Assessing Cardiomyocyte Subtypes Following Transcription Factor-mediated Reprogramming of Mouse Embryonic Fibroblasts
Summary
This manuscript describes a step-by-step protocol for the generation and quantification of diverse reprogrammed cardiac subtypes using a retrovirus-mediated delivery of Gata4, Hand2, Mef2c, and Tbx5.
Abstract
Direct reprogramming of one cell type into another has recently emerged as a powerful paradigm for regenerative medicine, disease modeling, and lineage specification. In particular, the conversion of fibroblasts into induced cardiomyocyte-like myocytes (iCLMs) by Gata4, Hand2, Mef2c, and Tbx5 (GHMT) represents an important avenue for generating de novo cardiac myocytes in vitro and in vivo. Recent evidence suggests that GHMT generates a greater diversity of cardiac subtypes than previously appreciated, thus underscoring the need for a systematic approach to conducting additional studies. Before direct reprogramming can be used as a therapeutic strategy, however, the mechanistic underpinnings of lineage conversion must be understood in detail to generate specific cardiac subtypes. Here we present a detailed protocol for generating iCLMs by GHMT-mediated reprogramming of mouse embryonic fibroblasts (MEFs).
We outline methods for MEF isolation, retroviral production, and MEF infection to accomplish efficient reprogramming. To determine the subtype identity of reprogrammed cells, we detail a step-by-step approach for performing immunocytochemistry on iCLMs using a defined set of compatible antibodies. Methods for confocal microscopy, identification, and quantification of iCLMs and individual atrial (iAM), ventricular (iVM), and pacemaker (iPM) subtypes are also presented. Finally, we discuss representative results of prototypical direct reprogramming experiments and highlight important technical aspects of our protocol to ensure efficient lineage conversion. Taken together, our optimized protocol should provide a stepwise approach for investigators to conduct meaningful cardiac reprogramming experiments that require identification of individual CM subtypes.
Introduction
The heart is the first functional organ to develop in the embryo1,2. In conjunction with the circulatory system, it supplies oxygen, nutrients, and a waste disposal mechanism during development. Three weeks after fertilization, the human heart beats for the first time and its proper regulation is maintained by cardiomyocytes (CMs). The irreversible loss of these specialized cells is therefore the fundamental issue underlying progressive heart failure. While some organisms such as the zebrafish and Xenopus have the potential for cardiac regeneration, the adult mammalian heart is more limited3,5,6. Thus, given the critical function of the heart, it is not astonishing that heart disease is the leading cause of death in the world, accounting for 600,000 deaths in the United States alone7. Therefore, cell-based therapies to efficiently repair or replace the injured myocardium are of great clinical interest.
The seminal study of Yamanaka and colleagues8 showed that forced expression of four transcription factors is sufficient to convert fully differentiated fibroblast cells to pluripotent stem cells. However, the tumorigenic capacity of all pluripotent stem cell strategies has been a critical concern in their use for therapeutic purposes. This motivated the scientific field to search for alternative methods to transdifferentiate cells while avoiding a pluripotent stage. Recently, several groups have shown the feasibility of this strategy by displaying direct conversion of mouse fibroblasts to induced cardiomyocyte-like cells (iCLMs) with the ectopic expression of the transcription factors Gata4, Mef2c, Tbx5, and later on, Hand2 (GMT and GHMT, respectively)9,10. Furthermore, the same strategy can be performed in vivo and in human-derived tissues9,11,12. Recent studies have highlighted additional factors or signaling pathways that can be modulated to further improve cardiac reprogramming efficiency13,14,15. Taken together, these studies demonstrate the potential of directed transdifferentiation for regenerative therapies. However, the low efficiency of CM reprogramming, the unknown molecular mechanisms, inconsistent reproducibility due to methodological differences16, and the heterogeneous nature of iCLMs remain unaddressed.
In order to directly evaluate iCLM heterogeneity, we designed a discrete and robust single-cell assay for the identification of sarcomere development and cardiac lineage specification-two necessary characteristics of functional cardiomyocytes. There are at least three major types of CM in the heart as defined by their location and unique electrical properties: atrial (AM), ventricular (VM) and pacemaker (PM)17,18,19,20. In an orchestrated combination, they allow the proper pumping of blood. During heart injury, one or all subtypes might be affected, and the type of cell therapy would need to be addressed on a case-by-case basis. Currently, most strategies focus on the overall generation of cardiomyocytes, while little work is being done to study the molecular mechanisms that regulates subtype specification.
The following study details how to properly quantify well-organized sarcomeres and identify a diverse set of cardiomyocyte subtypes. Using a pacemaker (PM)-specific reporter mouse, we are able to apply an immunocytochemical approach to distinguish induced atrial-like myocytes (iAM), induced ventricular-like myocytes (iVM), and induced PM-like myocytes (iPMs)21. Based on our observations, only cells that exhibit sarcomere organization are capable of spontaneous beating. This unique reprogramming platform allows for assessing the role of certain parameters in sarcomere organization, subtype specification, and efficiency of CM reprogramming at single-cell resolution.
Protocol
All experimental procedures involving animal practices were approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center.
1. Isolation of Hcn4-GFP E12.5 Mouse Embryonic Fibroblast (MEFs)
- Set up timed matings between homozygous Hcn4-GFP males and CD-1 females.
- Sacrifice pregnant female at E12.5 by carbon dioxide euthanasia and subsequent cervical dislocation.
- Remove uterine horns with dissecting forceps as described previously22,23, and place them into a Petri dish on ice with 1x phosphate-buffered saline (PBS) without Ca2+ or Mg2+.
- Perform all subsequent steps in the tissue culture hood using sterile technique.
