Building Double-decker Traps for Early Detection of Emerald Ash Borer
Summary
Effective traps to attract and capture the emerald ash borer (EAB) are a key element of detecting and managing this invasive pest. Double-decker traps, placed in full sun near ash trees, incorporate visual and olfactory cues and were more likely to capture EAB than other trap designs in field trials.
Abstract
Emerald ash borer (EAB) (Agrilus planipennis Fairmaire), the most destructive forest insect to have invaded North America, has killed hundreds of millions of forest and landscape ash (Fraxinus spp.) trees. Several artificial trap designs to attract and capture EAB beetles have been developed to detect, delineate, and monitor infestations. Double-decker (DD) traps consist of two corrugated plastic prisms, one green and one purple, attached to a 3 m tall polyvinyl chloride (PVC) pipe supported by a t-post. The green prism at the top of the PVC pipe is baited with cis-3-hexenol, a compound produced by ash foliage. Surfaces of both prisms are coated with sticky insect glue to capture adult EAB beetles. Double-decker traps should be placed near ash trees but in open areas, exposed to sun. Double-decker trap construction and placement are presented here, along with a summary of field experiments demonstrating the efficacy of DD traps in capturing EAB beetles. In a recent study in sites with relatively low EAB densities, double-decker traps captured significantly more EAB than green or purple prism traps or green funnel traps, all of which are designed to be suspended from a branch in the canopy of ash trees. A greater percentage of double decker traps were positive, i.e., captured at least one EAB, than the prism traps or funnel traps that were hung in ash tree canopies.
Introduction
The emerald ash borer (EAB) (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) has killed hundreds of millions of ash (Fraxinus spp.) trees since it was first identified in 2002 in the greater Detroit, Michigan metropolitan area and in nearby Windsor, Ontario, Canada. Inadvertent spread of infested ash trees, logs and firewood, along with natural dispersal of beetles, have resulted in EAB establishment in at least 27 states, and two Canadian provinces to date1. Recent reports indicate the EAB has also invaded Moscow, Russia, where it is killing landscape ash trees2,3, generating additional concern about its potential spread into Europe. Interspecific variation in EAB host preference and resistance among North American ash species has been documented4,5,6,7,8,9, but virtually all ash species in North America are likely to be suitable hosts. Catastrophic levels of ash mortality have been recorded in areas of Michigan and Ohio10,11,12, with associated ecological and economic impacts13,14,15,16.
Effective methods to detect new EAB infestations and monitor low density populations are critical aspects of managing this invasive pest in urban, residential and forested settings. Early detection provides time to develop a strategy, secure funding and implement activities to reduce impacts of the EAB. For example, municipal officials and homeowners can begin treating valuable ash in landscapes with systemic insecticides before damage caused by increasing larval densities limits efficacy of these products17,18. Similarly, identification of a new infestation and reliable information about local EAB distribution gives foresters and property owners the opportunity to implement timber sales, species conversion or other activities to reduce economic costs or ecological effects of ash mortality.
Early detection, delimitation, and effective monitoring of low-density EAB populations, however, remain difficult. Visual surveys to identify newly infested ash trees are not reliable because ash rarely exhibit external signs or symptoms of EAB infestation until larval densities build to moderate or even high levels4,19. The most effective means of detecting very low densities of EAB involve using girdled ash detection trees19,20,21,22. Ash trees are girdled in spring or early summer by removing a band of outer bark and phloem around the circumference of the trunk, which stresses trees, increasing their attraction to adult EAB beetles. Girdled trees can be debarked in autumn or winter to identify EAB larval presence and density. Although girdled ash trees have been used operationally for EAB detection19,23,24,25, they are problematic. Debarking girdled trees can be labor intensive and locating suitable trees for girdling can be difficult, especially in urban or residential areas or when surveys must be conducted for multiple years19.
Artificial traps baited with EAB attractants eliminate many concerns associated with using girdled ash detection trees. In contrast to other important forest pests such as gypsy moth (Lymantria dispar L) and some Scolytinae bark beetles that produce long distance sex or aggregation pheromones, to date, no effective long-distance pheromones have been found for EAB. A short range sex pheromone, cis-lactone, may facilitate mating26,27, but in field trials, cis-lactone lures have not consistently increased EAB attraction to artificial traps28. Adult beetles rely on volatile compounds emitted by ash leaves, bark and wood to identify their host trees29,30,31 and encounter potential mates. Several volatile compounds have been evaluated for use in lures to attract adult EABs to artificial traps27,32. Currently, traps used operationally for EAB detection surveys in the U.S. are baited with lures containing cis-3-hexenol, a common green leaf volatile produced by ash foliage30,33. In previous years, EAB traps used for U.S. surveys have also been baited with Manuka oil, which is extracted from the New Zealand tea tree (Leptospermum scoparium Forst and Forst ) or Phoebe oil, an extract of the Brazilian walnut tree (Phoebe porosa Mez.); both contain several sesquiterpenes that are also present in ash bark29. Problems with inconsistent supplies of these natural oils, however, have restricted their use.
