Subpial Adeno-associated Virus 9 (AAV9) Vector Delivery in Adult Mice
Summary
The goal of the present study was to develop and validate the potency and safety of spinal adeno-associated virus 9 (AAV9)-mediated gene delivery by using a novel subpial gene delivery technique in adult mice.
Abstract
The successful development of a subpial adeno-associated virus 9 (AAV9) vector delivery technique in adult rats and pigs has been reported on previously. Using subpially-placed polyethylene catheters (PE-10 or PE-5) for AAV9 delivery, potent transgene expression through the spinal parenchyma (white and gray matter) in subpially-injected spinal segments has been demonstrated. Because of the wide range of transgenic mouse models of neurodegenerative diseases, there is a strong desire for the development of a potent central nervous system (CNS)-targeted vector delivery technique in adult mice. Accordingly, the present study describes the development of a spinal subpial vector delivery device and technique to permit safe and effective spinal AAV9 delivery in adult C57BL/6J mice. In spinally immobilized and anesthetized mice, the pia mater (cervical 1 and lumbar 1-2 spinal segmental level) was incised with a sharp 34 G needle using an XYZ manipulator. A second XYZ manipulator was then used to advance a blunt 36G needle into the lumbar and/or cervical subpial space. The AAV9 vector (3-5 µL; 1.2 x 1013 genome copies (gc)) encoding green fluorescent protein (GFP) was then injected subpially. After injections, neurological function (motor and sensory) was assessed periodically, and animals were perfusion-fixed 14 days after AAV9 delivery with 4% paraformaldehyde. Analysis of horizontal or transverse spinal cord sections showed transgene expression throughout the entire spinal cord, in both gray and white matter. In addition, intense retrogradely-mediated GFP expression was seen in the descending motor axons and neurons in the motor cortex, nucleus ruber, and formatio reticularis. No neurological dysfunction was noted in any animals. These data show that the subpial vector delivery technique can successfully be used in adult mice, without causing procedure-related spinal cord injury, and is associated with highly potent transgene expression throughout the spinal neuraxis.
Introduction
The use of AAV vectors to treat a variety of spinal cord and CNS neurodegenerative disorders is becoming a well-accepted platform to effectively upregulate or silence the expression of gene(s) of interest. One of the key limitations to the more effective utilization of this technology to treat CNS/spinal cord disorders is the limited ability to deliver AAV vector(s) to the deep brain or spinal cord parenchyma in adult mammals.
It was demonstrated, for example, that the systemic delivery of AAV9 in adult rodents, cats, or non-human primates is only moderately effective at inducing transgene expression in neurons in the brain and spinal cord1,2,3. The more effective intrathecal delivery of AAV9 vectors has also been shown to lead to only limited transgene expression in anatomically-defined pools of neurons. More specifically, it has been demonstrated that cisternal or lumbo-sacral intrathecal AAV9 delivery in non-human primates, pigs, or rodents leads to a high level of transgene expression in spinal α-motoneurons and segmental dorsal root ganglion neurons. However, minimal or no expression in spinal interneurons or ascending or descending axons in the white matter is seen4,5,6,7. Collectively, these data show that a highly effective biological-anatomical barrier exists, which prevents the diffusion of intrathecally delivered AAV into deeper spinal parenchyma.
In a previous study using adult rats and pigs, a novel subpial vector delivery technique was developed8. Using this approach, highly potent and multi-segmental transgene expression was demonstrated after a single-bolus subpial AAV9 delivery. Intense GFP expression was consistently seen in neurons, glial cells, and descending/ascending axons through the injected spinal segments. This study demonstrated for the first time that the pia mater represents the primary barrier limiting effective AAV9 diffusion into the spinal parenchyma from the intrathecal space. While this previously developed technique and subpial injection device is relatively easy to use in large rodents (like rats) or adult pigs, the system is not suitable for use in small animals, such as adult mice. Because of the high number of available transgenic mouse models of a variety of neurodegenerative disorders, there is a clear need for the development of an effective spinal-parenchymal vector delivery technique in mice. The availability of such a technique would permit the study of the effect of specific gene silencing (e.g., using shRNA) or upregulation using cell-non-specific (e.g., cytomegalovirus-CMV or Ubiquitin) or cell-specific (e.g., synapsin or glial fibrillary acidic protein (GFAP)) promoters during early postnatal development or under diseased conditions.