- Remove the embryos from the uterus and amniotic sac using scissors and dissecting forceps. Keep the placenta attached for better handling. Pregnant CD-1 females typically give birth to between 10 and 14 pups.
- Using dissecting forceps, take the isolated embryos and quickly rinse them twice in 70% (v/v) EtOH.
NOTE: The washes should be fast to minimize cell death. - Remove the head, limbs, tail, and internal organs, including the heart from the isolated embryos.
- Place the remaining tissue in a 10-cm dish with 1 mL of 1x PBS and finely mince using a sterile razor blade to approximately 1 mm3 in size.
- Transfer minced tissue into a 50 mL conical tube with PBS.
- Spin at 300 x g for 3 min. Carefully aspirate excess PBS.
- Add 1 mL of sterile 0.25% trypsin-EDTA per embryo. Incubate cells in a 37 °C water bath for 15 min. Gently mix the tube every 4 min. Over-digestion of the tissue will significantly lower the yield.
- Vortex cell mixture at maximum speed (3,200 rpm) for 4 s.
- Add 2 mL of fibroblast media per embryo and mix. Filter through a 100 µm cell strainer using a pipette to aid the cells through the strainer. Refer to Table 1 for the formulation of all subsequent mediums.
- Spin at 300 x g for 4 min. Carefully aspirate supernatant.
- Add 10 mL of fresh fibroblast media per 3 embryos and triturate 6-10 times.
- Plate the cells in 1 15-cm tissue culture dish for every 3 embryos prepared. Culture overnight in a 37 °C, 5% CO2 incubator.
- After the overnight incubation, replace the media with fresh 30 mL of media per plate. Place cells back into the incubator overnight.
NOTE: Check for Hcn4-GFP+ cell contamination under a fluorescent microscope. The culture should be GFP– and only become GFP+ upon reprogramming. - The next day, harvest cells with 3 mL of fresh pre-warmed 0.25% trypsin-EDTA. Count and freeze cells. Typically, freeze cells at 3 x 106 cells per mL. The expected yield should be 3 x 106 cells per embryo.
2. Retrovirus Production and Reprogramming
Caution: The following protocol requires production and handling of infectious retroviruses. Perform the following steps in a Biosafety Level 2 cabinet under BSL-2 guidelines and sterile technique. Use 10% bleach to dispose of all materials exposed to retroviruses.
- Retrovirus production and MEFs preparation
NOTE: The following protocol is for retroviral production in 6-well plates and MEF infection in 24-well plates. For other formats, refer to Table 2. MEFs are plated at Day -1, so the timing will need to be coordinated appropriately for each experiment (Refer to section 2.3 and Figure 2).- Maintain Plat-E (PE) cells as per manufacturer's recommendations. Briefly, culture PE cells in DMEM supplemented with 10% FBS, 1 µg/mL puromycin, 10 µg/mL blasticidin, penicillin, and streptomycin. Passage cells 1:4 every two days when the culture reaches 70-90% confluency.
- Day -2: The day before transfection, seed Plat-E cells at 1 x 106 cells/well on a 6-well plate in transfection media. Cells should be 70-80% confluent at the time of transfection.
- Transfection using a commercial transfection agent.
NOTE: The commercial reagents should be at room temperature (RT) before transfection. For the DNA transfection, add each retroviral plasmid DNA individually (G, H, M, and T) to form a GHMT cocktail9.- Day -1: In a 15 mL conical polystyrene tube, mix 60 µL of reduced serum media with 6 µL of transfection reagent per reaction for a 6-well plate format. Incubate the mixture for 5 min at room temperature.
NOTE: Since the transfection reagent used here binds to plastics, add directly to the reduced serum media to avoid any decrease in transfection efficiency. - Add a total of 2 µg of GHMT cocktail per reaction and gently tap to mix it. Do not vortex. Incubate the reaction for 15 min at RT.
- Add the mixture from step 2.2.3 to the PE cells in a drop wise manner.
- Incubate the transfected Plat-E cells overnight in a 37 °C, 5% CO2 incubator. Record the time of transfection.
- Day -1: In a 15 mL conical polystyrene tube, mix 60 µL of reduced serum media with 6 µL of transfection reagent per reaction for a 6-well plate format. Incubate the mixture for 5 min at room temperature.
- Seeding of Hcn4-GFP mouse embryonic fibroblasts.
- 1 h before plating MEFs, prepare a 24-well plate for immunocytochemistry.
- Add a 12 mm fibronectin coverslip per well.
- Coat wells with 300 µL of bovine collagen solution (e.g., SureCoat) and incubate in a 37 °C incubator for 1 h.
- Aspirate coating solution immediately before MEF plating.
- Thaw a frozen vial of Hcn4-GFP MEFs and wash x1 with pre-warmed fibroblast media at 500 x g for 5 min.
- Determine cell viability using trypan blue exclusion or similar dyes. Calculate the number of viable cells per mL of culture using the following formula:
% viable cells = [1.00 – (Number of blue cells/ Number of total cells)] x 100- Calculate total number of viable cells using the following formula:
Viable cells = % viable cells x dilution factor x 10,000 x total volume of cell suspension
- Calculate total number of viable cells using the following formula:
- Seed 3 x 104 cells per well onto a 24-well plate with previously prepared bovine collagen solution-fibronectin coverslip.
- 1 h before plating MEFs, prepare a 24-well plate for immunocytochemistry.
- Transduction and reprogramming of MEFs
NOTE: According to the manufacturer's notes, properly maintained Plat-E cells produce an average titer of 1 x 107 infection units/mL. Although the titer is not directly measured for each experiment, a GFP control is always included as a surrogate for infection efficiency. High GFP expression and intensity (GFP+ > 95%) typically correlates with successful GHMT-mediated generation of iCLMs.- Day 0: 24 h post-transfection, filter the PE retroviral medium through a 0.45 µm-pore size surfactant-free cellulose acetate filter and transfer to a 15 mL conical tube. Add polybrene to a final concentration of 8 µg/mL. Carefully replenish cells with 2 mL of fresh transfection medium.
