Preparation of Authigenic Pyrite from Methane-bearing Sediments for In Situ Sulfur Isotope Analysis Using SIMS
Summary
Analyses of the sulfur isotopic composition (δ34S) of pyrite from methane-bearing sediments have typically focused on bulk samples. Here, we applied secondary ion mass spectroscopy to analyze the δ34S values of various pyrite generations to understand the diagenetic history of pyritization.
Abstract
Different sulfur isotope compositions of authigenic pyrite typically result from the sulfate-driven anaerobic oxidation of methane (SO4-AOM) and organiclastic sulfate reduction (OSR) in marine sediments. However, unravelling the complex pyritization sequence is a challenge because of the coexistence of different sequentially formed pyrite phases. This manuscript describes a sample preparation procedure that enables the use of secondary ion mass spectroscopy (SIMS) to obtain in situ δ34S values of various pyrite generations. This allows researchers to constrain how SO4-AOM affects pyritization in methane-bearing sediments. SIMS analysis revealed an extreme range in δ34S values, spanning from -41.6 to +114.8‰, which is much wider than the range of δ34S values obtained by the traditional bulk sulfur isotope analysis of the same samples. Pyrite in the shallow sediment mainly consists of 34S-depleted framboids, suggesting early diagenetic formation by OSR. Deeper in the sediment, more pyrite occurs as overgrowths and euhedral crystals, which display much higher SIMS δ34S values than the framboids. Such 34S-enriched pyrite is related to enhanced SO4-AOM at the sulfate-methane transition zone, postdating OSR. High-resolution in situ SIMS sulfur isotope analyses allow for the reconstruction of the pyritization processes, which cannot be resolved by bulk sulfur isotope analysis.
Introduction
Methane emissions from sediments are common along continental margins1,2. However, most of the methane in areas of diffusive seepage is oxidized at the expense of sulfate within the sediments, a process known as SO4-AOM (Equation 1)3,4. The production of sulfide during this process commonly results in the precipitation of pyrite. Also, OSR also drives the formation of pyrite by releasing sulfide (Equation 2)5.
CH4 + SO42– → HS– + HCO3– + H2O (1)
2CH2O + SO42– → H2S + 2HCO3– (2)
It has been found that authigenic sulfide in the sulfate-methane transition zone (SMTZ) reveals high δ34S values, which was suggested to be caused by enhanced SO4-AOM in areas of seepage6,7,8. In contrast, pyrite induced by OSR commonly displays lower δ34S values9. However, it is challenging to identify different pyrite generations induced by these processes (i.e., OSR and SO4-AOM) if only a bulk sulfur isotope measurement is used, since the successively formed interfingering pyrite generations are characterized by different isotopic compositions. Therefore, microscale in situ sulfur isotope analysis is required to improve our understanding of the actual mineralizing processes10,11,12. As a versatile technique for in situ isotope analysis, SIMS requires only a few nanograms of sample, which sparked its designation as a nondestructive technique. A primary ion beam sputters the target, causing the emission of secondary ions that are subsequently transported to a mass spectrometer for measuring13. In an early in situ sulfur isotope analysis application of SIMS, Pimminger et al. successfully analyzed the δ34S values in galena by using a 10 – 30 µm-diameter beam14. This approach has been increasingly applied to the microanalysis of sulfur isotopic compositions in sulfides, with significant improvements in both measurement precision and resolution11,12,13,14,15,16,17,18,19,20. Pyrite with various morphologic attributes and distinct sulfur stable isotope patterns has been reported from seep and non-seep environments21,22,23,24. However, to the best of our knowledge, prior to our recent SIMS study6, only one study used the in situ sulfur isotope analysis of pyrite from seep environments and revealed large sulfur isotope variability in biogenic pyrite25.
In this study, we applied SIMS to analyze the δ34S values of different generations of authigenic pyrite from a seepage site in the South China Sea, which allowed for microscale discrimination of OSR- and SO4-AOM-derived pyrite.
Protocol
1. Collection of Samples from a Sediment Core
Note: The core HS148 was obtained from a site near the gas hydrate drilling zone in the Shenhu area, South China Sea, during a cruise of the R/V Haiyang Sihao in 2006.
- Cut the piston core (here, HS148) into sections at intervals of 0.7 m from the top to the bottom (onboard the vessel) and transfer the sections to a cold room (4 °C) for storage after retrieval.
- Transfer the core sections to a cold room (4 °C) in the land-based lab for storage after the cruise. Take the sections out of the cold room and use a saw to cut them into halves lengthwise.
- Clean the surface of the sediment and collect a set of sediment samples (15 cm in length; 1/4 of the sediment core) across the entire length using a knife. Pack the wet samples individually in zippered plastic bags and label them using a marker.