Accordingly, in the present study, we have developed and validated a miniature subpial vector delivery system that can effectively be used in adult mice. Similarly, as in previous rat and pig studies, this work demonstrates potent transgene expression throughout the spinal parenchyma after a single-bolus subpial AAV9 delivery in mice. The simplicity of this approach, the very good tolerability of injected mice to subpial AAV9 delivery, and the high potency of transgene expression in the spinal parenchyma suggest that this technique can effectively be implemented in any laboratory setting and used in experiments targeting spinal gene expression.
Protocol
These studies were carried out under a protocol approved by the Institutional Animal Care and Use Committee of the University of California, San Diego and were in compliance with the Association for Assessment of Laboratory Animal Care guidelines for animal use. All studies were performed in such a manner as to minimize group size and animal suffering.
1. General Animal and Surgical Preparation
- Before starting the surgical procedure, thaw the virus (AAV9-UBI-GFP; 5 µL aliquots)8. Prepare a 5% dextran (10,000 MW) solution by mixing dextran powder in distilled water. Mix the virus solution with 5% dextran solution 1:1 to a final dextran concentration of 2.5%.
- Store the virus solution on ice (4 °C).
- Use adult C57BL/6J mice (male and female, 20-30 g). Anesthetize the mice using 5% isoflurane (in O2, 1 L/min) and maintain them at 2-3% inhaled isoflurane (in O2, 1 L/min) by nose cone during surgery, depending on the breathing rate and paw pinch response.
- Shave the back of the animals with shaving clippers and clean the skin with 2% chlorhexidine.
- In chronic recovery studies follow a strict sterile technique.
- If lumbar subpial injections are to be performed, cut the skin overlaying the Th8-L1 vertebrae with a scalpel and detach the paravertebral muscle from Th10-12 spinal vertebrae using scissors.
- Mount the animal into a standard stereotaxic frame using mouse spinal clamps.
- Shave the both sides of the lamina of the Th10-12 vertebrae using a dental drill (drill bit: 0.9 mm, speed: 20,000 rpm) until cracks appear.
- Remove cracked bone fragments with forceps and expose the dorsal surface of the lumbar spinal cord.
- Cut open the dura about 1 cm using a 30 G stainless steel needle and forceps.
- If cervical subpial injections are to be performed, incise the dorsal neck skin 1.5-2 cm using scissors and expose the C1-C2 segments.
- Remove the atlanto-occipital membrane of the cisterna magna using a 23G stainless steel needle and forceps.
- Clean the incision site of any tissue and bone debris using cotton swabs.
- Cut open the dura about 2-3 mm using a 30 G stainless steel needle and forceps.
2. Opening the Pial Membrane and Inserting the Subpial Needle for AAV9 Delivery
- Mount the 34 G pia-penetrating needle into the Z-arm of an XYZ manipulator using a glass capillary holder (Figure 1A and B).
NOTE: To manufacture the pia-penetrating needle, the original beveled tip of the 34G needle is sharpened using a glass capillary beveller with diamond abrasive plate – coarse (5.0 µm to 50 µm tip sizes) and grinding angle of 15 – 20°. The tip of the needle (1 mm length, measured from the tip) is then gently bent to about 90° (Figure 1B, left insert). - Using a surgical dissecting scope, set to 8-10 X magnification, penetrate the pia with the pia-penetrating needle by about 1 mm (Figure 1C) using the X-arm.
- Keep the angle of the penetrating needle to the tissue surface at 5-10°.
- After the pia opening, remove the pia-penetrating needle horizontally from the subpial space (Figure 1 D) using the X-arm.
NOTE: Remember the penetrated site by a landmark, such as a blood vessel. - Load a blunt 36G injection needle with AAV9-UBI-GFP virus using a 50-µL microsyringe connected to the injection needle with PE-10 or PE-20 tubing.
- Mount the needle into the Z-arm of a second XYZ manipulator (Figure 1A and B) using a glass capillary holder (Figure 1B, right insert).