In addition to host-produced volatiles, adult EAB beetles respond to visual stimuli, including color and light20,32,34,35. Early studies showed EAB adults, which are relatively agile fliers, were rarely captured by black funnel traps baited with various ash volatiles (DGM and TMP, unpublished data). Other trap designs, such as cross-vane traps, were evaluated but the aversion of EAB beetles to dark spaces and shadows limited the effectiveness of these traps.
Development of a three-sided prism, which can be coated with clear insect trapping glue35 to capture beetles, was a substantial improvement in trap design. Attraction of adult EABs to color has also been extensively evaluated in field trials and in laboratory studies with a retinograph34. Results show EAB beetles are consistently attracted to specific shades of green and purple32,36. Prism traps fabricated from colored corrugated plastic are now widely used in EAB survey activities in the U.S. and Canada.
Because EAB adults are strongly attracted to light, beetles are much more likely to colonize open-grown trees than shaded trees20,21. Guidelines for EAB detection surveys in the U.S. required individual prism traps to be suspended from a mid-canopy branch in an ash tree growing along a road or the edge of a wooded area37. In theory, this should ensure that at least one panel of the prism is exposed to sunlight. Operationally, however, prism traps may be partially shadowed by overhead branches or by adjacent or nearby trees. Sticky panel surfaces are frequently blown into foliage, resulting in leaves adhering to and obscuring at least a portion of one or more panels.
Double-decker (DD) traps were developed to integrate multiple visual and olfactory cues to enhance attraction of EAB beetles. Each DD trap is comprised of one green and one purple corrugated plastic prism attached to a 3 m tall schedule 40 polyvinyl chloride (PVC) pipe (10 cm diameter), which is supported by sliding the PVC pipe over a t-post. Using both green and purple prisms is designed to attract both sexes of EAB beetles32,36,38,39. Additionally, rather than being suspended from a branch in the canopy of an ash tree, the DD traps are placed in full sun, 5-10 m away from ash trees along the edge of a wooded area or in the midst of scattered, open-grown ash trees.
Protocol
1. Prepare the Green and Purple Panels
- Acquire green and purple corrugated plastic panels (120 cm × 60 cm) for EAB trapping from a commercial distributor of pest management supplies. Use a box-cutter or utility knife to score two fold lines in each panel by partially cutting through the plastic along the vertical corrugations, 40 cm from the two short edges of the panel. This allows the panel to be folded into a 3-sided prism (each face will be 40 x 60 cm). Panels are usually scored by the distributor to facilitate folding, so this step may not be necessary.
- Drill two pairs of holes into each panel using a ½ inch (1.3 cm) bit as indicated in Figure 1. Drill holes 4 inches (10 cm) from the top of the panel and 4 inches on either side of the left fold. Drill the second pair of holes 19 inches (48 cm) from the top of the panel and directly below the top holes (i.e., 4 inches on either side of the left fold) .
2. Prepare the PVC Pipe
- Obtain one section of schedule 40 polyvinyl chloride (PVC) pipe, 3 m long and 10 cm in diameter, from a commercial hardware store or other supplier for each trap. Drill four holes into the PVC pipe, as shown in Figure 2. Using a ½ inch (1.3 cm) drill bit, drill a pair of holes 4 inches (10 cm) from the top of the PVC pipe and 3½ inches (8.9 cm) apart. Align the holes horizontally.
- Drill a second pair of holes, 3½ inches apart, and 52 inches (1.3 m) below the top of the pipe, directly below the first set of holes .
3. Install a T-post
- Use a post pounder to install a t-post in the desired location. Drive the post deep enough so that the flange is just below the soil surface, but there is no need to go any deeper. The t-post will provide plenty of support for the DD trap, even in high winds. Guy wires or additional supports are not necessary. Although the t-post does not need to be set deeply into the soil, it is a good idea to check for underground utilities or buried pipes before driving the post into the ground.
4. Assemble the DD Trap
- Fold a purple panel into a prism. If panels are pre-glued, be sure the sticky side is on the outside. Feed the two small flaps through the slits and use two 8 inch (20 cm) cable ties to fasten the panel ends together. Repeat with a green panel.
- Support the PVC pipe horizontally above the ground. Slide the purple prism onto the PVC pipe and down to the lower set of holes in the PVC pipe.
- Feed a large, 2 foot (60 cm) long cable tie horizontally through the top left hole in the prism, then through the pair of holes in the PVC pipe, and finally through the adjacent hole on the right side of the prism. Be sure the prism is snug against the solid portion of PVC pipe that lies between the two holes. Close and tighten the cable tie, making sure the cable tie is not twisted.
