Treatment of Ligament Constructs with Exercise-conditioned Serum: A Translational Tissue Engineering Model
Summary
We present a model of ligament tissue in which three-dimensional constructs are treated with the human exercise-conditioned serum and analyzed for collagen content, function, and cellular biochemistry.
Abstract
In vitro experiments are essential to understand biological mechanisms; however, the gap between monolayer tissue culture and human physiology is large, and translation of findings is often poor. Thus, there is ample opportunity for alternative experimental approaches. Here we present an approach in which human cells are isolated from human anterior cruciate ligament tissue remnants, expanded in culture, and used to form engineered ligaments. Exercise alters the biochemical milieu in the blood such that the function of many tissues, organs and bodily processes are improved. In this experiment, ligament construct culture media was supplemented with experimental human serum that has been 'conditioned' by exercise. Thus the intervention is more biologically relevant since an experimental tissue is exposed to the full endogenous biochemical milieu, including binding proteins and adjunct compounds that may be altered in tandem with the activity of an unknown agent of interest. After treatment, engineered ligaments can be analyzed for mechanical function, collagen content, morphology, and cellular biochemistry. Overall, there are four major advantages versus traditional monolayer culture and animal models, of the physiological model of ligament tissue that is presented here. First, ligament constructs are three-dimensional, allowing for mechanical properties (i.e., function) such as ultimate tensile stress, maximal tensile load, and modulus, to be quantified. Second, the enthesis, the interface between boney and sinew elements, can be examined in detail and within functional context. Third, preparing media with post-exercise serum allows for the effects of the exercise-induced biochemical milieu, which is responsible for the wide range of health benefits of exercise, to be investigated in an unbiased manner. Finally, this experimental model advances scientific research in a humane and ethical manner by replacing the use of animals, a core mandate of the National Institutes of Health, the Center for Disease Control, and the Food and Drug Administration.
Introduction
Tendon and ligament injuries are common and can have debilitating consequences on normal mobility and quality of life. Surgical intervention is often necessary but can have limited and varied success4,5. The current understanding of how tendons and ligaments develop, mature, and respond to injury is incomplete, and thus effective research models are needed to provide insight into the development of more effective treatments5. To address this knowledge gap, animal models may be used but in vivo studies are inherently complex with difficulty in controlling the environment and directly targeting interventions to the intended tissue. In contrast, the experimental environment can easily be controlled and monitored in vitro with traditional monolayer cell culture. However, this technique may oversimplify the chemical and mechanical environment and thus may not recapitulate the in vivo behavior of the cells. Tissue engineering is able to marry the advantages of the complex in vivo environment in animal models with the control of the in vitro environment and provides an additional tool to study physiology. In addition, armed with a better understanding of ligament development, tissue engineering may also provide a source of graft tissue when surgical reconstruction is required6. Thus, the method described herein validates an in vitro 3D engineered tissue that can be used to study ligament function and morphology.
Fibrin-based tendon or ligament constructs have been used as an in vitro model to study physiological processes including collagen fibrillogenesis7 and tendon development8, as well as translational applications in which their utility as graft tissue has been evaluated in a sheep model of anterior cruciate ligament (ACL) reconstruction9. Our lab has previously established a 3D engineered ligament model spanning two brushite, a calcium phosphate bone-substitute material, cement anchors. This model can be subjected to different experimental conditions with ease simply by supplementing the culture media with biological factors10 or applying mechanical stimulation11. Importantly, this bone-to-bone ligament model allows for the in-depth analysis of the enthesis, the interface between boney and sinew elements, which is susceptible to injury.
In the study highlighted1 here to present this methodology, we were interested in the effect of exercise-induced changes in the biochemical milieu on ligament function. Exercise improves cellular and organ function in a variety of tissues throughout the body2,3,12, an effect that may be attributed to the release of various known (e.g., IL-613, IL-1514, Meteorin-like15, exosomes16,17), and other unknown, biochemical factors released into systemic circulation. Furthermore, the post-exercise biochemical milieu is enriched with exercise-responsive hormones, the release of which is stimulated by sympathetic nervous system stimulation of secretory glands (e.g., cortisol and catecholamines from the adrenal gland18, and growth hormone from the anterior pituitary19). However, in vivo, it is impossible to differentiate the effects of the mechanical stimulus of exercise from exercise-induced biochemical changes. While some studies have characterized the expected rise of certain circulating hormones and cytokines in response to exercise as mentioned above, there are too many factors, both known and unknown, to faithfully recapitulate in vitro. That is, isolating a few factors for an in vitro study inadequately addresses the complexity of the biochemical response. In this study, we investigated how changes in the serum biochemical milieu, prompted by exercise, affects engineered ligament function. To isolate the effects of the biochemical changes, we obtained serum from human participants before and after a bout of resistance exercise and used it treat 3D engineered ligaments formed using human anterior cruciate ligament (ACL) fibroblasts. Using this model, we can obtain functional data, including effects on mechanical properties and collagen content, as well as quantify effects on molecular signaling.
Protocol
The following procedures adhere to a protocol that was approved by the Institutional Review Board of University of California, Davis; consult with the local ethics board prior to beginning research.
1. Isolate Primary Fibroblasts from Human ACL Remnants
NOTE: Maintain sterility and perform all steps in a biological safety cabinet (BSC).
- Obtain approval from the appropriate ethics review board for the collection and use of human tissues as described below.
- Prepare 5x antibiotic/antimycotic (ABAM) solution by diluting 100x antibiotic/antimycotic solution in 1X phosphate-buffered saline (PBS)
- Collect ACL tissue fragments in 5X ABAM solution in a 50 mL conical tube, store at 4 °C until the digestion step. Cut tissue into smaller fragments with a razor blade if necessary to a maximum size of 1 x 1 x 1 cm3.
CAUTION: Comply with local biohazard regulations for proper use of biohazard material and decontamination and disposal of biohazard waste. - Prepare a sufficient volume of collagenase solution to submerge the tissue fragments. Dissolve collagenase type II (1 mg/mL) in high glucose Dulbecco's modified Eagle medium (DMEM) containing 1x penicillin/streptomycin, and 20% fetal bovine serum (FBS) and filter at 0.22 µm.
- Rinse ACL tissue 3 times in PBS.
- Digest tissue. Transfer ACL tissue to a new 50 mL conical tube and add a sufficient volume of collagenase solution to submerge the tissue. Incubate at 37 °C overnight (~17 h).
- Before the digestion duration is complete, prepare growth media (GM) by supplementing DMEM high glucose media with 10% FBS and 100 U/mL penicillin.
- 15 min before the digestion time is complete, briefly vortex the tissue-containing 50 mL tube 3 times every 5 min.
- Using a 25 mL serological pipette, triturate the digested tissue vigorously to break the tissue up further and dislodge cells.
- Centrifuge at 1,500 x g for 5 min. Aspirate the supernatant and resuspend pellet in 10 mL GM.
- Repeat steps 1.8-1.9 three more times.
- After the last centrifugation, resuspend the pellet in 5-10 mL GM. Use a small sample of the cell suspension to perform a cell count with a hemocytometer and assess cell viability using Trypan Blue.
- Plate the cell suspension onto 15 cm tissue culture plates at a density of 3-4 x 105 cells per plate.
- Culture to 70% confluence in a sterile incubator maintained at 37 °C and 5% CO2, changing the GM every three days. Use or store (see below) cells within 5 passages.