NOTE: Cells easily detach from the plate if media is changed too rapidly. - Aspirate the medium of the cultured MEFs and add the freshly collected retroviral medium; it should yield 1.7 mL of media per well of a 6-well plate. Add ~800 µL per well of a 24-well plate. Return MEF plate to the incubator and incubate overnight.
- Day 1: Repeat steps 2.4.1 and 2.4.2. Discard cells after the 2nd virus collection. Return infected MEFs to the incubator and let them rest overnight.
- Day 2: 48 h post-induction, aspirate the cell conditioned media and wash x1 with 1x PBS. Add 500 µL pre-warmed iCLM media per well of a 24 well plate.
- Replace iCLM media every 2 – 3 days. Process plate 14 days after viral induction for immunocytochemistry (ICC) analysis of cardiac reprogramming.
- Day 0: 24 h post-transfection, filter the PE retroviral medium through a 0.45 µm-pore size surfactant-free cellulose acetate filter and transfer to a 15 mL conical tube. Add polybrene to a final concentration of 8 µg/mL. Carefully replenish cells with 2 mL of fresh transfection medium.
3. Immunostaining of Reprogrammed MEFs
- 14-days post-induction, carefully aspirate the media.
- Rinse each well with 300 µL of ice-cold 1x PBS. Aspirate excess solution.
- Fix cells with 250 µL of 4% paraformaldehyde (PFA) solution per well of a 24 well plate. Incubate 15 min at RT.
NOTE: Fixed cells can be stored in PBS at 4 °C for 1 – 2 weeks before staining. - Permeabilize cells by washing wells x3 with 300 µL 0.1% PBS-TritonX 100 (PBST). Incubate 5 min at RT between washes. Aspirate excess solution after the last wash.
- Block for 10 min at RT with 1x Universal Block Buffer at 300 µL/well.
- Prepare (ICC) staining buffer: Add 1:1 of 1x PBS and 1x Universal blocking buffer. Dilute primary antibodies in ICC staining buffer and incubate antibodies overnight at 4 °C. Refer to the material section for recommended dilutions.
- Stain one pair of slides with mouse α-actinin, chicken αGFP, and rabbit Nppa for iPM and iAM identification.
- Stain one pair of slides with mouse α-actinin, chicken αGFP, and rabbit Myl2 for iPM and iVM identification.
- The following day, wash wells x3 with 300 µL 0.1% PBST. Incubate 5 min at RT between washes. Aspirate excess solution after the last wash.
- Prepare the secondary antibody dilutions in ICC staining buffer. Refer to the material section for recommended dilutions. Incubate secondary antibodies 1 h at RT, protected from light.
- Stain all slides with the following secondary antibodies: mouse Alexa-555, chicken Alexa-488, and rabbit Alexa-647.
- Wash wells x3 with 300 µL 0.1% PBST. Incubate 5 min at RT between washes. Protect from light.
- Add 2.4 µL of mounting media containing 1.5 µg/mL of 4',6-diamidino-2-phenylindole (DAPI) to a glass microscope slide. Carefully remove the coverslip from the well of the 24-well plate, remove excess solution, and transfer to the glass slide with mounting media. Gently press the coverslip to remove excess volume and air.
- Seal slides with preferred nail polish or plastic sealant. Store mounted slides at 4 °C protected from light.
4. Identification of Cardiac Subtypes Using Confocal Microscopy
NOTE: For imaging, a confocal microscope equipped with at least 2 fluorescent detectors capable of spectral detection at 405, 488, 555, and 639 nm wavelengths is necessary in order to identify iPMs, iAMs, and iVMs. Image cells using a Plan-Apochromat 20X/0.75 objective or better. Using the manufacturer's image analysis software, scanning zoom images can achieve 40X-oil immersion quality images.
- Image library: take 8-bit images with DAPI, Alexa-488, Alexa-555, and Alexa-647 channels (ch.). Pixel dwell time of 6 s, 1024 frame size, line step at 2, and averaging of 2 is sufficient for high resolution images.
- For each slide, start from one edge and start scanning up and down in the red fluorescent channel (ch.) for α-actinin+ Sarcomere+ cells (Refer to Figure 3 and Figure 5A for examples). Sarcomere striations are easier to identify visually in the 555 nm wavelength.
- Once an α-actinin+ Sarcomere+ cell has been identified, switch to the green ch. and keep note if it is positive (iPM). Switch to the computer to assess the far-red 647 ch. (iAM or iVM).
NOTE: Cells that are α-actinin+/Sarcomere+/Hnc4-GFP+/Nppa–/Myl2– are designated as iPMs. GFP expression will be seen throughout the cell (Figure 4A). - Stain slides with α-actinin (mouse-Alexa555), Hcn4-GFP (chicken-Alexa488), Nppa (rabbit-Alexa647), and DAPI. Cells that are α-actinin+/Sarcomere+/Hnc4-GFP–/Nppa+ are iAM. Nppa staining will appear perinuclear and punctate (Figure 4B).
- Stain slides with α-actinin (mouse-Alexa555), Hcn4-GFP (chicken-Alexa488), Myl2 (rabbit-Alexa647). Cells positive for α-actinin+/Sarcomere+/Hnc4-GFP–/Myl2+ are iVMs. Myl2 staining will exhibit a striated form along the sarcomere filament. Due to variations in the quality of the staining and the Z-plane, striations may not always be easily visible (Figure 4C).
- Once an α-actinin+ Sarcomere+ cell has been identified, switch to the green ch. and keep note if it is positive (iPM). Switch to the computer to assess the far-red 647 ch. (iAM or iVM).