- Feed a second large cable tie through the bottom hole in the prism, around the pipe and through the corresponding hole on the adjacent side of the prism. Close the cable tie and tighten, again taking care to ensure the cable tie does not twist before it is tightened.
- Slide the green prism onto the PVC pipe and attach it to the upper set of holes in the PVC pipe with two 60-cm cable ties, following the same steps as before. There will be a 21 inch (54 cm) gap between the upper and lower prisms.
5. Apply Insect Glue and Set Up the Trap
- Apply clear insect trapping glue to panels that are not pre-glued. Even pre-glued panels often require additional glue to capture EAB beetles. To apply insect trapping glue, set the base of the PVC pipe on the ground and prop the inner face of the upper prism on the t-post.
- Using latex (or similar) gloves, scoop up two to three handfuls of glue and smear it across the surface of a panel, making sure the surface of the panel is thoroughly coated. Rotate the PVC pipe to access all three surfaces on both prisms. Glue does not need to be applied to the PVC pipe.
- Pick up the PVC pipe, hold it vertically and slide the lower end over the t post. Avoid touching the sticky panels.
6. Bait the DD Trap
- As pre-glued prisms usually arrive with a small hole (4 mm) near the bottom edge of one panel of the prism, use a 4 inch (10 cm) cable tie to attach one pouch of cis-3-hexenol to the lower edge of the top (green) prism. If a hole is not present, use a handheld hole punch or a pocketknife to cut a small hole or slit into the plastic, then use the small cable tie to attach the lure.
NOTE: The cis-3-hexenol compound in the lures is slowly released over about a 10 week period. If traps need to remain effective for more than 10 weeks, replace the lures as needed.
7. Checking Traps for EAB Beetles
- Check traps at 2-4 week intervals if possible, to avoid losing captured beetles, which occasionally drop off the traps, especially if heavy rain occurs. Examine all three sides of the lower prism for EAB beetles. Then, using disposable latex gloves, lift the PVC pipe straight up and off the t-post and prop the inner face of the upper prism on the t-post, exposing one panel of both prisms.
- Use forceps to remove any suspect EAB beetles and place them in a re-sealable plastic specimen bag.
- Rotate the PVC pipe to examine all three panels on both prisms for suspect EAB beetles, then slide the pipe back over the t-post.
NOTE: Flies, click beetles and various other insects will inevitably be captured on the traps. It takes a keen eye to spot an EAB beetle. Carrying a photo reference card can be helpful when checking traps for the first time. - To confirm species identification, return suspect beetles to the lab and soak them in a non-toxic histological clearing agent for a day or two to remove the insect trapping glue. Beetles can then be examined under a dissecting microscope.
Representative Results
In a large scale study, three artificial trap designs as well as girdled ash trees were deployed systematically, 10-20 m apart, across a newly infested 16 ha forested area with a very low density of EAB25. Artificial trap designs tested included purple prisms baited with Manuka oil and suspended >3 m high from a branch in the canopy of ash trees, 3 m tall DD traps with two green panels, and DD traps with two purple panels supported by t-posts. Double-decker traps of both colors were baited with a blend of ash volatiles including cis-3-hexenol on the top panel and Manuka oil on the lower panel. Traps were checked to collect captured EAB throughout the summer and girdled trees were debarked in fall. A Χ2 goodness-of fit (GOF) test with Bonferroni correction for pairwise comparisons was used to test whether total EAB captures differed among the four trap types. Purple double-decker traps captured more EAB beetles than the purple canopy traps, green double-decker traps, or sticky bands on girdled trees. Green double-decker traps captured more EAB beetles than purple canopy traps. One or more EAB beetles were captured on 25% of the purple canopy traps, 56% of the green double-decker traps, and 81% of the purple double-decker traps, while all of the girdled trees were colonized25.
More recently, EAB captures and detection success of different trap designs were monitored in 2015 on eight blocks of traps established in two forested sites with low to moderate densities of EAB (Figure 3). Each block consisted of six traps, spaced 10-20 m apart. Traps evaluated included a purple prism, a dark green prism, a light green prism, and a green funnel trap, each suspended from a branch >5 m high in ash trees growing on the edge of wooded areas. Each block also included a DD trap with two purple prisms and a DD trap with a green prism on top and a purple prism on the bottom. As before, the DD traps were 3 m tall and supported on t-posts set in the ground. All traps were baited with cis-3-hexenol and the light and dark green prism traps also included cis-lactone lures. The mean number of EAB captured per trap was analyzed among treatments by two-way ANOVA with main effects for replicate and treatment followed by the Tukey-Kramer means separation test. Every DD trap captured one or more EAB beetles, while 62% of the green funnel traps and 37 to 75% of the prism traps in the canopies of ash trees captured at least one EAB. Both color combinations of DD traps captured significantly more male and female EABs than any of the trap designs hung in the ash canopies (Figure 3). While there was no significant difference between traps with two purple panels and traps with green and purple panels in this study, other experiments have found that green and purple DD traps captured more EAB than traps with two purple panels28 and that when placed at higher locations, green prism traps captured more EAB than purple prism traps34. Therefore, inclusion of green panels in the upper position on double decker traps may improve attraction to EAB.