- Freeze cells for future use.
- Trypsinize cells at 70% confluence as follows. Aspirate media and wash cells with PBS. Add enough pre-warmed (37 °C) 0.05% trypsin to just cover the bottom of tissue culture plates. Place plates in the culture incubator for ~5 min until cells are detached (verify cells are floating using a light microscope). Use a pipette to collect cells and dispense into a Falcon tube.
- Centrifuge to pellet cells, and resuspend cells in DMEM high glucose media containing 20% FBS and 10% dimethyl sulfoxide (DMSO). Aliquot cell suspension into cryovials and cool at -1 °C/min for at least 24 h. Store frozen cryovials in liquid nitrogen.
2. Prepare Silicone-coated Plates
- Prepare 35 mm tissue culture plates: Remove lids and lay out open plates on a flat surface.
- Mix silicone elastomer kit according to manufacturer's instructions.
- Use a 10 mL syringe to dispense approximately 2 mL per 35 mm plate.
- Allow silicone to cure at room temperature for 2-3 days.
3. Prepare Brushite Cement Anchors
- In advance, prepare silicone reverse-molds containing cylindrical wells for anchor formation. Reverse molds can be made to the specifications of the desired anchor shape and size.
- Determine the desired height and diameter of the final anchor. This protocol uses a custom-made mold formed from craft silicone in a 35 mm tissue culture dish in which plastic cylinders of about 3.25 mm in diameter were placed, allowing for a final mold height of about 6.5 mm. Final anchor dimensions are approximately 3-3.5 mm in height and about 3.4 mm in diameter with 1.5 mm pin protruding from the bottom of the anchor.
- Add uncured silicone to a 35 mm dish in which the mold will be made. Place plastic cylinders accordingly.
NOTE: The size of the plastic cylinders will determine the diameter of the final anchors. The placement of the plastic cylinders and the amount of silicone used can be modified to produce anchors of different heights. The thickness between the bottom of the wells in the mold and the bottom of the mold itself will determine how much of the pin can protrude from the bottom of the anchor allowing the anchor to later be pinned securely in the silicone-coated dish. - After allowing the silicone to cure, remove the plastic cylinders and remove the mold from the 35 mm dish.
- Prepare a 3.5 M orthophosphoric/100 mM citric acid solution. Dissolve 0.961 g citric acid in 11.5 mL orthophosphoric acid. Bring the volume of the solution up to 50 mL with MilliQ water. Store solution at room temperature and protect from light.
- Prepare molds: Place one minutien pin in the center of each cylindrical well in the molds.
- Combine β-tricalcium phosphate and orthophosphoric/citric acid solution at a 1 g/mL concentration in a plastic weigh boat on ice.
- Mix the cement vigorously using a plastic cell scraper.
- Triturate the cement to continue mixing and pipette mixture into the mold
- Centrifuge the filled mold for 1 min at 2,250 x g.
- Allow brushite cement anchors to set at room temperature overnight.
- Remove anchors from the mold and pin two anchors 12 mm apart in each silicone-coated plate.
- Sterilize pinned plates by spraying with 70% ethanol, filling both the plates and lids, and placing into a BSC. After at least 30 min, aspirate plates and replace lids, storing in the BSC until needed.
4. Obtain Human Serum
- Ensure that approval by the appropriate ethics review board has been obtained for this protocol.
- Ensure that written informed consent has been obtained from human subjects to participate in a given intervention (e.g., exercise, food or drug intervention) that will effect desired changes in serum. Here, we describe the collection at rest and 15 min after resistance exercise.
- Using a trained phlebotomist, obtain a resting blood sample from a participant by venipuncture into an appropriate evacuated container.
- Collect a post-exercise blood sample 15 min after having the participants engage in the desired exercise protocol. As previously described1, use the resistance exercise protocol in this experiment to stimulate an endogenous biochemical response.
- Have participants perform five sets of leg press with one-minute rest between sets. Next, have the participants perform a set of knee extensions and a set of hamstring curls consecutively with no rest and then repeat the back-to-back exercises three times with 1 min rest between sets.
- Allow blood to clot before centrifuging at 1,500 x g for 10 min. Under sterile conditions, transfer serum to sterile tubes for future media supplementation (serum stored at 4 °C) and biochemical analysis (a small aliquot of serum stored at -20 °C).
5. Form Engineered Ligaments
NOTE: In advance, expand primary fibroblasts and prepare silicone-coated plates with pinned brushite anchors.
- Prepare reagents:
- Prepare thrombin. Dissolve bovine thrombin at 200 U/mL in DMEM high glucose media. Filter at 0.22 µm, aliquot, and store at -20 °C.
- Prepare fibrinogen. Dissolve bovine fibrinogen at 20 mg/mL in DMEM high glucose media. Incubate for 3-4 h in a 37 °C water bath, swirling every 30 min to aid dissolution. Filter at 0.22 µm (multiple filters may be needed), aliquot, and store at -20 °C.
- Prepare aprotinin. Dissolve aprotinin in 10 mg/mL in water. Filter at 0.22 µm, aliquot, and store at -20 °C.
- Prepare aminohexanoic acid. Dissolve aminohexanoic acid at 0.1g/mL in water. Filter at 0.22 µm, aliquot, and store at 4 °C.
- Prepare ascorbic acid. Dissolve ascorbic acid in DMEM high glucose media at a concentration of 50 mM. Filter at 0.22 µm and store at 4 °C.
- Prepare L-proline. Dissolve L-proline in PBS at a concentration of 50 mM. Filter at 0.22 µm and store at 4 °C.
- Prepare transformation growth factor-β1 (TGF-β1). Reconstitute TGF-β1 according to the manufacturer's directions at a concentration of 10 µg/mL. Aliquot and store at -20 °C.
- Determine the number of constructs required for the experiment and ensure sufficient numbers of pinned plates are prepared. Both biological and technical replicates are recommended. In the study1 highlighted here, use duplicate technical replicates and 12 biological replicates (serum from 12 individuals at rest and after exercise).
NOTE: Perform the following steps under sterile conditions in a BSC. - Expand cells by culturing in 15 cm plates to 70% confluence. 2.5 x 105 cells are required per construct.
- Trypsinize cells and resuspend in GM at a concentration of 3.67 x 105 cells/mL.
- Generate a master mix for the number of constructs required: For 1 construct, the master mix contains 681 µL cell suspension (containing 2.5 x 105 cells), 29 µL thrombin, 2 µL aprotinin, and 2 µL aminohexanoic acid.
- After mixing the master mix well, add 714 µL to each pinned plate in a 'figure 8' pattern around the brushite cement anchors. Ensure that the master mix directly contacts the sides of the anchors.
- Gently tap each plate to distribute the master mix evenly across the plate.
- For one plate at a time, quickly add 286 µL fibrinogen in a dropwise fashion evenly over one plate and immediately slide the plate back and forth and side to side over the surface of the BSC to distribute the fibrinogen to form the cell-embedded fibrin gels. Proceed to the next plate.
- Place the constructs in a sterile incubator maintained at 37 °C and 5% CO2 and incubate for at least 15 min to allow polymerization of the fibrinogen.
- Prepare sufficient feed media (FM) for 2 mL per construct. Supplement GM with 200 µM ascorbic acid, 50 µM proline, and 5 ng/mL TGF-β1.