5. Quantification
NOTE: In order to assess the actual number of potentially reprogrammed MEFs, 2 wells of a 24-well plate are seeded in parallel to the experimental wells and are harvested one day after plating. The total number of cells plated is then determined by averaging the two wells. This becomes the actual total cells plated (aTotal).
- Sarcomere+
- Visually inspect each cell on a coverslip for proper α-actinin+/Sarcomere+ (Right panels Figure 3) and record (Figure 5B-i).
- Tabulate the total number of α-actinin+/Sarcomere+ on each coverslip and divide by the actual total cells plated (aTotal) (Figure 5B-iii). For example, if aTotal = 12,500 cells, and 100 cells were α-Actinin+/Sarcomere+ then, 0.8% of the plated MEFs were reprogrammed. An average reprograming experiment will yield 1% α-Actinin+/Sarcomere+ cells (Figure 5C).
- Subtype+
NOTE: For the following steps, refer to Figure 5B/C for a representative iCLM quantification workflow. Briefly, for each sarcomere+ cell, tabulate if it is unique for either subtype (Figure 5B-i). Calculate % Subtype (Figure 5B-iii) by dividing the number of subtype+ cells over the average sarcomere+ cells x 100 (Figure 5B-i). To calculate the absolute % subtype efficiency (Figure 5B-iv), divide the subtype+ cell number from Figure 5B-i by the total number of cells seeded x 100 (Figure 5B-ii).- For each of the α-actinin+/Sarcomere+ cells, assess if they are GFP+, Nppa+, or Myl2+.
- Tabulate total number of α-actinin+/Sarcomere+/Hnc4-GFP+/Nppa–/Myl2–, actinin+/Sarcomere+/Hnc4-GFP–/Nppa+, and α-actinin+/Sarcomere+/Hnc4-GFP–/Myl2+ cells (Figure 5B-i).
- To calculate the percentage of each subtype, divide the number of Subtype+ cells by the total number of α-actinin+/Sarcomere+ cells in that well and multiply by 100. GHMT generates iPMs, iAMs, and iVMs at roughly equal ratios (Figure 5B-iii).
- To calculate the absolute number of subtype+ cells, divide the total number of subtype+ cells for the experimental condition by aTotal and multiply by 100 (Figure 5B-ii). On average iPMs represent 0.3% of the total infected cell population, iAMs 0.3%, and iVMs 0.25% (Figure 5B-iv).
Representative Results
Taking advantage of the PM-specific reporter mouse, we developed a multiplex immunostaining strategy to identify diverse endogenous myocytes as depicted in Figure 1. Following the reprogramming steps shown in Figure 2, induction of subtype-specific CMs can be detected as early as day 421, albeit at a low-rate. By day 14, the experiment can be stopped and assessed for sarcomere organization (Figure 3) and subtype-specification (Figure 4). Figure 5 summarizes the workflow of slide preparation for ICC (Figure 5 Panel A), and the quantification of iCLM subtype-specific cells (Figure 5 Panel B/C).
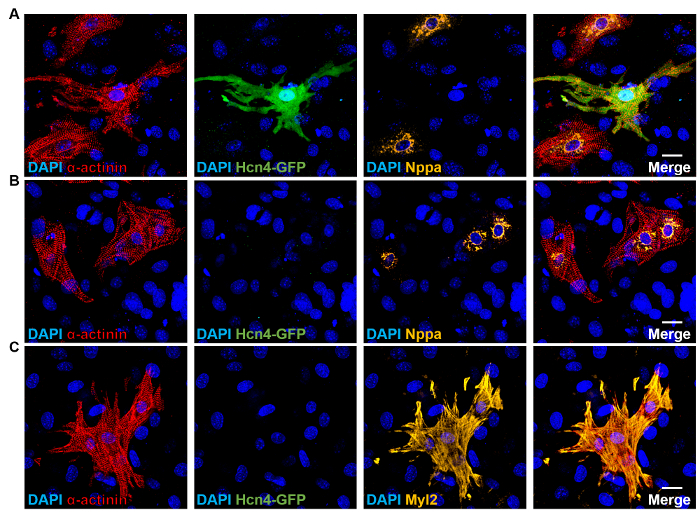
Figure 1: Subtype Diversity of Endogenous Cardiomyocytes. (A-B) Immunocytochemistry (ICC) staining of neonatal atrial cardiomyocytes from Hcn4-GFP reporter mice for α-actinin (sarcomere marker, red), Hcn4-GFP (PM marker, green), and Nppa (atrial marker, orange). (C) Immunocytochemistry staining of neonatal ventricular cardiomyocytes from Hcn4-GFP reporter mice for α-actinin (sarcomere marker, red), Hcn4-GFP (PM marker, green), and Myl2 (ventricular marker, orange). DAPI (blue): nuclear staining. Scale bars: 20 µm. Please click here to view a larger version of this figure.
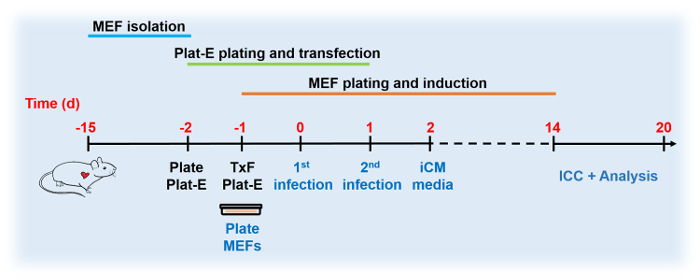
Figure 2: Reprogramming Timeline Schematic. Schematic representation of GHMT-induced Hcn4-GFP MEFs. The three major stages are depicted. Please click here to view a larger version of this figure.