Number of EAB beetles captured per prism was also evaluated, given that DD traps have twice as much surface area (14,400 cm2) as the individual prism canopy traps (7,200 cm2) and 30% more area than the combined surfaces of all 12 funnels of a funnel trap (11,160 cm2). The visual two-dimensional silhouette area of a prism trap is equivalent to one panel of the 3-sided prism (2,400 cm2) and the visual silhouette area of a DD trap is twice that (4,800 cm2) of a prism trap and 20% greater than the two-dimensional visual silhouette area of a funnel trap (2000 cm2) (equivalent to the diameter of a funnel (20 cm) times the total height of 12 partially nested funnels [100 cm]) when the trap is hung and expanded. Even when individual prisms were considered, however, the DD traps captured more EAB beetles than the other trap designs. Overall, individual prisms on the DD traps at the two sites captured 49 to 111 adult EABs (totals of 146 and 214 EAB per DD trap), while individual prism traps and funnel traps in ash canopies captured a total of 11 to 28 EAB beetles, respectively.
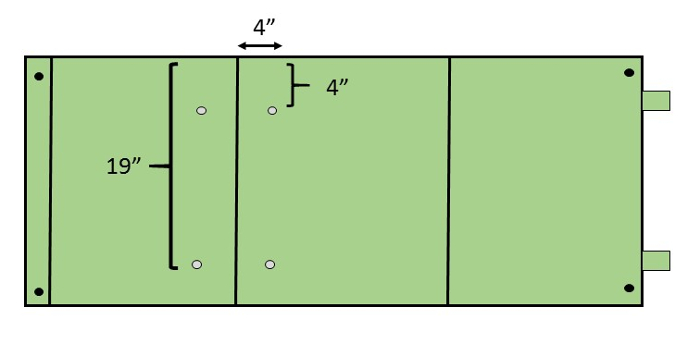
Figure 1: Preparing panels. Two corrugated plastic panels (120 cm x 60 cm), typically green and purple, are used for each double-decker trap to capture EAB beetles. Each panel will be folded into thirds to create a prism. Each prism face will be 40 cm x 60 cm. Please click here to view a larger version of this figure.
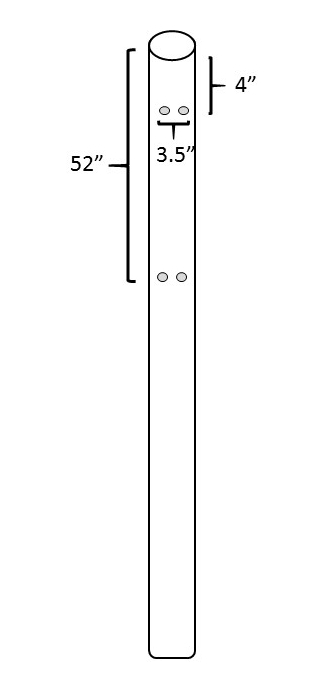
Figure 2: Preparing the PVC pipe. A polyvinyl chloride (PVC) pipe (schedule 40) that is 3 m tall and 10 cm in diameter will be used to support the two prism traps for capturing EAB beetles. A total of four holes will need to be drilled into each PVC pipe. Cable ties to attach the prisms to the PVC pipe will pass through these holes.
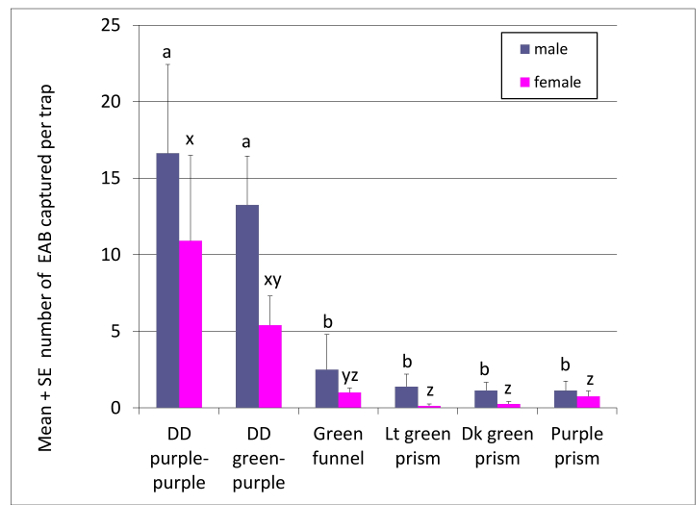
Figure 3: Captures of EAB beetles on DD and other traps. Mean (+ SE) number of male and female EAB beetles captured on green funnel traps or light purple prism traps baited with cis-3-hexenol and hung in ash canopies, light green prism traps or dark green prism traps baited with cis-3-hexenol and cis-lactone and hung in ash canopies, or double-decker traps (3 m tall) with two purple prisms, or with a green prism on top and a lower purple prism, both baited with cis-3-hexenol. Bars topped by different letters within the same sex are significantly different (P <0.05). N = 8 replicates at two northern Michigan sites in 2015. Please click here to view a larger version of this figure.