- Add 2 mL FM to cover each construct. Culture the constructs in a sterile incubator maintained at 37 °C and 5% CO2 for a total of 14 days or to the desired endpoint, refreshing the media every second day with 2 mL FM
6. Tensile Testing Engineered Ligaments
NOTE: Tensile testing was performed using a custom-built tensile tester in a PBS bath; reverse-molded grips that are coupled to the force transducer hold brushite cement anchors in place during the test.
- Determine the length and width of the ligament constructs using digital calipers; calculate the cross-sectional area of the tissue.
- Unpin the ligament construct from the plate and place the anchors in the reverse molded grips, ensuring the construct is submerged in PBS.
- Adjust the distance between the grips, setting the length of the construct to its initial length.
- Begin the test: strain the construct to failure at a strain rate of 0.4 mm/s (or ~3%/s).
- After completion of the test, process the tissue remnants for collagen content (see section 7).
- From the resultant load-deformation data, calculate stress-strain data and quantify mechanical properties of interest; for example, maximal tensile load, ultimate tensile strength and modulus (i.e., elastic property over a linear region of the stress-strain curve).
7. Quantification of Collagen Content of Engineered Ligaments
- Remove engineered ligaments from brushite cement anchors and dry at 120 °C for 25 min.
- Determine the dry mass of constructs and place into individual 1.5 mL tubes. Dry constructs may be stored at room temperature until further processing.
- To each construct, add 200 µL 6 M HCl. Boil at 120 °C in a heating block for 2 h in a fume hood.
CAUTION: HCl is highly corrosive and acidic, the use of boil-proof/safe-lock tubes or other method of securing tubes is recommended. - Centrifuge the tubes briefly to collect liquid, and leave them uncapped to evaporate at 120 °C in a heating block for 1.5 h in a fume hood.
- Resuspend the resultant pellet in 200 µL hydroxyproline buffer. Store at -20 °C until needed.
- Prepare hydroxyproline buffer. In 300 mL water, add 16.6 g citric acid, 4 mL acetic acid, 11.4 g NaOH and stir until dissolved. pH to 6-6.5 and bring volume up to 500 mL. Add 250 µL toluene as a preservative and store at 4 °C protected from light.
- Prepare reagents.
- Prepare trans-4-Hydroxy-L-proline. Dissolve in water to make a 4 mg/mL solution.
- Prepare chloramine-T. Dissolve in water to make a 14.1 mg/mL solution.
- Prepare aldehyde-perchlorate. Dissolve 1.5 g 4-dimethylaminobenzaldehyde in 6 mL 1-propanol, 2.6 mL perchloric acid (CAUTION: corrosive, strong oxidizer, use appropriate precautions), and 0.5 mL water.
- In a set of new 1.5 mL tubes, dilute a sample of each resuspended pellet in hydroxyproline buffer to a volume of 200 µL.
NOTE: Dilutions may range from 1:4 to 1:50 of sample:buffer depending on the expected collagen content of the sample; thus some trial-and-error testing may be needed to determine a dilution factor that is appropriate for the given sample set (i.e., place the samples toward the middle of the standard curve). - Prepare hydroxyproline standards. Dilute hydroxyproline in hydroxyproline buffer (see section 7.5.1) to 80 µg/mL. Perform serial dilutions to make 6-8 200 µL standards between 0-20 µg/mL.
- Add 150 µL 14.1 mg/mL Chloramine T solution to each standard and diluted sample. Vortex and incubate at room temperature for 20 min.
- Add 150 µL aldehyde-perchlorate solution to each sample and diluted sample. Vortex and incubate in a heating block at 60 °C for 15 min. Dispose of the excess aldehyde-perchlorate solution as hazardous waste according to local regulations (contains perchloric acid).
- Allow standards and samples to cool, before aliquoting 200 µL of each, in duplicate, into 96-well plates.
- Read plate at 550 nm in a spectrophotometer. Dispose of plate and the remaining volume in 1.5 mL tubes as hazardous waste according to local regulations (contains perchloric acid).
- Calculation of total collagen and collagen fraction.
- Convert the absorbance value for each sample to micrograms of hydroxyproline using the hydroxyproline standard curve.
- Multiply each well by 2.5 to calculate amount of hydroxyproline in the diluted sample. Recall that only 200 of the 500 µL total mixture (200 µL diluted sample + 150 µL chloramine T +150 µL AP solution) is added to each sample well.
- Multiply by dilution factor (Section 7.7) to calculate amount of hydroxyproline in original sample.
- Divide by 0.137 to calculate the amount of collagen (assumes that collagen contains 13.7% hydroxyproline20).
NOTE: Mammalian hydroxyproline abundance in collagen varies slightly across tissues and mammalian species; for example, pig and sheep Achilles tendon contain 13.5 and 13.7% (hydroxyproline mass/dry tissue mass), respectively21. Here, use 13.7% to estimate percent hydroxyproline in collagen, which is used to calculate the collagen content of a tissue sample using the following equation:
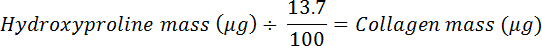
- Divide by the dry mass to calculate the collagen fraction and convert to a percentage.
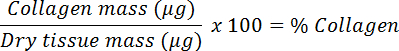
8. Quantification of Molecular Endpoints
NOTE: In addition to primary outcomes of tensile testing and collagen content, molecular endpoints can be measured on 2D or 3D tissue to add mechanistic insight. Bioassays can be used to determine molecular endpoints (see the following section below for context The impact of the post-exercise serum milieu on in vitro ligament function).
- For 3D tissue:
- Prepare constructs as per Step 5 above.
- After construct treatment/intervention, snap-freeze constructs in liquid nitrogen.
- Using a mortar and pestle cooled on dry ice, grind constructs into a powder. Continue at step 8.4/5).
- For 2D tissue:
- Culture human ACL fibroblasts to confluence in a monolayer in six-well plates containing DMEM.
- Aspirate DMEM and apply treatment media according to your experimentation strategy (e.g., time course or dose response experiments).
- Aspirate treatment media and wash cells with PBS.
- Scrape cells to collect using an appropriate extraction buffer/reagent (see next).
- Protein expression analysis: Use a cytosolic extraction buffer (e.g., 250 mM sucrose, 50 mM Tris pH 7.4, 5 mM MgCl2, and protease/phosphatase inhibitor cocktail) to obtain protein lysates. Perform protein concentration assay and continue analysis according to standard western blot procedures.
- Gene expression analysis: Isolate total RNA using 500 µL RNA isolation reagent according to the manufacturer's instructions to obtain high-quality RNA. Perform reverse-transcription and real-time quantitative PCR analysis according to standard procedures.
- DNA isolation: Isolate and quantify genomic DNA using DNA isolation reagent according to the manufacturer's instructions. Quantify DNA concentration by using a spectrophotometer to measure the sample absorbance at 260 nm.