NOTE: To manufacture the subpial AAV9 injection needle, the blunt tip of a 36 G needle is polished using a glass capillary beveller with diamond abrasive plate – coarse (5.0 µm to 50 µm tip sizes) to remove the sharp edges. The tip of the needle (2-3 mm length, measured from the tip) is then gently bent to about 90°. The pia-penetrating and subpial injection needles are inserted into 1-2 cm-long 20G slave stainless steel tubing (10 mm from the end of the needle) and glued with epoxy. The use of 20 G (0.91 mm-diameter) tubing is required for a secure attachment to the glass capillary holder. - By manipulating the X, Y, and Z arms of the second manipulator, position the tip of the AAV9 injection needle into the pia-penetrated site and then advance it about 2-3 mm into the subpial space through the previous pial membrane opening using the X-arm ( Figure 1E and F).
NOTE: 1) The AAV9-UBI-GFP is prepared according to previously reported protocols9,10, and the final titers are adjusted to 1.2 x 1013 genome copies per mL (gc/mL). 2) There is no need to mark the site of the pial opening because it is readily identifiable (Figure 1D). - Inject the AAV9-UBI-GFP (1.5, 3, or 5 µL) into the subpial space using a 50 µL microsyringe (see Table 1 for experimental groups).
- Remove the injection needle from the subpial space after the AAV9-UBI-GFP injection is complete.
- Close the muscle and skin using 4.0 monofilament suture and surgical clips.
NOTE: There is no need to seal the open vertebra. - Allow the animals to recover on a heating pad.
- For pain control inject Buprenorphine 0.05 mg/kg/sc every 12 h for 2-3 days post-surgery.
3. Perfusion-fixation, Tissue Cryoprotection, and Immunofluorescence Staining
- At a predetermined time point after the subpial AAV9 injections, deeply anesthetize the mice with euthanasia solution (see the Table of Materials, 0.3 mL) and transcardially perfuse them with 20 mL of heparinized saline followed by 20 mL of 4% paraformaldehyde in PBS.
- Dissect the spinal cords and brains using a bone rongeur and post-fix them in 4% formaldehyde in PBS overnight at 4 °C.
- Cryoprotect the spinal cords and brains with 30% sucrose in PBS for a minimum of 5-7 days.
- Cut coronal, transverse, or longitudinal/horizontal frozen sections (30 µm-thick) on a cryostat and store them in PBS at 4 °C.
4. Immunofluorescence Staining of Spinal Cord and Brain Sections (See the Table of Materials)
- Incubate free-floating sections in primary antibodies overnight.
- After incubation with primary antibodies, wash the sections three times in PBS and incubate with fluorescence-conjugated donkey anti-rabbit, donkey anti-chicken, and donkey anti-goat secondary antibodies.
- Mount the sections on microscopy slides, dry them at room temperature, and cover them with an anti-fade medium.
- Capture images using epifluorescence fluorescence microscope (objectives: 10X, NA-0.3; 20X, NA-0.8; and 63X, NA-1.4).
Representative Results
Potent Transgene Expression in Subpially AAV9-injected Segments:
The analysis of transgene (GFP) expression in spinal cord sections at 14 days after AAV9 delivery showed AAV9-dose dependent GFP expression throughout the spinal parenchyma. First, two bilateral 3 µL injections of AAV9-UBI-GFP injected into the upper lumbar subpial space were associated with the near-complete infection of the white and gray matter in the whole lumbar spinal cord, extending to the upper thoracic segments (Figure 2A and 2B, left and middle columns). Two bilateral 1.5 µL injections of AAV9-UBI-GFP into the upper lumbar subpial space were associated with a similar near-complete infection of the white and gray matter in the whole lumbar spinal cord (as seen after injections of 3 µL); however, the mid-thoracic segments showed only occasionally infected neurons (Figure 2B, right column). Staining with α-motoneuron-specific (ChAT) and neuron-specific (NeuN) antibodies showed consistent GFP expression in the entire population of lumbar α-motoneurons (Figure 2C) and interneurons localized in the dorsal horn (Figure 2D), intermediate zone (Figure 2E), and ventral horn (Figure 2F). Second, two bilateral cervical injections of AAV9 (5 µL for each injection) led to similar GFP expression in the white and gray matter in the whole cervical spinal cord (gray and white matter) and in the upper thoracic segments (Figure 3A and 3B). Analysis of lumbar spinal cord sections in the same animals showed a high density of GFP+ descending axons terminating in the vicinity of the lumbar GFP-negative α-motoneurons and interneurons (Figure 3C and 3D). The delivery of two bilateral cervical and two bilateral upper lumbar injections of AAV9 (5 µL for each injection) was associated with GFP expression in the entire spinal cord, from the upper cervical to sacral segments, and was homogenously present in the white and gray matter (Figure 3E-3H).