Discussion
Both the design and placement of DD traps exploit the attraction of adult EAB beetles to specific shades of color and to light. The green prism on the top of the PVC pipe is most attractive to male beetles, who spend their life span feeding on ash leaves, as well as mating32,36,38,39. The lower purple prism ensures traps are also attractive to female beetles32. Like males, female beetles feed on ash foliage throughout their life span, but mature females spend large amounts of time on the bark of branches or the trunk where they lay eggs39. While detecting new infestations only requires capturing a beetle of either sex, the ability to attract and capture female EABs may be particularly important. Mature EAB females are physiologically capable of flying further than males40 and population models suggest long-distance dispersal of mated females contributes substantially to the expansion of new EAB infestations41,42,43.
Placing DD traps in the open serves multiple purposes. Once assembled, DD traps are highly apparent and the silhouette of the tall, vertical trap resembles that of a small ash tree. Using two prisms enhances the silhouette of the trap, but perhaps more importantly, doubles the surface area available for trapping beetles. The green and purple prisms on the stand-alone DD traps are unlikely to be obscured by foliage from trees or vegetation. In contrast to baited prism traps hung from the branch of an ash tree, lures on the DD traps do not have to compete with compounds emitted by live ash surrounding the trap. Instead, lures on the DD traps provide the beetles with a distinct point source of ash-related volatiles they can readily identify and locate. Moreover, setting the DD trap in the open exploits the strong and consistent preference for sunny conditions exhibited by EAB beetles.
Finally, the DD traps are simple to install, easy to check and withstand strong winds and summer thunderstorms. The traps are not only highly apparent to EAB beetles; they are also easy for survey personnel to locate for mid-season checks or recovery. Vandalism has never been problem in any of the dozens of sites where DD traps have been monitored over the past ten years. The unappealing sticky panels likely serve to quell the enthusiasm of would-be vandals.
The DD traps can also serve as outreach tools to increase public awareness of EAB. Displaying a sign can be helpful. Simple and inexpensive signs with a photo of a DD trap and an explanation of the purpose of the trap can be printed with a desktop color printer, laminated, glued to a wood or fiberboard backing, then attached to a t-post in areas where people are likely to encounter traps at the site. Explanatory signs and the obvious effort underway to detect EAB typically generate goodwill as well as functioning as an educational tool.
Obviously, DD traps with two panels, a t-post and PVC pipe cost at least twice as much as suspending a single prism trap in the canopy of ash trees. The PVC pipe and t-post, however, can be re-used for many years. Additionally, much of the cost of detection surveys for invasive forest pests such as EAB reflects the time needed for survey crews to locate a suitable site, install the trap, check the traps periodically, and to sort and identify trap catches. Costs of false negatives, e.g., failure to detect a low density infestation of EAB, must also be considered if a less effective trap is used. Results from studies encompassing more than 30 different sites have shown that in recently infested areas with low density EAB populations, DD traps were consistently more likely to capture EAB beetles than prism traps hanging in the canopies of ash trees22,31,44.
Distinct advantages and disadvantages are associated with each EAB trap design evaluated in previous field trials. Funnel traps are initially more expensive than the other trap designs, but can be re-used for multiple years. Prism traps are the least expensive design, while DD traps are intermediate in cost. The most expensive components of DD traps (t-posts and PVC pipe) are readily available in hardware and home improvement stores and can be re-used for multiple years. Non-toxic, non-ethanol antifreeze or insecticidal strips are needed to capture insects attracted to funnel traps. Prisms, whether suspended in ash canopies or on DD traps, must be coated with sticky insect glue to capture insects and cannot be reused. All insects captured by funnel traps must be collected and returned to the laboratory for sorting and identification. Panels on prism and DD traps must be examined and suspect beetles removed with forceps for subsequent species confirmation in the laboratory. Some expertise is useful for distinguishing EABs (or at least buprestid beetles) in the field, but training surveyors is not difficult. Hanging funnel traps and prism traps on branches in ash tree canopies can require considerable time, especially if trees are large. Crews often need long poles or large slingshots to access branches and braided line or cord is needed to suspend the traps. Double-decker traps, which are placed in the open near ash trees, can be easily set up anywhere. High winds have occasionally blown prisms out of trees or caused lines bearing funnel or prism traps to wrap around upper branches, preventing access to the traps from the ground. In contrast, DD traps have remained intact during severe storms, including storms with straight line winds22,44.