Representative Results
Overview of engineered ligament formation and experimental intervention
Figure 1 shows an overview of the formation of engineered ligaments. Brushite cement, a bone substitute material22 is prepared by combining an orthophosphoric acid/citric acid solution with β-tricalcium phosphate in cylindrical wells. Alternatively, if not directly measuring mechanical function of the tissues, 3-0 silk sutures can be used as anchors in the formation of a tissue. These are pinned 12 mm apart in silicone-coated 35 mm dishes and sterilized by soaking in 70% ethanol. Fibroblasts are isolated from anterior cruciate ligament remnants obtained during ACL reconstruction surgery. After expansion, 2.5 x 105 cells are encapsulated in a fibrin gel formed in the brushite cement anchor-pinned dish. After formation, ligament constructs can be examined for changes in mechanical properties, collagen content, cell proliferation, gene expression, protein levels, and tissue morphology.
Throughout culture, the cells contract the fibrin gel and form a linear tissue between the two anchors (Figure 2A). After 1-2 days in culture, the cells have attached to the fibrin gel, extended cell processes, and started to exert traction forces (Figure 2B). As the fibrin gel is contracted by traction forces and broken down by cellular enzymes, tension is generated between the two anchor points and our cells align parallel to this axis (Figure 2B) and begin to deposit collagen. After 4-5 days, the constructs have contracted around the anchors forming a linear cylindrical tissue (Figure 2A; at this point external stimuli may be applied to the system (intervention at this time avoids disrupting the linear tissue formation process). Interventions may consist of supplementing the culture media with human or animal serum after a given intervention, exogenous cytokines and growth factors, employing mechanical stimulation, or changing other environmental factors such as oxygen tension. Using growth media (DMEM with 10% FBS and 100 U/mL penicillin) supplemented with 200 µM ascorbic acid, 50 µM L-proline, and 5 ng/mL TGF-β1, we have determined that cell proliferation continues throughout a 2 week culture period (Figure 2C) and indeed, light microscopy reveals a dense tissue containing highly aligned cells at 14 days of culture (Figure 2B).
Assessment of engineered ligaments
At the end of the culture period, engineered ligaments can be assessed in a variety of ways. A major advantage of this system is the ability to determine functional changes to the tissue via mechanical testing, a vital assessment given the mechanical role of native ligament. Uniaxial tensile testing can be utilized to measure mechanical properties including load to failure, ultimate tensile strength, and Young's modulus. Viscoelastic properties can also be measured with stress relaxation and creep tests. Figure 3A depicts an engineered ligament held in reverse molded grips in a custom-built uniaxial tensile tester. The right grip is attached to a force transducer to measure the load across the ligament as the tissue is strained to failure. Figure 3B shows a representative stress-strain plot for a test to failure. After undergoing mechanical testing, the same constructs can be dried and processed for a hydroxyproline assay23 to assess total collagen content as well as other biochemical assays. With a sufficient number of additional samples per condition, a thorough examination of an experimental intervention can be conducted, including its effects on cell proliferation, gene and protein expression, and histological morphology. While 14 days is a typical endpoint for our studies, engineered ligaments continue to improve in their mechanical properties and collagen content through 28 days of culture as shown in Figure 3C and can survive for at least 3 months in culture24.
Donor variability is an important consideration for experimental repeatability. Figure 4 shows a representative experiment reported by Lee et al.25 comparing 7 different ACL donors (n = 3 male and n = 4 female) demonstrating typical tensile properties and collagen content after a 2-week culture in the previously described supplemented growth media. Using cells from similar ACL collections, age of donor, time after injury, gender, etc., the engineered ligaments demonstrate low variability between donors and similar characteristics between male and female donors with the exception of the difference in collagen fraction. In the aforementioned study, engineered ligaments were used as an in vitro model to investigate why women have a significantly greater risk of ACL injury than men, and demonstrated that ACL fibroblasts isolated from female donors do not inherently form weaker and less collagenous engineered ligaments25.
The impact of the post-exercise serum milieu on in vitro ligament function
We have previously demonstrated the ability of engineered ligaments to be used to probe physiological processes1,25. In the following representative experiment as reported by West et al.1, we determined the biochemical effects of exercise on ligament function and highlight the methodology and findings here. We formed engineered ligaments using human ACL cells and applied an intervention, at day 7 of culture, consisting of culture media conditioned with human serum collected pre- or post-exercise. Briefly, we recruited healthy young male participants and collected blood samples before and after an acute bout of resistance exercise that increases circulating hormones and cytokines including human growth hormone (GH). Human serum was isolated from pre- and post-exercise blood samples and used in place of fetal bovine serum in the culture media for the second week of engineered ligament culture (Figure 5A). Pre- and post-exercise serum samples were analyzed using ELISA for changes in GH and insulin-like growth factor 1 (IGF-1), the concentration of which can be altered by exercise (Figure 5B). This information was used to correlate serum effects on the engineered ligaments with changes in the serum in response to exercise. After a 14 day culture period, the ligament constructs were evaluated using mechanical testing and a hydroxyproline determination of collagen content and demonstrated a significant increase in both maximal tensile load and collagen content in response to the post-exercise serum. In aiming to assess whether this effect was related to exercise-induced releases of GH or IGF-1, engineered ligaments were formed in a separate experiment and treated with a dose response of either human recombinant GH or IGF-1. Interestingly, while serum GH increased in the blood (Figure 5B-i), progressively increasing recombinant GH concentration in the culture media did not increase collagen content (Figure 5D-i) or mechanical properties (data not shown) in engineered ligaments. In contrast, serum IGF-1 levels did not increase after exercise, but a dose-response experiment revealed that increasing levels in the culture media improved the collagen content of ligament constructs (Figure 4D-ii). Thus, whereas exercise did result in robust increases in post-exercise GH, the dose-response experiment using rhGH raises doubt as to whether GH is directly responsible for the phenotypic enhancement of the engineered ligaments (at least, the 22 kDa isoform alone does not appear to be responsible). Conversely, whereas serum IGF-1 was not altered at 15 min post-exercise, testing rhIGF-1 over a broad range of concentrations revealed that IGF-1 is capable of improving collagen content; however, it should be noted that increasing rhIGF-1 concentrations through a range that estimated physiological levels did not significantly increase collagen content. Thus, the unique post-exercise serum environment was important toward improving the mechanics and collagen of engineered ligaments.
In the study highlighted here1, the volume of experimental serum was limited due to ethical considerations; so, short-term 2D bioassays, which had lower serum demands, were used to further probe the molecular mechanisms responsible for the increase in collagen that was observed. ACL fibroblasts were cultured to confluence in 6-well plates and treated for 1 hour with rest or post-exercise serum, and compared with dose responses of recombinant GH, IGF-1, TGF-β1 and the activation of targets in the PI3K/mTORC1, ERK1/2, and Smad signaling pathways were determined. In the presence of post-exercise serum, the PI3K/mTORC1 and ERK1/2 pathway showed greater activation as assessed by phosphorylation of S6K (Figure 5D-i) and ERK1/2 (Figure 5D-ii), respectively. Compared to the hormone and cytokine dose responses, while GH had a small positive effect on mTOR signaling (Figure 5D-i) and IGF-1 showed a positive effect at the lowest dose, the three treatments of GH, IGF-1, and TGF-β1 did not account for the increase in PI3K/mTORC1 and ERK1/2 signaling. Taken together, our 3D engineered ligament model and 2D bioassay data suggests that the post-exercise serum environment is able to improve engineered ligament function and collagen content through activation of the PI3K/mTORC1 and ERK1/2 pathways.