Retrograde and Anterograde Transport-mediated Transgene Expression in Supraspinal Motor and Sensory Centers:
Widespread GFP expression in the lumbar or cervical spinal cord after subpial AAV9 delivery was associated with robust retrograde and anterograde infection-mediated GFP positivity in the supraspinal descending axons and their projecting neurons and in axons and terminals of ascending tracts (Figure 4). Thus, intense GFP positivity was seen in neurons localized in the reticular formation (RF), nucleus ruber (NR), and motor cortex (MC) (Figure 4B-4D). Similarly, clear GFP immunoreactivity was seen in the terminals of the spinocerebellar (SCT), spinoreticular, and spinothalamic tracts (STT) (Figure 4B-4D).
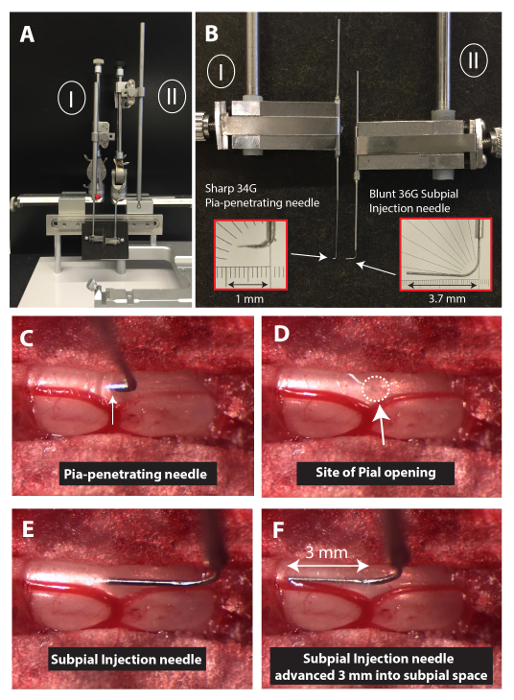
Figure 1: Experimental Setup to Perform Spinal Subpial Injections in an Adult Mouse. (A) To perform spinal subpial injections in adult mice, two separate XYZ manipulators are used (I and II). (B) The Z-arm of each manipulator holds a glass capillary holder. One capillary holder holds the pia-penetrating 34 G needle, and the second one holds the blunt 36 G subpial injection needle. (C, D, E, and F) Images depicting the position of the pia-penetration needle just after the pia puncture (C; dura matter is already removed), after removal of the pia-penetrating needle (D), after the insertion of the tip of the subpial injection needle (E), and after advancing the subpial injection needle 3 mm into the subpial space (F). Please click here to view a larger version of this figure.