It is important to note that EAB detection and survey methods are not mutually exclusive. Different detection methods can be integrated into survey strategies appropriate for specific situations or local conditions. For example, low value or declining ash trees along fence lines, roads or in forested settings can be girdled and debarked, to function as detection tools. Less expensive prism traps or reusable funnel traps can be distributed more broadly in large scale systematic surveys. Free-standing DD traps may be especially appropriate for high risk areas such as campgrounds or recreation areas where potentially infested ash firewood presents an ongoing threat. Surveyors can elect to place DD traps in a variety of locations or settings, as long as the traps are near ash trees and in the open, so the prisms are exposed to sun. Double-decker traps have been successfully used in highway medians and along railroads, in powerlines running through forested areas, around the perimeter of sawmills and waste wood disposal yards, and along rivers, drainage ditches and other riparian areas. Although the DD traps are baited with an ash volatile (cis-3-hexenol) and were designed for EAB surveys, native buprestid species are also frequently captured on the sticky panels. Further studies to assess potential modifications to DD traps or lures that might increase attraction of other buprestid species could be useful.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Several technicians and graduate students at Michigan State University have helped to develop, refine and evaluate the DD trap design over the years, including Andrea Anulewicz, Robert McDonald and Nathan Siegert. We thank James Wieferich and Jeremy Lowell (MSU) for their assistance in developing the instructions for DD installation. James Wieferich and Molly Robinett (MSU) reviewed an earlier draft of this manuscript and we appreciate their suggestions. Joseph Francese and Damon Crook (USDA APHIS) generously shared their observations on EAB response to color and host volatiles. Funding for DD trap development and evaluation was provided by grants from the USDA Forest Service, Northeastern Area, Forest Health Protection.
Materials
| Light green corrugated plastic panel: 120 cm x 60 cm | Great Lakes IPM; www.greatlakesipm.com | IPM-EAB GR | All three surfaces of each prism need to be covered with clear insect trapping glue, even if the panels are pre-glued. Pre-glued panels are often not sticky enough to consistently capture or retain EAB beetles. Other clear insect trapping glue products are available but are considerably more difficult to apply. | ||||||||||||
| Light purple corrugated plastic panel: 120 cm x 60 cm | Great Lakes IPM; www.greatlakesipm.com | IPM-EAB LP | |||||||||||||
| Large cable tie (4): 60 cm with a 79 kg capacity | Cabletiesandmore.com; http://www.cabletiesandmore.com/cableties.php | CT-24-NU-100PK | |||||||||||||
| Medium cable ties (4): 20 cm with a 22.7 kg capacity | Cabletiesandmore.com; http://www.cabletiesandmore.com/cableties.php | CT261 | |||||||||||||
| Small cable tie: 10 cm with a 8.2 kg capacity | Cabletiesandmore.com; http://www.cabletiesandmore.com/cableties.php | CT204 | |||||||||||||
| cis-3-hexanol pouch | Synergy Semiochemicals; http://www.semiochemical.com/html/buprestids.html) | 3136 | Lures used to bait DD traps consist of pouches containing cis-3-hexenol, a non-toxic compound present in ash leaves. One pouch is attached to the lower edge of the top prism using a small cable tie. Each pouch of cis-3-hexenol has a release rate of approximately 50 mg/day. Note that cis-3-hexenol is sometimes written as Z-3-hexenol. | ||||||||||||
| Aphinity Hexenol | Sylvar Technologies | ||||||||||||||
| Lure GLV4 emerald ash borer | Chemtica, Heredia, Costa Rica | ||||||||||||||
| cis-3-hexanol pouch | WestGreen Global Technologies; http://www.westgreenglobaltechnologies.com/ | ||||||||||||||
| Clear insect trapping glue | Hummert International; http://www.hummert.com/product-details/8196/pestick | 01-3522-1 | |||||||||||||
| Histoclear II histological clearing agent | National Diagnostics; www.nationaldiagnostics.com | HS-202 | Histoclear II will be needed to remove the sticky insect glue from suspect beetles. Other histological clearing agents are available but may not remove the glue and some products dissolve plastic, an important consideration if plastic containers are used for soaking the beetles. | ||||||||||||