In summary, using an engineered ligament model combined with exercise-conditioned serum, we were able to i) investigate the effect of post-exercise serum environment on engineered ligament function and collagen, ii) correlate changes in ligament phenotype with changes serum hormone concentration, with the aim to determine which changes in the serum led to changes in engineered ligaments, and iii) further the scope of the work by using 2D bioassays to probe molecular targets of the serum biochemical milieu to determine molecular mechanisms that are activated by post-exercise serum that lead to improvements in ligament function.
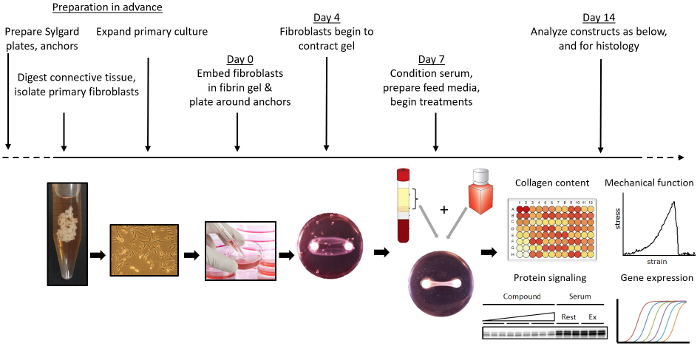
Figure 1: Overview of the formation and use of engineered ligaments. Brushite cement anchors are manufactured and pinned into silicone-coated plates. Primary fibroblasts are isolated and expanded from ACL remnants. Engineered ligaments are formed by encapsulating fibroblasts in a fibrin gel around two brushite cement anchors. Engineered ligaments are cultured and treated with whichever specific chemical or mechanical (e.g., via a bioreactor) stimuli desired. At the desired endpoint, engineered ligaments can be collected and assessed for mechanical properties, gene expression, collagen content, protein expression, and histology. Please click here to view a larger version of this figure.
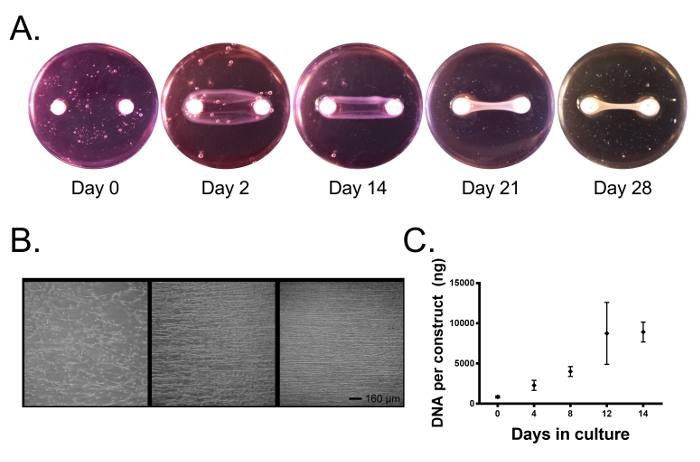
Figure 2. Primary ligament fibroblasts form a fibrin-based bone-to-bone engineered ligament spanning two brushite cement anchors. (A) Over time, the fibroblasts contract the fibrin gel around the brushite cement anchors forming a linear tissue. (B) In the first three days, the cells attach to the fibrin gel and exert traction forces, aligning the cells with the long axis of the construct. Over 14 days, the cells form a highly aligned tissue. Scale bar = 160 µm. (C) The DNA content of the engineered ligaments continues to increase over 14 days in culture as the cells proliferate. Data is presented as mean ± SD with n = 3-4 constructs per group. groups. Please click here to view a larger version of this figure.
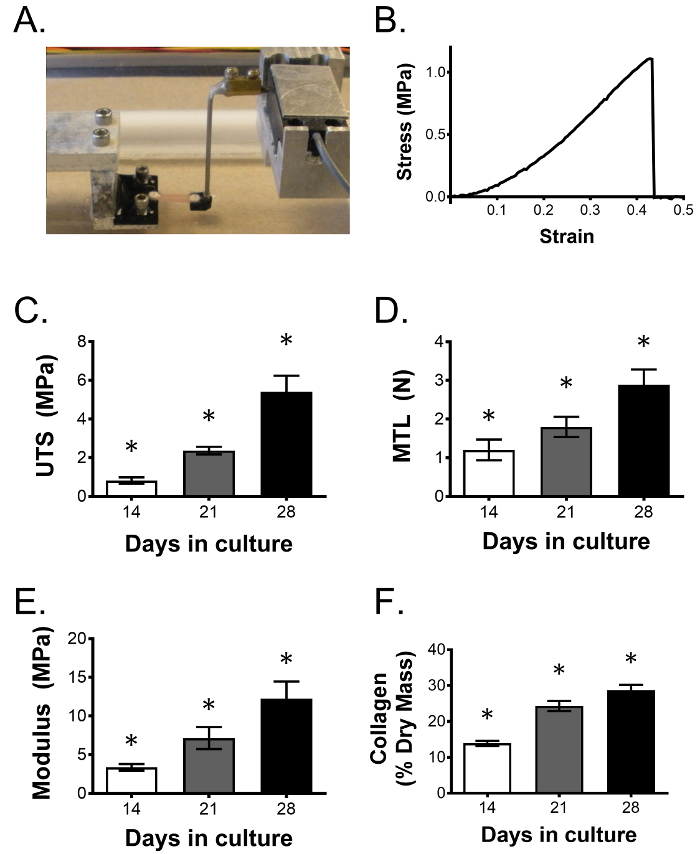
Figure 3: Engineered ligament mechanical properties and collagen content improve over time. (A) Ligament constructs are uniaxially tensile tested to determine the effect of a given intervention on ligament function. As shown, two reverse molded, 3D printed grips hold reciprocally shaped anchors that are bridged by the engineered sinew. Anchors are coupled to a stepper motor and force transducer to generate stress/strain curves of the tested tissue, allowing mechanical properties can be determined. (B) Representative stress-strain curve from an engineered ligament strained to failure. Over the course of 28 days, (C) ultimate tensile strength (UTS), (D) maximal tensile load (MTL), (E) Young's modulus, and (F) collagen fraction continue to improve. Data are presented as mean ±SD with n = 5 constructs per group. * indicates significant difference from all other groups. Please click here to view a larger version of this figure.
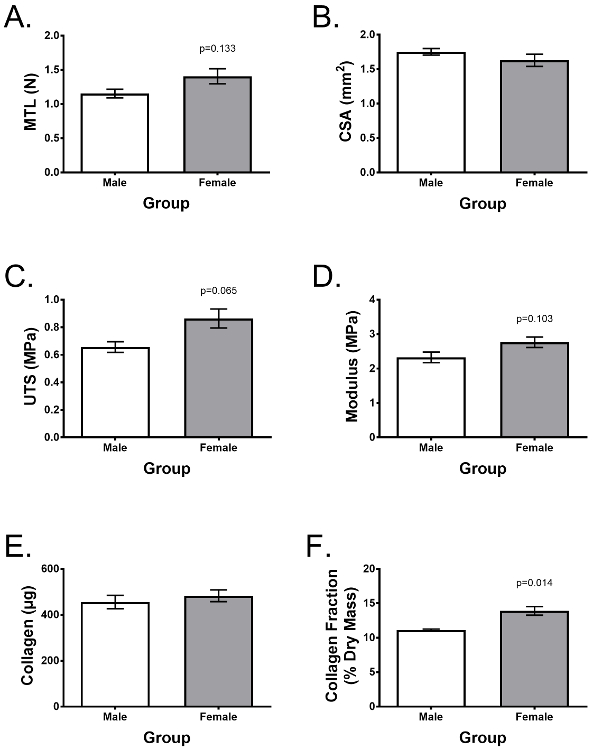
Figure 4: Engineered ligaments can be assessed for functionality and biochemical content, displaying low donor variability. Engineered ligaments were formed 7 different donors (n = 3 male, n = 4 female). After 2 weeks of culture, they were assessed for differences in (A) maximum tensile load, (B) ultimate tensile strength (UTS), (C) Young's modulus, (D) cross-sectional area (CSA), (E) total collagen content per construct, and (F) collagen as a fraction of dry mass. Data are presented as mean ± SD and statistical significance was with Student's t-test. * indicates significant difference from other groups (p <0.05). Figure adapted from Lee et al.25 Please click here to view a larger version of this figure.