- Place the wet sediment samples (~30 g) in pre-cleaned beakers and dry them at 40 °C in a drying oven for 24 h. After drying, separate the sediments into two aliquots: one for the collection of pyrite aggregates (i.e., authigenic pyrite), and the other for bulk sulfur extraction (see step 3).
- Put one aliquot of dry sediment into beakers and add distilled water to soften the sediment for 2 h. Transfer the slurry (including the sediments and water in the beaker) to a 0.063-mm sieve washed with distilled water.
- Sift the sediment with distilled water so that all fine grains (<0.063 mm) are washed through. Collect the coarse fraction (e.g., quartz grains, fossil shells, and authigenic minerals) in beakers and dry them at 40 °C in a drying oven for 24 h.
- Place some of the coarse segment fractions on a glass slide under a binocular microscope (20X magnification). Identify the pyrite aggregates from the coarse fraction. Handpick such pyrite aggregates using a needle and pack them individually into zippered plastic bags.
NOTE: Most of the pyrite aggregates are black in color and tubular in shape. - Pulverize a second aliquot of dry sediment sample into a fine powder (<0.074 mm) using an agate mortar for further bulk sulfur extraction (see step 3).
2. Observation of Variable Morphologies
- Select some representative pyrite tubes from the handpicked pyrite aggregates under a binocular microscope (20X magnification) for thick section preparation to examine the morphological and textural features of the pyrite aggregates.
- Stick double-sided tape on a slide and place the selected pyrite tubes on the tape. Put a mounting tube (25 mm in diameter) on the slide to cover all pyrite aggregates. Mix 10 mL of epoxy resin with 1.3 mL of hardener at room temperature and pour the mixing liquid into the mounting tube.
- Place the slide and the mounting tube into a vacuum chamber. Pump the air out of the chamber until the pressure in the chamber is below 0.2 bar, so that all the pore spaces of the samples are filled with epoxy. Move the slide and the mounting tube out of the chamber and let the epoxy cure at room temperature for 12 h.
- After the epoxy has cured, hand-grind the pyrite tubes on a fixed, 9-µm diamond mesh pad until the pyrite grains are exposed. Hand-polish the pyrite grains to produce a smooth and flat surface, using 5-, 3-, and 1-µm diamonds successively.
- Observe the morphology and the texture of the pyrite under a reflected light microscope at 200X magnification, with a ~3-mm working distance.
- Perform petrographic observation under a reflected light microscope6 and then coat the thick sections with a 25-nm layer of carbon. Examine their morphological and textural features using a thermal field emission scanning electron microscope with secondary electron imaging and backscattered electron modes6,19.
NOTE: This step was performed at the School of Earth Science and Geological Engineering, Sun Yat-sen University.
3. Bulk Sulfur Isotope Analyses
Note: The total sulfur (as sulfide) was extracted as hydrogen sulfide via wet chemical sequential extraction26,27 at the Institut für Geologie und Paläontologie, Westfälische Wilhelms-Universität Münster.
- Place 4 g of the dried sample powder or 10 mg of pyrite aggregates per flask into round-bottom flasks and add 10 mL of ethanol into each flask as a catalyst.
- Prepare zinc acetate (3%) acetic acid solution in a 500-mL glass flask to trap hydrogen sulfide. Connect the zinc acetate-containing flask to the sample-containing flask. Check the connections of the flasks and flush nitrogen into the flasks to remove the air.
- Inject 20 mL of HCl solution (25%) into the round-bottom flasks using a syringe to liberate the acid-volatile (mono)sulfides (AVS) from the sample; allow the samples to react for 1 h at room temperature.
NOTE: Here, the analysis revealed that no AVS was present in the studied samples. - Inject 30 mL of 1 M CrCl2 solution into the round-bottom flasks when the above reaction is completed; allow the samples to react for 2 h at 85 °C.
NOTE: Chromium reducible sulfur (CRS, pyrite) reduces to hydrogen sulfide (H2S) after the reaction and precipitates as zinc sulfide in the zinc acetate trap. - Transfer all the solutions containing zinc sulfide precipitates to beakers and convert the zinc sulfide precipitates to silver sulfide (Ag2S) by adding a 0.1 M AgNO3 solution to the beakers. Place the beakers on the heating plate and heat them to 90 °C so that the finely disseminated Ag2S better coagulates.
- Collect the Ag2S precipitates by filtration (<0.45 µm) after the solution has cooled down to room temperature and dry the filtrate overnight at 40 °C.
- Weigh 200 µg of Ag2S precipitate and mix it with an equal amount of V2O5 in tin cups. Have the sulfur composition analyzed on SO2 molecules via combustion using a mass spectrometer connected to an elemental analyzer (EA-IRMS)6.
NOTE: The above step was performed at the Institut für Geologie und Paläontologie, Westfälische Wilhelms-Universität Münster.