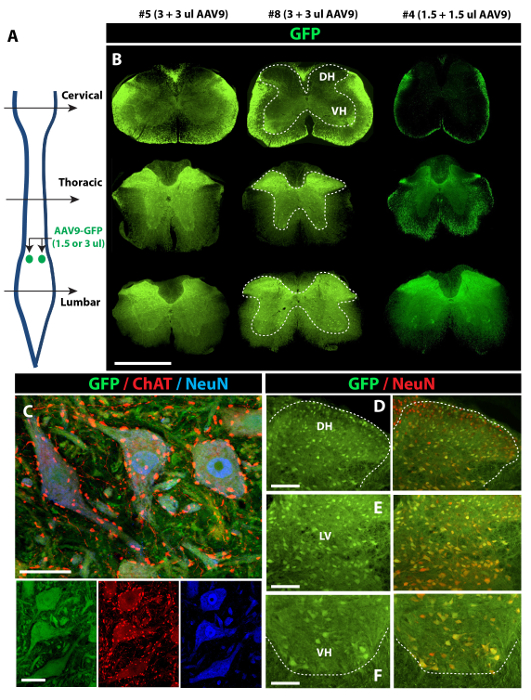
Figure 2: Potent Spinal Parenchymal GFP Expression after Lumbar Subpial AAV9-UBI-GFP Delivery in Adult Mice. (A) Two bilateral injections of AAV9-UBI-GFP (1.5 or 3 µL injections each) were delivered into the upper lumbar subpial space, and animals were perfusion-fixed 14 days after AAV9 delivery. (B) Intense GFP expression in the gray (inside the dotted area) and white matter, extending from the lumbar to the upper thoracic segments, can be seen in animals injected with 3 + 3 µL of AAV9 (left and middle columns). (C, D, E, and F) Co-staining of transverse spinal cord sections taken from the lumbar enlargement in animals injected with 3 + 3 µL of AAV9 show GFP expression in virtually all ChAT (α-motoneuron marker)-positive α-motoneurons (C and F) and NeuN-positive interneurons in the dorsal horn (D) and intermediate zone (E). Scale bars = 1,000 µm (B); 30 µm (C); 100 µm (D-F). DH: dorsal horn; LV: lamina V; VH: ventral horn. Please click here to view a larger version of this figure.
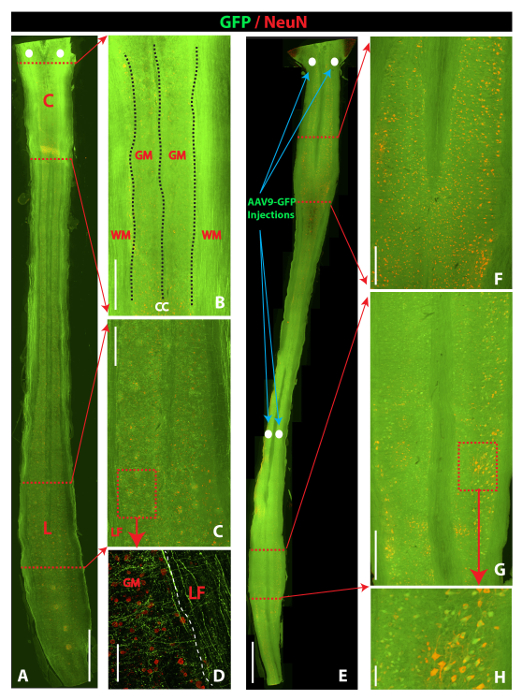
Figure 3: Comparison of Spinal GFP Expression After Spinal Subpial Cervical Versus Spinal Subpial Cervical plus Subpial Lumbar AAV9-UBI-GFP Delivery in Adult Mice. (A, B, C and D) Horizontal section cut through the whole length of the spinal cord in an animal that previously received upper cervical subpial injections of AAV9-UBI-GFP (5 + 5 µL). Intense GFP expression in the white and gray matter in the cervical region can be seen (B). In the lumbar spinal cord, a high density of GFP+ descending axons in the lateral funiculus (LF) and the gray matter between NeuN-positive but GFP-negative neurons can be identified (C and D). (E, F, G, and H) Horizontal section cut through the whole length of the spinal cord in an animal previously receiving upper cervical and upper lumbar subpial injections of AAV9-UBI-GFP (5 + 5 µL at the cervical and lumbar level). Intense GFP fluorescence throughout the whole spinal cord (white and gray matter) can be seen. Individual NeuN-stained interneurons and α-motoneurons co-expressing GFP can readily be identified in cervical (F) and lumbar (G and H) spinal gray matter. Scale bars = 2,000 µm (A and E); 500 µm (B, C, F, and G); 100 µm (D and H). C: cervical; L: lumbar; WM: white matter; GM: gray matter; LF: lateral funiculus). Please click here to view a larger version of this figure.