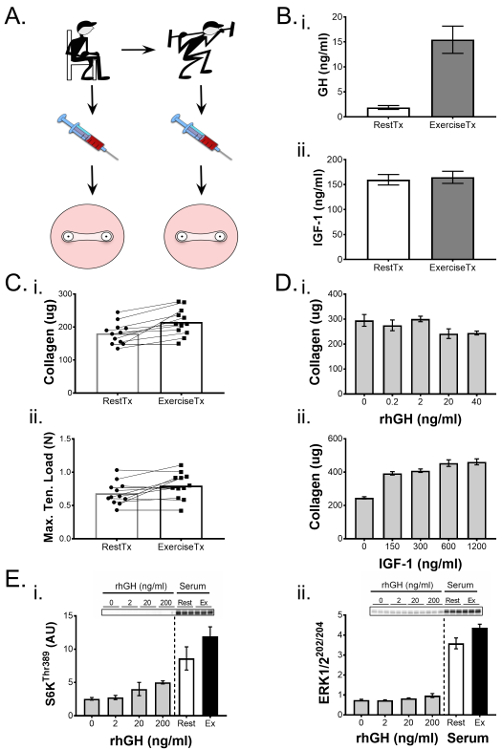
Figure 5. Engineered ligaments demonstrate mechanical and biochemical changes in response to biological interventions. (A) Serum was isolated from blood draws collected from subjects pre- (RestTx) and post-exercise (ExTx) and used to treat engineered ligaments during the second week of culture. (B) (i) Human growth hormone (GH) and (ii) insulin-like growth factor (IGF)-1 levels in the rest and exercise serum were quantified through ELISA. (C) Engineered ligaments treated with ExTx demonstrated improved (i) collagen content and (ii) maximal tensile load. Statistical significance of paired comparisons (RestTx and ExTx) was analyzed by a t-test with significance level set at p <0.05. (D) A dose-response of (i) GH and (ii) IGF-1 was used to determine possible contributions of these factors to the changes in collagen content due to ExTx. E) 2D bioassays were used to compare the effects of increasing doses of GH, RestTx, and ExTx on molecular signaling targets such as the phosphorylation of (i) S6KThr389 and (ii) ERK1/2Thr202/Tyr204. Statistical comparison of more than two experimental groups was performed using ANOVA and Tukey's HSD. Data are presented as presented as mean ± SD. * indicates a significant difference from control (p <0.05) and § indicates significant difference from 150 ng/mL and 300 ng/mL IGF-1. Figure adapted from West et al.1 Please click here to view a larger version of this figure.
Discussion
The present manuscript describes a model of ligament tissue that is a useful experimental platform for investigators with a broad spectrum of research topics, from tissue development to translational/clinical questions. The engineered ligament model described here is based on a versatile protocol that can be adapted at various points throughout the workflow (Figure 1 and Discussion Section). Further, the inherently reductionist nature of the in vitro environment can be brought nearer to the physiological realm by supplementing feed media with conditioned human or animal serum.
Constructs can be formed using fibroblasts from a variety of sources
While the methodology and representative results shown here are based on the use of primary ACL fibroblasts, the cell isolation protocol can be adjusted for collecting other types of primary fibroblasts. As described in Figure 4, engineered ligaments formed with primary cells isolated from young human donors show low donor variability. Primary cells are limited by initial isolation and passage restriction; the use of cell lines may improve the reproducibility of experiments. The use of other cell types may require modifications in cell culture media and fibrin gel formulation. For example, we have observed that human mesenchymal stem cells (MSCs) are not able to form linear tissues between the brushite cement anchors over the course of 2 weeks while equine superior digital flexor tendon fibroblasts, equine bone marrow stromal cells, chick embryonic tendon fibroblasts, and murine C3H10T1/2 MSCs rapidly contract and digest the fibrin gel to form a linear tissue (unpublished observations). This contrast may be a consequence of differences in cell contractility, proliferation, and fibrinolytic enzyme production.
Application of chemical and mechanical stimulation
In the method described herein, fibrin-based tissue forms around brushite cement anchors, allowing for the application of mechanical stimulation via a stretch bioreactor11, as well as for end-point tensile testing. The presence of brushite cement-soft tissue interface (enthesis) also presents an opportunity for further investigation and improvement22,26 (see Clinical applications section below). In this in vitro environment, the contribution of chemical and mechanical factors can be more readily identified; an example of this is shown in Figure 5, whereby the effect of the post-exercise serum environment was separated from the mechanical stimuli of exercise. Pilot studies may be needed to determine timeframe of experimental interventions, composition of treatments, and appropriate endpoints to expect an observable change. For example, in post-exercise serum study1, the length of experimental treatment was constrained by the supply of serum used to supplement media, from which constructs were fed every second day. Further, during the second week of culture, culture media was supplemented with rest or post-exercise serum with ascorbic acid and L-proline maintained while TGF-β1 was removed. TGF-β1 is a known pro-fibrotic growth factor which increases in serum after exercise27. Therefore, to avoid obscuring TGF-β1-related effects of the post-exercise serum, this cytokine was not maintained in the culture media.
This engineered ligament model can also be used to test the effect of mechanical stretch. By engineering reverse modeled grips to hold the brushite cement anchor ends (similar to the uniaxial tensile tester depicted in Figure 1), stretch bioreactors can be designed to accommodate engineered ligaments. Our lab has previously used this model to investigate the molecular signaling response of engineered ligaments to uniaxial tensile stretch in a custom-made bioreactor11 which will provide a better understanding for the rational design of an in vitro stretch paradigm or even, potentially, in vivo stretch/activity/therapeutic applications.
Assessment of engineered ligaments
As with traditional monolayer culture, 3D constructs can be assayed for gene/protein expression; additionally, their 3D morphology also provides the opportunity to assess functional and morphological changes and constructs can be maintained in culture for long-term studies (Figure 3). While engineered ligaments are not equivalent to native, mature ligaments, they bear similarity to developing tendons/ligaments and behave similarly to native tissue in response to nutrients26, growth factors10, hormones25, and exercise11,28. Thus, while caution is warranted before making broad generalizations from any in vitro model, results from ligament construct testing may reveal or inform a particular physiological mechanism that may otherwise be impossible to investigate in vivo.