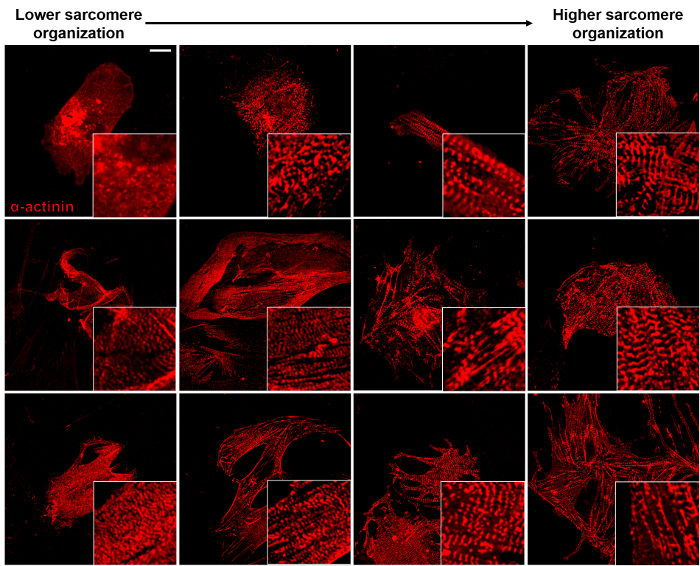
Figure 3: Degree of Sarcomere Organization. ICC staining of Hcn4-GFP MEFs 14 days after GHMT transduction for α-actinin (sarcomere marker, red) shows a diverse range of sarcomere organization. The degree of organization increases from left to right panels. Representative pictures of each level (n= 3). Scale bar: 20 µm. Please click here to view a larger version of this figure.
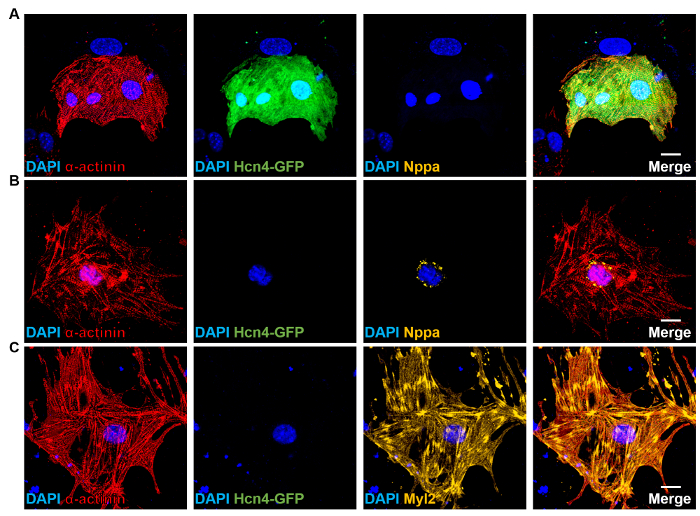
Figure 4: Subtype-specific Reprogrammed Cardiomyocytes. (A-C) ICC staining of GHMT-transduced Hcn4-GFP MEFs for α-actinin (sarcomere marker, red), Hcn4-GFP (PM marker, green), Nppa (atrial marker, orange), or Myl2 (ventricular marker, orange). DAPI (blue): nuclear staining. Scale bars: 20 µm. Please click here to view a larger version of this figure.
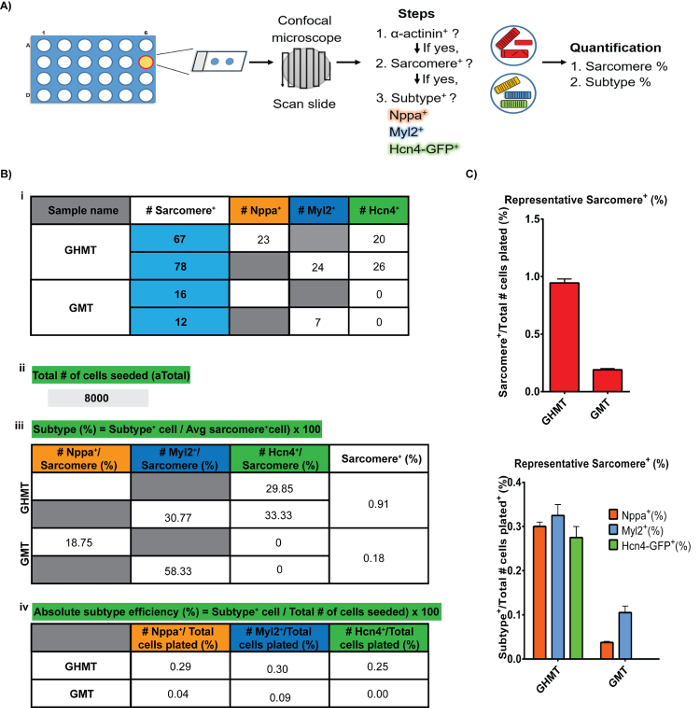
Figure 5: Image Acquisition and Analysis Workflow. Schematic representation for image analysis. Panel A depicts the priority order of assigning sarcomere+ and subtype-specificity to a cell. Panel B (i-iv) and C show the expected results from an average GHMT-iCLM experiment. Key points and formulas are shown in green. Please click here to view a larger version of this figure.