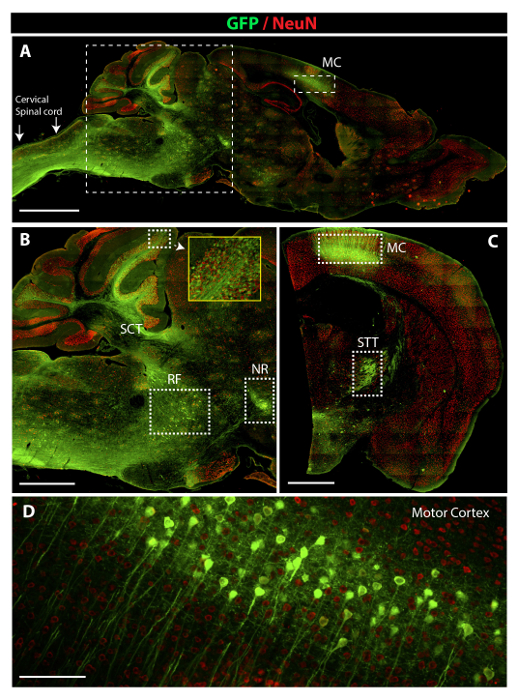
Figure 4: Potent Retrograde and Anterograde AAV9-UBI-GFP-mediated GFP Expression in Brain Motor and Sensory Centers. (A) A low-power image depicting the presence of intense GFP positivity in the cervical spinal cord, medulla oblongata, cerebellum, and motor cortex (MC). (B) A higher-power image taken from a sagittal brain section and showing the presence of GFP fluorescence in neurons in the reticular formation (RF), nucleus ruber (NR), and axons of the spino-cerebellar tract (SCT). (C) A lower-power image taken from coronal brain sections showing the presence of GFP fluorescence in pyramidal neurons in the motor cortex (MC) and in the terminals of the spinothalamic tract in areas of the reticular thalamic nuclei (STT). (D) A high-power image demonstrating an intense GFP expression in pyramidal neurons in the motor cortex. Scale bars = 2,000 µm (A); 1,000 µm (B and C) ; 60 µm (D). Please click here to view a larger version of this figure.
| Experimental Groups | Site/level of AAV9 delivery (*) | Volume of AAV9 inj, infusion rate | Survival time | Tissue analysis |
| Group A (n = 7) | C2 (bilateral) | 5 µL/5 min | 14 days | Brain + spinal cord |
| Group B (n = 12) | L1-L2 (bilateral) | 1.5 µL or 3µL, 60 sec/µL | 14 days | Brain + spinal cord |
| Group C (n = 6) | C2 + L1-L2 (bilateral at each level) | 5 µL/5 min | 14 days | Spinal cord |
| * – bilateral = two subpial injections one delivered into the right and one to the left subpial space of injected segment(s) are performed. | ||||
Table 1: Experimental Groups. All experiments were performed in adult C57BL/6J mice.
Discussion
The current study describes a technique of subpial vector (AAV9) delivery in adult mice. As demonstrated in the accompanying video, this approach and technique can effectively be used, provided that the required instruments and pia-penetrating needle and subpial injection needle are properly manufactured, according to the established and tested specifications.
Critical Technical Variables in Performing a Consistent and Safe Subpial Injection in Mice:
As demonstrated, a subpial injection can readily be performed in adult anesthetized mice after a dorsal cervical or lumbar laminectomy. There are several critical technical variables that define the success of the subpial injection technique. First, the tip of 34 G pia-penetrating needle needs to be consistently sharpened using a beveller to assure the smooth puncture of the pia. Any inconsistencies in the technique on how the needle is sharpened can lead to difficulties during pia penetration and/or can cause spinal injury by using too much force to puncture the pia. Under normal circumstances (as shown in the video and in Figure 1C and D), the site of the pia opening shows no sign of spinal cord injury or subpial bleeding. In some cases, some subpial bleeding can be seen, but this was found not be associated with neurological dysfunction or altered gene expression after AAV9 delivery. Second, it is important to keep the surgical site free of blood after the dura is removed by using electrocautery. Any spotty blood residue can obscure the reliable identification of the pia surface, even if a surgical microscope is used. As shown in Figure 1C-F, a blood-free laminectomy site can be effectively maintained using small gel-foam pieces to cover the laminectomized vertebra and surrounding soft tissue. Third, particular attention needs to be paid to the proper placement of anesthetized animals into the spinal frame and to the immobilization of the spinal column using spinal clamps. Using too much pressure during the placement of spinal clamps can cause vertebral rupture; using insufficient pressure can lead to a progressive loosening of the immobilized vertebra, which typically happens once a dental drill is used to perform the laminectomy.