Supplement feed media with conditioned serum for a flexible and dynamic model with broad applications
The human serum metabolome is a milieu of ~4,500 compounds including, but not limited to, glycoproteins, lipoproteins, lipid derivatives, energy substrates, metabolites, vitamins, enzymes, hormones, neurotransmitters, and a plethora of building blocks/intermediates.29 Further inspection of the human serum metabolome according to compound classes29 reveals additional benefits of integrating experimental serum into in vitro experiments. That is, the majority of the ~4500 compounds in serum are hydrophobic or lipid-derived, underscoring the importance of binding proteins for transport/solubilization. It follows that experimentally recapitulating endogenous compound transport dynamics, and hence bioavailability and compound-target interactions, would be nearly impossible. Thus, experimental serum is particularly effective for the study of compounds that are known to depend on accessory molecules for solubilization, transport, target binding, and mechanism of action.
Our lab has a long-standing interest in the health benefits of exercise. Exercise improves cellular and organ function in a variety of tissues throughout the body12, an effect that may be attributed to a variety of factors (e.g., IL-613, IL-1514, Meteorin-like15, exosomes16,17) that are released into systemic circulation. The post-exercise biochemical milieu reflects factors released both from contracting skeletal muscle exercise-responsive hormones as well as factors that are released as the result of sympathetic nervous system stimulation of secretory glands (e.g., cortisol and catecholamines from the adrenal gland18, and growth hormone from the anterior pituitary19). We recently used a model of pre- and post- exercise serum to investigate the effects of the exercise-induced biochemical milieu on engineered tissue.1 While numerous important exercise-related research questions remain, the model is by no means limited in this way. For example, serum could be obtained, either from animal or human sources, following dietary or pharmacological interventions, or from different age groups or clinical populations30. In this way, exogenous or endogenous compounds of interest will be present in the serum and treatment media, in bioavailable quantities and will interact with the target tissue in concert with the endogenous milieu (i.e., in a more physiological context). This approach is dynamic since it is highly likely that a given intervention will exert a multi-organ (and multi-compound) effect and thus the physiological milieu will be co-modified. While this approach presents certain challenges, since multiple systemic biochemical variables are altered simultaneously, it is an approach that may help overcome drawbacks of purely reductionist experimental methodology31,32. Taken together, implementing conditioned serum together with a tissue engineered (in vitro biomimetic) tissue can be used as a tool for physiology, nutrition and clinical research questions.
Clinical applications are numerous
The tissue engineering model presented here can be used to investigate anatomical and clinical research questions that traditional in vitro models cannot. A ligament or tendon in vivo contains a soft-to-hard tissue transition region called the enthesis. The enthesis, which is vulnerable to mechanical stress-related injury33, can be studied in cross-section via histochemical and electron microscopy techniques22,26. This unique interface is doubly important for those with low or constrained mobility since physical inactivity impairs the ability of connective tissue to transfer load to regions of low to high compliance34, ultimately resulting in an overall decrease in tissue compliance and increased injury risk.
Our lab has recently used this tissue engineering model25 to model another population, female athletes, who are at-risk for connective tissue injuries: the incidence of ACL injury is approximately five-fold greater than their male counterparts35. Potential mechanisms underpinning this sex-based disparity in injury were investigated by treating ligament constructs with physiological concentrations of the female sex hormone, estrogen, at concentrations that mimicked stages of the menstrual cycle. Interestingly, high concentrations of estrogen inhibited the gene expression and activity of lysl oxidase, the primary enzyme responsible for creating lysine-lysine cross-links in the collagen matrix of ligaments and tendons. Importantly, 48 h of high estrogen (to simulate the follicular phase) decreased ligament construct stiffness without altering the collagen density of the constructs. From a physiological perspective, this suggests that increases in ligament laxity in females may be due, at least in part, to decreased cross-link formation. From an experimental perspective, these findings25 highlight the utility of the 3D construct model, which permitted functional cross-linking activity to be examined. From a clinical perspective, this model can now be used to rapidly screen for interventions that can prevent the negative effects of estrogen of ligament function.
Closing remarks
Here we have presented a detailed methodology for the formation of engineered ligaments and their utility as a 3D in vitro tissue model. The model is highly adaptable to a wide range of objectives, providing flexibility in the cell type, interventions, and outcome measures of interest. Supplementing feed media with conditioned serum adds a physiological context that cannot be achieved in a traditional in vitro environment, improving the modeling of in vivo physiology. In short, we believe this to be a broadly applicable model with exciting implications for furthering both the fields of physiology and tissue engineering.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by an NSERC postdoctoral fellowship (DWDW), an ARCS Foundation Scholarship (AL), and a UC Davis College of Biological Sciences grant (KB).
Materials
| Austerlitz Insect pins, minutien stainless steel, size 0.20 | Entomoravia | N/A | For brushite cement anchors; include info on multiple sources and alternative products |
| β-tricalcium phosphate | Plasma Biotal Ltd (Derbyshire, UK) | N/A | For brushite cement anchors; include whether it's hazardous /toxic |
| o-phosphoric acid, 85% (w/w) | EMD Millipore | PX0995 | For brushite cement anchors; include info on preparation |
| Citric acid | Sigma-Aldrich | 251275-500g | For brushite cement anchors |
| Falcon 35mm tissue culture dishes | Fisher Scientific | 08-772A | For silicone-coated plates |
| Sylgard 184 silicone elastomer kit | Ellsworth Adhesives | 4019862 | For silicone-coated plates |
| 1X Phosphate-buffered saline (PBS) | Fisher Scientific | SH3002802 | For cell isolation and expansion |
| 100X antibiotic/antimycotic solution | VWR | 45000-616 | For cell isolation |
| Type II collagenase | Thermo Fisher Scientific | 17101015 | For cell isolation |
| 100X penicillin/streptomycin solution | Thermo Fisher Scientific | 15140122 | For cell isolation |
| Steriflip-GP, 0.22 µm pore, polyethersulfone, gamma irradiated | EMD Millipore | SCGP00525 | For reagent sterilization |
| DMEM high glucose with sodium pyruvate and L-glutamine | VWR | 10-013-CV | For cell and tissue culture |
| Fetal bovine serum | BioSera | FBS2000 | Component of tissue digestion media and growth media |
| Penicillin G Potassium Salt | MP Biomedicals | 0219453680 – 100 MU | Component of growth media. Dissolve in water to 100,000 U/mL, filter sterilize, aliquot, and store at -20°C. |