| iCLM media | ||
| Component | Volume (mL) | Final concentration |
| DMEM | 270 | |
| Medium 199 | 90 | |
| FBS | 50 | 10% |
| Insulin-Transferrin-Selenium G | 2.5 | 0.50% |
| MEM vitamin solution | 10 | 2% |
| MEM Amino Acids | 20 | 4% |
| Non-essential amino acids | 10 | 2% |
| Antibiotic-Antimycotics | 10 | 2% |
| B-27 supplement | 10 | 2% |
| Heat inactivated Horse Serum | 25 | 5% |
| Na-Pyruvate | 2.5 | 1.5 mM |
| Plat-E media (PE) | ||
| Component | Volume (mL) | Final concentration |
| DMEM | 450 | |
| FBS | 50 | 10% |
| Penicillin/Streptomycin | 5 | 1% |
| Puromycin | 0.05 | 1 μg/mL |
| Blasticidin | 0.5 | 10 μg/mL |
| Fibroblast medium (FB) | ||
| Component | Volume (mL) | Final concentration |
| DMEM | 450 | |
| FBS | 50 | 10% |
| Penicillin/Streptomycin | 5 | 1% |
| Glutamax | 5 | 1% |
| Transfection medium (TxF) – Filtered (0.45 μm) | ||
| Component | Volume (mL) | Final concentration |
| DMEM | 450 | |
| FBS | 50 | 10% |
| Immunocytochemistry (ICC) staining buffer | ||
| Component | Volume (mL) | Final concentration |
| 1x PBS | 5 | |
| 1x Universal blocking buffer | 5 | |
Table 1: Culture Medium. Table summary for the preparation of the several mediums used during GHMT-induced reprogramming.
| A) Cell seeding and transfection | ||||||
| Plate/Dish | Surface Area (cm2) | Seeding density (cells) | Growth medium (mL) | Total DNA amount to transfect (μg) | Transfection Reagent (μL) | Reduced Serum Media (μL) |
| 15 cm plate | 152 | 1.00E+06 | 20 | 25 | 75 | 600 |
| 10 cm plate | 55 | 5.50E+06 | 10 | 9 | 27 | 300 |
| 6 cm plate | 21 | 2.20E+06 | 4 | 3.5 | 10.5 | 105 |
| 6 well/x1 | 9 | 1.00E+06 | 2 | 2 | 6 | 60 |
| 12 well/x1 | 4 | 4.00E+05 | 1 | 0.5 | 1.5 | 15 |
| 24 well/x1 | 2 | 2.00E+05 | 0.5 | 0.3 | 0.9 | 9 |
| 48 well/x1 | 1 | 1.70E+05 | 0.25 | 0.15 | 0.45 | 4.5 |
| B) Fibroblast seeding and induction | ||||||
| Plate/Dish | Fibroblast seeding density (millions) | Approximate infection units for iCLM | ||||
| 6 cm plate | 0.22-0.33 | 5.00E+7 (~ 5 mL) | ||||
| 6 well/x1 | 0.1-0.15 | 3.00E+7 (~ 3 mL) | ||||
| 12 well/x1 | 0.04-0.06 | 1.30E+7 (~ 1 mL) | ||||
| 24 well/x1 | 0.02-0.03 | 6.50E+6 (~ 0.8 mL) | ||||
| 48 well | 0.001-0.015 | 3.00E+6 (~ 0.4 mL) | ||||
Table 2: Seeding, Transfection and Induction Formats. (A) Table summary for the plating and transfection of cells. (B) Seeding density and approximate infection units (or viral supernatant) needed to induce MEFs into cardiomyocyte-like cells.
Discussion
The present study provides a direct-reprogramming strategy for conversion of MEFs into a diverse set of cardiac subtypes via retrovirus-mediated expression of the cardiac transcription factors Gata4, Mef2c, Tbx5, and Hand2 (GHMT). Using a multiplex immunostaining approach in combination with a PM-specific reporter mouse, we are able to identify iAM, iVMs, and iPMs at single cell resolution. Such an assay allows for an experimental in vitro system capable of isolating the contributions of individual transcription factors towards subtype diversity and sarcomere development. In parallel, this could bring insight to new transcription factors or small molecules that bias iCLMs towards a particular lineage. Nevertheless, there are several critical steps for the successful completion of this assay. Below, we address the impact of viral titer, fibroblast quality, and imaging analysis in a general iCLM experiment.
In our study, we employ ecotropic-retroviruses to reprogram E12.5 MEFs. We noticed the retroviral titer is directly related to the quality of the cells. High passage number (> 35) and poor culturing techniques severely affect the quality of the retroviral particles; therefore, there are several considerations to keep in mind. Plat-E cells do not produce VSV-G pseudotyped virus, and are thus unable to withstand ultracentrifugation or freezing cycles24,25. In order to preserve the longevity of the cells, it is imperative to maintain the stock with antibiotic selection. However, they should be maintained in antibiotic free media during viral production. In our experience, the transfection reagent used here provides the highest transfection efficiencies in cells. If other transfection methods are to be used, comparing the viral titers produced is essential26. Although there are recommendations by the manufacturer to harvest the viral supernatant 48 h after transfection, we observed that two 24-h harvesting rounds yield higher reprogramming efficiencies while avoiding toxic effects usually associated with higher-titer viral preps. Furthermore, though several studies have shown the feasibility of commercial viral supernatant concentrators27, we have not employed these in our regular protocol in order to maintain a higher throughput.
In addition to high titer viral cocktails, fibroblast quality is of crucial importance for a successful reprogramming assay28. If timed correctly, freshly isolated MEFs should be utilized due to their higher efficiencies compared to frozen stocks. This could be related to the nature of retroviruses, as they need a highly-proliferative host in order to integrate29. Additionally, MEF seeding density plays a critical role. We have included a table with the seeding densities employed in our experiments (Table 2). Moreover, passaging the MEFs will also significantly decrease reprogramming efficiency.