The volume of subpially-injected AAV9 correlates with the magnitude of rostro-caudal transgene expression. Thus, the use of two lumbar bilateral subpial AAV9 injections (3 + 3 µL or 5 + 5 µL) was associated with consistent GFP expression in the whole lumbar gray and white matter, extending up to the upper thoracic segments. There was minimal variability across animals used. AAV9 injection volumes of 1.5 + 1.5 µL led to similar GFP expression in the segments localized in the vicinity of injections; however, the spread of the virus and the resulting GFP expression was limited in the thoracic segments (Figure 2B). Based on this data, the volume of delivered AAV9 is the critical variable determining the spread of subpially injected AAV9 in the rostrocaudal direction.
Limitation of the Subpial Gene Delivery Technique:
One of the relative limitations of the subpial injection technique (in comparison to intrathecal delivery) is the more invasive nature of this approach when a dorsal laminectomy must be performed to allow access to the dorsal surface of the spinal cord. However, the potent transgene expression seen throughout the spinal cord and in the supraspinal brain centers appears to demonstrate the clear advantage over intrathecal AAV delivery, which is characterized by selective transgene expression in a subpopulation of α-motoneurons and primary afferents (but is not present in neurons in the deeper spinal cord laminae)8.
Future Applications of the Subpial Gene Delivery Technique:
Similarly, as demonstrated in previous studies in adult rats and pigs, highly effective transgene expression can be achieved in the spinal white and gray matter in adult mice by using subpial AAV9 delivery. In addition, because of the relatively smaller dimensions (particularly the length) of the mouse spinal cord, the delivery of AAV9 at only two spinal cord levels (upper cervical and upper lumbar) is sufficient to lead to near-complete spinal cord infection. This characteristic can have an important implication in designing specific disease-modifying treatment approaches by either targeted gene silencing or upregulation.
For example, by using shRNA-silencing vectors, it is expected that a highly effective decrease in the expression of mutated genes (e.g., SOD in the case of the inherited form of ALS) will be achieved throughout the spinal cord as well as in spinally-projecting brain motor nuclei. Second, because of the highly effective infection of the spinal white matter axons, therapeutic genes (e.g., encoding growth factors) can be upregulated to promote axonal sprouting in spinal trauma-injured animals. Third, by manipulating the volume or the titer of subpially-delivered virus as well as the site of subpial injection, the expression of the transgene can be targeted to a discrete region of the spinal cord (e.g., the unilateral dorsal horn). Localized transgene expression can potentially be used in pain or muscle spasticity-modifying treatment by upregulating inhibitory neurotransmitter systems (e.g., GABA) or inhibiting excitatory systems (e.g., the glutamate-coupled receptor system). Fourth, in addition to AAV delivery, other molecules or vectors with poor blood-brain barrier permeability (e.g., micro RNA) will likely be more effectively delivered into the spinal parenchyma after subpial delivery. These can effectively be tested by using the technique described in this manuscript. Finally, as demonstrated in the current study, the subpial technique can successfully be used in adult mice with average bodyweights (BW) between 20 and 30 g. Thus, it is likely that the same technique and experimental setup can be employed in other animal species with similar BW (e.g., Sprague-Dawley (SD) rat pups). The BW of P6 and P21 SD rats is around 17 and 62 g, respectively. Using the rat pups during the early stage of post-natal development can be a useful tool to study the role of specific gene up- or downregulation in the development of spinal neural circuits and sensory and motor processing.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This study was supported by the SANPORC and ALSA Foundation grant (Martin Marsala); the National Sustainability Programme, project number LO1609 (Czech Ministry of Education, Youth and Sports); and RVO: 67985904 (Stefan Juhas and Jana Juhasova).
