki, G. J., Liu, Y., Von Drasek, S., Wang, J. X., Gill, B. S. Asphaltene Precipitation and Solvent Properties of Crude Oils. Pet. Sci. Technol. 16 (3-4), 251-285 (1998).
- Perrotta, A., McCullough, J. P., Beuther, H. Pressure-Temperature Microscopy of Petroleum-Derived Hydrocarbons. Prepr. Pap. Am. Chem. Soc., Div. Pet. Chem. 28 (3), 633-639 (1983).