| CELLSTAR polystyrene tissue culture dishes (145 x 20 mm) | VWR | 82050-598 | For cell culture |
| Trypan blue | Thermo Fisher Scientific | T10282 | For cell isolation |
| Trypsin-EDTA (0.25%) | Thermo Fisher Scientific | 25200056 | For cell culture. Dilute to 0.05% in PBS |
| Dimethyl sulfoxide | Sigma-Aldrich | 472301 | For cell freezing media |
| Nalgene Mr. Frosty Cryogenic Freezing Container | Thermo Fisher Scientific | 5100-0001 | For cell freezing |
| BD Vacutainer Red Plastic 10 ml | Fisher Scientific | 367820 | For human serum collection |
| Bound Tree Insyte Autoguard IV Catheters, 22ga x 1inch Needle | Fisher Scientific | 354221 | For human serum collection |
| Thrombin, bovine origin | Sigma-Aldrich | T4648-1KU | For engineered ligament formation. Dissolve at 200 U/mL in DMEM high glucose media. Filter at 0.22 μm, aliquot, and store at -20°C. |
| Fibrinogen, bovine origin | Sigma-Aldrich | F8630-5G | For engineered ligament formation. Dissolve at 20 mg/mL in DMEM high glucose media. Filter at 0.22 μm, aliquot, and store at -20°C. |
| Aprotinin from bovine lung | Sigma-Aldrich | A3428 | For engineered ligament formation. Dissolve at 10 mg/mL in water. Filter at 0.22 μm, aliquot, and store at -20°C. |
| 6-Aminohexanoic acid | Sigma-Aldrich | 07260-100g | For engineered ligament formation. Dissolve at 0.1g/mL in water. Filter at 0.22 μm, aliquot, and store at 4°C. |
| L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate | Sigma-Aldrich | A8960-5G | Component of feed media. Dissolve in DMEM high glucose media at a concentration of 50 mM. Filter at 0.22 μm and store at 4°C. |
| L-proline | Sigma-Aldrich | P5607-25G | Component of feed media. Dissolve in PBS at a concentration of 50 mM. Filter at 0.22 μm and store at 4°C. |
| Transforming growth factor-β1 | Peprotech | 100-21 | Component of feed media. Reconsistute according to manufacturer's instructions at a concentration of 10 μg/mL. Aliquot and store at -20°C. |
| Stericup-GP, 0.22 µm, polyethersulfone, 250 mL, radio-sterilized | EMD Millipore | SCGPU02RE | For reagent sterilization |
| Hydrochloric acid | Fisher Scientific | A144-212 | Dilute in water to 6M |
| 4-Dimethylaminobenzaldehyde | Sigma-Aldrich | 39070-50g | For hydroxyproline assay |
| Chloramine-T trihydrate | Sigma-Aldrich | 402869-100g | For hydroxyproline assay |
| trans-4-Hydroxy-L-proline | Sigma-Aldrich | H54409-100g | For hydroxyproline assay |
| 1-propanol | Sigma-Aldrich | 279544-1L | For hydroxyproline assay |
| Perchloric acid | Sigma-Aldrich | 311421-250ml | For hydroxyproline assay |
| Acetic acid, glacial | EMD Millipore | AX0073-9 | For hydroxyproline assay |
| Sodium hydroxide | Fisher Scientific | S318-500 | For hydroxyproline assay |
| Toluene, anhydrous | Sigma-Aldrich | 244511-1L | For hydroxyproline assay |
| Corning Costar Clear Polystyrene 96-Well Plates | Fisher Scientific | 07-200-656 | For hydroxyproline assay |
References
- West, D. W., et al. The exercise-induced biochemical milieu enhances collagen content and tensile strength of engineered ligaments. J Physiol. 593 (20), 4665-4675 (2015).
- Booth, F. W., Laye, M. J. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol. 587 (Pt 23), 5527-5539 (2009).
- Booth, F. W., Hargreaves, M. Understanding multi-organ pathology from insufficient exercise. J Appl Physiol (1985). 111 (4), 1199-1200 (2011).
- Shearn, J. T., et al. Tendon tissue engineering: progress, challenges, and translation to the clinic. J Musculoskelet Neuronal Interact. 11 (2), 163-173 (2011).
- Liu, C. F., et al. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng Part B Rev. 17 (3), 165-176 (2011).
- Vunjak-Novakovic, G., Altman, G., Horan, R., Kaplan, D. L. Tissue engineering of ligaments. Annu Rev Biomed Eng. 6, 131-156 (2004).
- Bayer, M. L., et al. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials. 31 (18), 4889-4897 (2010).
- Guerquin, M. J., et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. 123 (8), 3564-3576 (2013).
- Ma, J., et al. Three-dimensional engineered bone-ligament-bone constructs for anterior cruciate ligament replacement. Tissue Eng Part A. 18 (1-2), 103-116 (2012).
- Hagerty, P., et al. The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials. 33 (27), 6355-6361 (2012).
- Paxton, J. Z., Hagerty, P., Andrick, J. J., Baar, K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 18 (3-4), 277-284 (2012).
- Safdar, A., et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 108 (10), 4135-4140 (2011).
- Pedersen, B. K., Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 8 (8), 457-465 (2012).
- Crane, J. D., et al. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 14 (4), 625-634 (2015).
- Rao, R. R., et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 157 (6), 1279-1291 (2014).
- Aswad, H., et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 57 (10), 2155-2164 (2014).
- Safdar, A., Saleem, A., Tarnopolsky, M. A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 12 (9), 504-517 (2016).
- Maling, H. M., Stern, D. N., Altland, P. D., Highman, B., Brodie, B. B. The physiologic role of the sympathetic nervous system in exercise. J Pharmacol Exp Ther. 154 (1), 35-45 (1966).
- Pritzlaff, C. J., et al. Impact of acute exercise intensity on pulsatile growth hormone release in men. J Appl Physiol (1985). 87 (2), 498-504 (1999).
- Creemers, L. B., Jansen, D. C., van Veen-Reurings, A., van den Bos, T., Everts, V. Microassay for the assessment of low levels of hydroxyproline. Biotechniques. 22 (4), 656-658 (1997).
- Neuman, R. E., Logan, M. A. The determination of hydroxyproline. J Biol Chem. 184 (1), 299-306 (1950).
- Paxton, J. Z., Donnelly, K., Keatch, R. P., Baar, K., Grover, L. M. Factors affecting the longevity and strength in an in vitro model of the bone-ligament interface. Ann Biomed Eng. 38 (6), 2155-2166 (2010).
- Woessner, J. F. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 93, 440-447 (1961).
- Paxton, J. Z., Wudebwe, U. N., Wang, A., Woods, D., Grover, L. M. Monitoring sinew contraction during formation of tissue-engineered fibrin-based ligament constructs. Tissue Eng Part A. 18 (15-16), 1596-1607 (2012).
- Lee, C. A., et al. Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol (1985). 118 (10), 1250-1257 (2015).
- Paxton, J. Z., Grover, L. M., Baar, K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A. 16 (11), 3515-3525 (2010).
- Heinemeier, K., Langberg, H., Kjaer, M. Exercise-induced changes in circulating levels of transforming growth factor-beta-1 in humans: methodological considerations. Eur J Appl Physiol. 90 (1-2), 171-177 (2003).
- Mackey, A. L., Heinemeier, K. M., Koskinen, S. O., Kjaer, M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connect Tissue Res. 49 (3), 165-168 (2008).
- Psychogios, N., et al. The human serum metabolome. PLoS One. 6 (2), e16957 (2011).
- Nguyen, T., et al. The effects of resting and exercise serum from children with cystic fibrosis on C2C12 myoblast proliferation in vitro. Physiol Rep. 2 (6), e12042 (2014).
- Joyner, M. J., Pedersen, B. K. Ten questions about systems biology. J Physiol. 589 (Pt 5), 1017-1030 (2011).
- Joyner, M. J. Giant sucking sound: can physiology fill the intellectual void left by the reductionists?. J Appl Physiol (1985). 111 (2), 335-342 (2011).
- Benjamin, M., et al. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 208 (4), 471-490 (2006).
- Arruda, E. M., Calve, S., Dennis, R. G., Mundy, K., Baar, K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J Appl Physiol (1985). 101 (4), 1113-1117 (1985).
- Arendt, E., Dick, R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 23 (6), 694-701 (1995).