Materials
| C57BL/6J Mice | Jackson Labs | 664 | |
| Lab Standard Stereotaxic for Mice | Harvard Apparatus | 72-9568 | |
| Mouse Spinal Adaptor | Harvard Apparatus | 72-4811 | |
| XYZ Manipulator | Stoelting | 51604 | |
| Manual Infusion Pump | Stoelting | 51218 | |
| 34G Beveled Nanofill Needle | World Precision Instruments | NF34BV-2 | |
| 36G Blunt Nanofill needle | World Precision Instruments | NF-36BL-2 | |
| Fluriso, Isoflurane | MWI Veterinary Supply | 502017 | |
| Chlorhexidine Solution | MWI Veterinary Supply | 501027 | |
| 20G Stainless Steel Needle | Becton-Dickinson | 305175 | |
| 23G Stainless Steel Needle | Becton-Dickinson | 305145 | |
| 30G Stainless Steel Needle | Becton-Dickinson | 305128 | |
| Cotton Tipped Applicator | MWI Veterinary Supply | 27426 | |
| Glass Capillary Beveller | Narishige International | SM-25B | |
| Slide Microscope Superfrost | Leica Microsystems | M80 | |
| 50μl Microsyringe | Hamilton | 81242 | |
| BD Intramedic PE-20 Tubing | Becton, Dickinson | 427406 | |
| BD Intramedic PE-10 Tubing | Becton, Dickinson | 427401 | |
| 4-0 monofilament suture | VetOne | V1D397 | |
| Glass Capillary Beveller | Narishige | Pipet Micro Grinder EG-40 | |
| 5 min Epoxy (Epoxy Clear) | Devcon | 14310 | |
| Euthanasia Solution | MWI Veterinary Supply | 11168 | |
| Heparin Inj 1000U/mL | MWI Veterinary Supply | 54254 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| Sucrose | Sigma-Aldrich | S0389 | |
| Anti NeuN Antibody | EMD-Millipore | ABN78 | Primary Rabbit Polyclonal Antibody, 1:1000 |
| Anti-Choline Acetyltransferase (CHAT) Antibody | EMD-Millipore | AB144P | Primary Goat Polyclonal Antibody, 1:100 |
| Anti GFP Antibody | Aves Labs | GFP-1020 | Primary Chicken Polyclonal Antibody, 1:1000 |
| Donkey anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 594 | ThermoFisher Scientific | A21207 | Secondary Antibody, 1:1000 |
| Donkey anti-Rabbit IgG Secondary Antibody, Alexa Fluor 680 | ThermoFisher Scientific | A10043 | Secondary Antibody, 1:1000 |
| Donkey anti-Chicken IgY Secondary Antibody, Alexa Fluor 488 | Jackson Immunoresearch Labs | 703-545-155 | Secondary Antibody, 1:1000 |
| Donkey Anti-Goat IgG H&L (Alexa Fluor 647 | Abcam | ab150131 | Secondary Antibody, 1:1000 |
| Slide Microscope Superfrost | Fisher Scientific | 12-550-143 | |
| ProLong Gold Antifade Mountant | Fisher Scientific | P36930 | |
| Epifluorescence Microscope | Zeiss | Zeiss AxioImager M2 | |
| Fluorescence Confocal Microscope | Olympus | Olympus FV1000 | |
| Dextran | Polysciences, Inc | 19411 | |
| AAV9-UBC-GFP | UCSD Viral Vector Core Laboratory | ||
References
- Foust, K. D., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 27 (1), 59-65 (2009).
- Duque, S., et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 17 (7), 1187-1196 (2009).
- Gray, S. J., et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 19 (6), 1058-1069 (2011).
- Meyer, K., et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther. 23 (3), 477-487 (2015).
- Foust, K. D., et al. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol Ther. 21 (12), 2148-2159 (2013).
- Passini, M. A., et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther. 25 (7), 619-630 (2014).
- Bell, P., et al. Motor neuron transduction after intracisternal delivery of AAV9 in a cynomolgus macaque. Hum Gene Ther Methods. 26 (2), 43-44 (2015).
- Miyanohara, A., et al. Potent spinal parenchymal AAV9-mediated gene delivery by subpial injection in adult rats and pigs. Mol Ther Methods Clin Dev. 3, 16046 (2016).
- Xu, Q., et al. In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery. PLoS One. 7 (3), 32581 (2012).
- Xiao, X., Li, J., Samulski, R. J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 72 (3), 2224-2232 (1998).