| Histoclear II histological clearing agent | Great Lakes IPM; www.greatlakesipm.com | 10011 | Histoclear II will be needed to remove the sticky insect glue from suspect beetles. Other histological clearing agents are available but may not remove the glue and some products dissolve plastic, an important consideration if plastic containers are used for soaking the beetles. | ||||||||||||
| t-post: 1.5 m | multiple sources | A t-post (5 feet tall) (1.5 m) is used to support the PVC pipe. | |||||||||||||
| post pounder | multiple sources | Use a post pounder to set t-posts into the ground. No additional support is necessary. | |||||||||||||
| HDPE (high density polyethylene) PVC pipe : 3 m x 10 cm diameter | multiple sources | ||||||||||||||
| Forceps (rigid) | multiple sources | Forceps (tweezers) will be needed to remove suspect beetles from the traps. Rigid forceps work better than flexible forceps. | |||||||||||||
| Latex gloves | multiple sources | Latex gloves are needed for applying the insect trapping glue to the prisms and for checking the traps to collect EAB beetles. | |||||||||||||
| Baby oil or baby wipes | multiple sources | Baby oil or baby wipes are helpful for removing the trapping glue from hands and equipment. | |||||||||||||
| Re-sealable plastic specimen bags: 5 cm x 8 cm | multiple sources | Small re-sealable plastic specimen bags are useful for collecting beetles from traps. Each bag should be labelled, either with pre-made, adhesive labels or with soft felt pens. | |||||||||||||
| Guides to help with distinguishing EAB from beetles native to North America are available on the national EAB website at www.emeraldashborer.info. | |||||||||||||||
References
- Baranchikov, Y., Mozolevskaya, E., Yurchenko, G., Kenis, M. Occurrence of the emerald ash borer, Agrilus planipennis in Russia and its potential impact on European forestry. OEPP/EPPO Bulletin. 38, 233-238 (2008).
- Orlova-Bienkowskaja, M. J. Ashes in Europe are in danger: the invasive range of Agrilus planipennis in European Russia is expanding. Biol. Invasions. 16, 1345-1349 (2014).
- Anulewicz, A. C., McCullough, D. G., Cappaert, D. L. Emerald ash borer (Agrilus planipennis) density and canopy dieback in three North American ash species. Arbor. Urban For. 33, 338-349 (2007).
- Chen, Y., Poland, T. M. Nutritional and defensive chemistry of three North American ash species: possible roles in host performance and preference by emerald ash borer adults. Grt. Lakes Entomol. 43, 20-33 (2010).
- Pureswaran, D. S., Poland, T. M. Host selection and feeding preference of Agrilus planipennis (Coleoptera: Buprestidae) on ash (Fraxinus spp). Environ. Entomol. 38, 757-765 (2009).
- Rebek, E. J., Herms, D. A., Smitley, D. R. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp). Environ. Entomol. 37, 242-246 (2008).
- Tanis, S. R., McCullough, D. G. Differential persistence of blue ash and white ash following emerald ash borer invasion. Can. J. For. Res. 42, 1542-1550 (2012).
- Tanis, S. R., McCullough, D. G., G, D. Host resistance of five Fraxinus species to Agrilus planipennis (Coleoptera: Buprestidae) and effects of paclobutrazol and fertilization. Environ. Entomol. 44, 287-299 (2015).
- Burr, S. J., McCullough, D. G. Condition of green ash (Fraxinus pennsylvanica) overstory and regeneration at three stages of the emerald ash borer invasion wave. Can. J. For. Res. 44, 768-776 (2014).
- Knight, K. S., Brown, J. P., Long, R. P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol. Invasions. 15, 371-383 (2013).
- Klooster, W. S. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions. 16, 859-873 (2014).
- Flower, C. E. Native bark-foraging birds preferentially forage in infected ash (Fraxinus spp.) and prove effective predators of the invasive emerald ash borer (Agrilus planipennis Fairmaire). For. Ecol. Manage. 313, 300-306 (2014).
- Gandhi, K. J. K., Herms, D. A. North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biol. Invasions. 12, 1839-1846 (2010).
- Kovacs, K. F. Cost of potential emerald ash borer damage in U.S. communities, 2009-2019. Ecol. Econ. 69, 569-578 (2010).
- Kovacs, K. The influence of satellite populations of emerald ash borer on projected economic damage in U.S. communities, 2010-2020. Environ. Manage. 92, 2170-2181 (2011).
- Herms, D. A., McCullough, D. G. Emerald ash borer invasion of North America: history, biology, ecology, impact and management. Ann. Rev. Entomol. 59, 13-30 (2014).
- Herms, D. A., McCullough, D. G., Smitley, D. R., Sadof, C. S., Cranshaw, W. . Insecticide options for protecting ash trees from emerald ash borer. , 16 (2014).
- Mercader, R. J., McCullough, D. G., Bedford, J. M. A comparison of girdled ash detection trees and baited artificial traps for Agrilus planipennis (Coleoptera: Buprestidae) detection. Environ. Entomol. 42, 1027-1039 (2013).