Immunocytochemistry (ICC) is our standard technique for analysis of sarcomere organization and subtype specification. With the help of a PM-GFP reporter mouse, we were able to form an antibody panel for the detection of three major cardiac subtypes (AM, VM, and PM). However, due to constraints of antibody species availability and the limitation of 4-channels on a standard confocal microscope set-up, two coverslips per subtype are needed to quantify the prevalence of all three subtypes. One coverslip will stain for α-Actinin/GFP(Hcn4)/Myl2, and one for α-Actinin/GFP(Hcn4)/Nppa. Based on our previous observation that sarcomeric structure is a common characteristic of all CMs and a potential prerequisite for subtype specification21, the first step in our analysis is determining sarcomere+ cells. Yet, due to its subjective nature, establishing the level of sarcomere organization is perhaps the most difficult part of this assay; this can be limited by averaging multiple observer's quantifications or by developing computational cell segmentation software to automate the process30. Using endogenous cells as a point of reference, we discovered a threshold for well-organized sarcomere+ and utilized that to score iCLMs (Figure 3). Given these parameters, an average experiment will give rise to 20 – 30% α-Actinin+ cells but only 1% are α-Actinin+/Sarcomere+. Of the 1% sarcomere+ cells, ~ 30% will be Nppa+, Myl2+,or Hcn4-GFP+.
Given that cardiomyocytes are structurally complex, population-based gene expression (e.g. qRT-PCR) or flow cytometry analyses cannot capture the intricate morphological changes that occur during iCLM reprogramming. In contrast, patch clamping and calcium transient imaging are highly stringent single-cell functional assays, but specialized skills and equipment are required to conduct these experiments. Thus, the described methodology is unique in that it provides a straightforward approach to study key structural and functional parameters of iCLM reprogramming without significantly compromising throughput.
Despite the many recent advances in direct reprogramming, much work remains to be done to better understand the molecular mechanisms that regulates cardiac reprogramming, and more specifically, subtype specification. These mechanisms will become especially important to translate direct reprogramming for clinical applications. As such, in this study we describe a platform capable of directly modulating discrete parameters to assess the contribution towards sarcomere development, subtype specification, and iCLM maturity. Moreover, this system can be further developed to work in a high-throughput format allowing for complex screening of small molecules or extracellular matrixes for the next step in regenerative cardiology.
Divulgations
The authors have nothing to disclose.
Acknowledgements
A.F.-P. was supported by the National Science Foundation Graduate Research Fellowship under Grant No.2015165336. N.V.M was supported by grants from the NIH (HL094699), Burroughs Wellcome Fund (1009838), and the March of Dimes (#5-FY14-203). We acknowledge Young-Jae Nam, Christina Lubczyk, and Minoti Bhakta for their important contributions to protocol development and data analysis. We also thank John Shelton for valuable technical input and members of the Munshi lab for scientific discussion.
Materials
| DMEM | Sigma | D5796 | Component of iCLM media, Plat-E media, fibroblast, and Transfection media |
| Medium 199 | Thermo Fisher Scientific | 11150059 | Component of iCLM media |
| Fetal bovine serrum (FBS) | Sigma | F2442 | Component of iCLM media, Plat-E media, fibroblast, and Transfection media |
| Insulin-Transferrin-Selenium G | Thermo Fisher Scientific | 41400-045 | Component of iCLM media |
| MEM vitamin solution | Thermo Fisher Scientific | 11120-052 | Component of iCLM media |
| MEM amino acids | Thermo Fisher Scientific | 1601149 | Component of iCLM media |
| Non-Essential amino acids | Thermo Fisher Scientific | 11140-050 | Component of iCLM media |
| Antibiotic-Antimycotics | Thermo Fisher Scientific | 15240062 | Component of iCLM media |
| B-27 supplement | Thermo Fisher Scientific | 17504044 | Component of iCLM media |
| Heat-Inactivated Horse Serum | Thermo Fisher Scientific | 26050-088 | Component of iCLM media |
| NaPyruvate | Thermo Fisher Scientific | 11360-70 | Component of iCLM media |
| Penicillin/Streptomycin | Thermo Fisher Scientific | 1514022 | Component of Plat-E media and fibroblast media |
| Puromycin | Thermo Fisher Scientific | A11139-03 | Component of Plat-E media |
| Blasticidin | Gemini Bio-Products | 400-128P | Component of Plat-E media |
| Glutamax | Thermo Fisher Scientific | 35050-061 | Component of Fibroblast media |
| Confocal laser scanning LSM700 | Zeiss | For confocal analysis | |
| FuGENE 6 transfection Reagent | Promega | E2692 | Transfection reagent |
| Opti-MEM Reduced Serum Medium | Thermo Fisher Scientific | 31985-070 | Transfection reagent |
| Polybrene | Millipore | TR-1003-G | Induction reagent. Use at a final concentration of 8um/mL |
| Platinium-E (PE) Retroviral Packagin Cell Line, Ecotropic | CellBiolabs | RV-101 | Retroviral pacaking cell line |
| Trypsin 0.25% EDTA | Thermo Fisher Scientific | For MEFs and Plat-E dissociation | |
| Mouse anti α-Actinin (Clone EA-53) | Sigma | A7811 | Antibody for confocal analysis. Use at 1:200 |
| Chicken anti-GFP IgY | Thermo Fisher Scientific | A10262 | Antibody for confocal analysis. Use at 1:200 |
| Rabbit Pab anti-NPPA | Abgent | AP8534A | Antibody for confocal analysis. Use at 1:400 |