- McCullough, D. G., Poland, T. M., Anulewicz, A. C., Emerald Cappaert, D. Emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) attraction to stressed or baited ash (Fraxinus spp.) trees. Environ. Entomol. 38, 1668-1679 (2009).
- McCullough, D. G., Poland, T. M., Cappaert, D., Anulewicz, A. C. Emerald ash borer (Agrilus planipennis) attraction to ash trees stressed by girdling, herbicide and wounding. Can. J. For. Res. 39, 1331-1345 (2009).
- McCullough, D. G., Siegert, N. W., Poland, T. M., Pierce, S. J., Ahn, S. Z. Effects of trap type, placement and ash distribution on emerald ash borer captures in a low density site. Environ. Entomol. 40, 1239-1252 (2011).
- Hunt, L., Mastro, V., Lance, D., Reardon, R., Parra, G. Emerald ash borer state update: Ohio. , (2007).
- Mercader, R. J. Estimating local spread of recently established emerald ash borer, Agrilus planipennis, infestations and the potential to influence it with a systemic insecticide and girdled ash trees. For. Ecol. Manage. , (2016).
- Rauscher, K., Mastro, V., Reardon, R., Parra, G. The 2005 Michigan emerald ash borer response: an update. , (2005).
- Ryall, K. Detection and sampling of emerald ash borer (Coleoptera: Buprestidae) infestations. Can. Entomol. 147, 290-299 (2015).
- Silk, P. J., Ryall, K. Semiochemistry and chemical ecology of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Can. Entomol. 147, 277-289 (2015).
- Poland, T. M. Recent development and advances in survey and detection tools for emerald ash borer. , (2016).
- Crook, D. A. Development of a host-based semiochemical lure for trapping emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 37, 356-365 (2008).
- de Groot, P. Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. J. Chem. Ecol. 34, 1170-1179 (2008).
- Poland, T. M., McCullough, D. G. Comparison of trap types and colors for capturing emerald ash borer adults at different population densities. Environ. Entomol. 43, 157-170 (2014).
- Crook, D. J., Mastro, V. C. Chemical ecology of the emerald ash borer Agrilus planipennis. J. Chem. Ecol. 36, 101-112 (2010).
- Rodriguez-Saona, C. Behavioral and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian ash, Fraxinus mandshurica. Chemoecology. 16, 75-86 (2006).
- Crook, D. J. Laboratory and field response of the emerald ash borer (Coleoptera: Buprestidae) to selected regions of the electromagnetic spectrum. J. Econ. Entomol. 102, 2160-2169 (2009).
- Francese, J. A. Optimization of trap color for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). J. Econ. Entomol. 103, 1235-1241 (2010).
- Crook, D. J., Khrimian, A., Cossé, A., Fraser, I., Mastro, V. C. Influence of trap color and host volatiles on capture of the emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 105, 429-437 (2012).
- . Emerald Ash Borer Survey Guidelines Available from: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/survey_guidelines.pdf (2013)
- Grant, G. G., Poland, T. M., Ciaramitaro, T., Lyons, D. B., Jones, G. C. Comparison of male and female emerald ash borer (Coleoptera: Buprestidae) responses to phoebe oil and (Z)-3-hexenol lures in light green prism traps. J. Econ. Entomol. 104, 173-179 (2011).
- Cappaert, D., McCullough, D. G., Poland, T. M., Siegert, N. W. Emerald ash borer in North America: a research and regulatory challenge. Am. Entomol. 51, 152-165 (2005).
- Taylor, R. A. J., Bauer, L. S., Poland, T. M., Windell, K. Flight performance of Agrilus planipennis (Coleoptera: Buprestidae) on a flight mill and in free flight. J. Insect Behav. 23, 128-148 (2010).
- Mercader, R. J. Evaluation of the potential use of a systemic insecticide and girdled trees in area wide management of the emerald ash borer. For. Ecol. Manage. 350, 70-80 (2015).
- Mercader, R. J., Siegert, N. W., Liebhold, A. M., McCullough, D. G. Simulating the effectiveness of three potential management options to slow the spread of emerald ash borer, (Agrilus planipennis) populations in localized outlier sites. Can. J. For. Res. 41, 254-264 (2011).
- Mercader, R. J., Siegert, N. W., Liebhold, A. M., McCullough, D. G. Influence of foraging behavior and host spatial distribution on the localized spread of the emerald ash borer, Agrilus planipennis. Pop. Ecol. 53, 271-285 (2011).
- Poland, T. M., McCullough, D. G., Anulewicz, A. C. Evaluation of an artificial trap for Agrilus planipennis (Coleoptera: Buprestidae) incorporating olfactory and visual cues. J. Econ. Entomol. 104, 517-531 (2011).