| Rabbit Pab anti Myl2 IgG | ProteinTech | 10906-1-AP | Antibody for confocal analysis. Use at 1:200 |
| Vectashield solution with DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride) | Vector Labs | H-1500 | Dye for confocal analysis |
| Superfrost Plus Microscope slides | Thermo Fisher Scientific | 12-550-15 | 25 x 75 x 1.0 mm |
| BioCoat Fibronectin 12mm coverslips | NeuVitro Corp | GG-12-1.5 | Coverslips for confocal analysis |
| 100um cell strainer | Thermo Fisher Scientific | 08-771-19 | |
| 0.45um Syringes filters SFCA 25MM | Thermo Fisher Scientific | 09-740-106 | For virus filtration |
| 6ml Syringes | Covidien | 8881516937 | For virus filtration |
| Goat anti-Chicken IgY (H&L) A488 | Abcam | AB150169 | Secondary antibody for confocal analysis. Use at 1:400 |
| Donkey anti-rabbit A647 IgG(H+L) | Thermo Fisher Scientific | A31573 | Secondary antibody for confocal analysis. Use at 1:400 |
| Goat anti-mouse IgG(H+L) A555 | Thermo Fisher Scientific | A21422 | Secondary antibody for confocal analysis. Use at 1:400 |
| Triton X-100 | Sigma | 93443-100ml | For cell permeabilization |
| Dulbecco's PBS without CaCl2 and MgCl2 (D-PBS) | Sigma | D8537 | |
| Power Block 10X Universal Blocking reagent | Thermo Fisher Scientific | NC9495720 | Dilute to 1X in H20 |
| 16% Paraformaldehyde aqueous solution (PFA) | Electro Microscopy Sciences | 15710 | Use at 4% diluted in dH20 |
| 6 cm plates | Olympus | 25-260 | |
| 6-well plates | Genesee Scientific | 25-105 | |
| 24-well plates | Genesee Scientific | 25-107 | |
| 10 cm Tissue culture dishes | Corning | 4239 | |
| 15 cm Tissue culture dishes | Thermo Fisher Scientific | 5442 | |
| 15 ml Conical tubes | Corning | 4308 | |
| 50 ml Conical tubes | Corning | 4249 | |
| 0.4% Trypan blue solution | Sigma | T8154 | For viability |
| Ethyl Alcohol 200 proof | Thermo Fisher Scientific | 7005 | |
| Bleach | Thermo Fisher Scientific | 6009 |
References
- Sissman, N. J. Developmental landmarks in cardiac morphogenesis: comparative chronology. Am J Cardiol. 25 (2), 141-148 (1970).
- Buckingham, M., Meilhac, S., Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 6 (11), 826-835 (2005).
- Ali, S. R., et al. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 111 (24), 8850-8855 (2014).
- Bergmann, O., et al. Evidence for cardiomyocyte renewal in humans. Science. 324 (5923), 98-102 (2009).
- Senyo, S. E., et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 493 (7432), 433-436 (2013).
- Lin, Z., Pu, W. T. Strategies for cardiac regeneration and repair. Sci Transl Med. 6 (239), 239rv231 (2014).
- Writing Group, M., et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 133 (4), e38-e360 (2016).
- Takahashi, K., Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4), 663-676 (2006).
- Song, K., et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 485 (7400), 599-604 (2012).
- Ieda, M., et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 142 (3), 375-386 (2010).
- Qian, L., et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 485 (7400), 593-598 (2012).
- Fu, J. D., et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Reports. 1 (3), 235-247 (2013).
- Zhou, H., Dickson, M. E., Kim, M. S., Bassel-Duby, R., Olson, E. N. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc Natl Acad Sci U S A. 112 (38), 11864-11869 (2015).
- Zhou, Y., et al. Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming. Cell Stem Cell. 18 (3), 382-395 (2016).
- Zhao, Y., et al. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat Commun. 6, 8243 (2015).
- Miki, K., Yoshida, Y., Yamanaka, S. Making steady progress on direct cardiac reprogramming toward clinical application. Circ Res. 113 (1), 13-15 (2013).
- Atkinson, A., et al. Anatomical and molecular mapping of the left and right ventricular His-Purkinje conduction networks. J Mol Cell Cardiol. 51 (5), 689-701 (2011).
- Bootman, M. D., Smyrnias, I., Thul, R., Coombes, S., Roderick, H. L. Atrial cardiomyocyte calcium signalling. Biochim Biophys Acta. 1813 (5), 922-934 (2011).
- Miquerol, L., Beyer, S., Kelly, R. G. Establishment of the mouse ventricular conduction system. Cardiovasc Res. 91 (2), 232-242 (2011).
- Später, D., Hansson, E. M., Zangi, L., Chien, K. R. How to make a cardiomyocyte. Development. 141 (23), 4418-4431 (2014).
- Nam, Y. J., et al. Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development. 141 (22), 4267-4278 (2014).
- Conner, D. A. . Current Protocols in Molecular Biology. , (2001).
- Jozefczuk, J., Drews, K., Adjaye, J. Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J Vis Exp. (64), (2012).
- Burns, J. C., Friedmann, T., Driever, W., Burrascano, M., Yee, J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 90 (17), 8033-8037 (1993).
- Ichim, C. V., Wells, R. A. Generation of high-titer viral preparations by concentration using successive rounds of ultracentrifugation. J Transl Med. 9, 137 (2011).
- Qian, L., Berry, E. C., Fu, J. D., Ieda, M., Srivastava, D. Reprogramming of mouse fibroblasts into cardiomyocyte-like cells in vitro. Nat Protoc. 8 (6), 1204-1215 (2013).
- Yang, R., et al. Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors. Nat Commun. 5, 5807 (2014).
- Muraoka, N., Ieda, M. Direct reprogramming of fibroblasts into myocytes to reverse fibrosis. Annu Rev Physiol. 76, 21-37 (2014).
- Coffin, J. M., Hughes, S. H., Varmus, H. E., Coffin, J. M., Hughes, S. H., Varmus, H. E. . Retroviruses. , (1997).
- Bass, G. T., et al. Automated image analysis identifies signaling pathways regulating distinct signatures of cardiac myocyte hypertrophy. J Mol Cell Cardiol. 52 (5), 923-930 (2012).