4. In Situ SIMS Analysis
- Select representative pyrite aggregates with characterized crystal habits (e.g., framboids, overgrows, and euhedral crystals) from different sediment samples after petrographic study. Stick the selected pyrite aggregates and pieces of Sonora pyrite standard to double-sided tape. Mold them within 5 mm of the center of a 25-mm epoxy mount.
NOTE: The epoxy disc production process is the same as in step 2.2.- After the epoxy has cured, hand-grind the disc on a fixed 9-µm diamond mesh pad to the desired level so that pyrite grains are exposed. Hand-polish the epoxy discs to produce a smooth, flat surface, successively using 5-, 3-, and 1-µm diamonds, which is required for high-precision isotope ratio analysis by SIMS28.
- Clean the surface of the epoxy disc with deionized water and ethanol. Place the epoxy disc in a gold-coating machine and coat the dry surface sections with a 25-nm layer of gold.
- Observe the sample again under a scanning electron microscope at 1,000X magnification, with a 9-mm working distance, to select spots characterized with different crystal habits (e.g., framboids, overgrows, and euhedral crystals) for SIMS analysis.
NOTE: High-spatial resolution SIMS sulfur isotope analysis was applied to reveal the sulfur isotope variability of the different pyrite types.
- Observe the sample again under a scanning electron microscope at 1,000X magnification, with a 9-mm working distance, to select spots characterized with different crystal habits (e.g., framboids, overgrows, and euhedral crystals) for SIMS analysis.
- Perform SIMS analysis15,16.
NOTE: Performed at the SIMS lab of the Guangzhou Institute of Geochemistry, Chinese Academy of Sciences.- Use a Cs+ primary ion beam to measure the sulfur isotope ratios (34S/32S) of pyrite. Focus the Cs+ primary ion beam onto a 15 µm × 10 µm spot at an energy of 10 kV, with 2.5-nA current. Use three off-axis Faraday cups for the simultaneous measurement of 32S, 33S, and 34S in multi-collector mode, with an entrance slit width of 60 µm and an exit slit width of 500 µm on each of the three Faraday cup detectors.
- Carry out sulfur isotope analyses in automated sequences, with each analysis consisting of 30 s of pre-sputtering, 60 s of secondary ion automated centering, and 160 s of data acquisition and sulfur isotope signal integration (40 cycles × 4 s).
- Analyze Sonora pyrite as a standard at regular intervals, every 5 – 6 sample analyses.
NOTE: See Chen et al.19 for more detailed analytical methods and instrument parameters.
- Analyze Sonora pyrite as a standard at regular intervals, every 5 – 6 sample analyses.
Representative Results
Data Expression – Bulk Sulfur Isotopes:
The bulk sulfur isotope ratio is expressed in relation to the Vienna Canyon Diablo Troilite (V-CDT) standard, and the analytical precision is better than ±0.3‰. The sulfur isotope measurements were calibrated with international reference materials: IAEA-S1 (δ34S = -0.30‰), IAEA-S2 (δ34S = -21.55‰), IAEA-S3 (δ34S = -31.4‰), and NBS 127 (δ34S = 20.30‰).
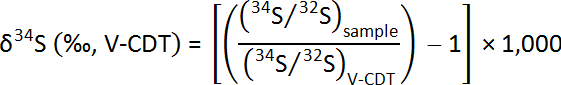
Data Expression – SIMS Sulfur Isotopes:
Measured ratios of 34S/32S from SIMS analyses are normalized using the V-CDT standard and are calculated as "raw" δ34S values following standard delta notation:
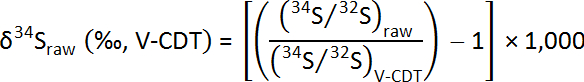
The correction factor (α) of the instrumental bias of the δ34S values for pyrite measurements was calculated using the δ34Sraw values of the bracketing analyses of Sonora pyrite17 (δ34S= +1.61‰), as follows:
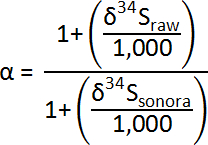
The δ34S values of the samples are calibrated with the measured δ34S values and the correction factor (α), as follows:
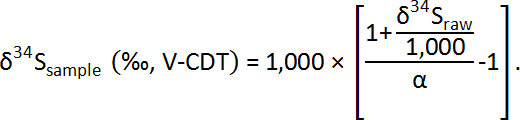
Outcomes:
Most pyrite aggregates hand-picked from the sediment are black in color and tubular in shape, varying from 3 to 8 mm in length and 0.2 to 0.6 mm in diameter (Figure 1). The pyrite aggregates mainly consist of three types of pyrite, with different morphologies: (1) framboids, (2) overgrowth layers surrounding the framboidal cores, and (3) euhedral crystals. In the shallow sediment above a depth of 483 cmbsf, most pyrite occurs as framboids, while overgrowth layers and euhedral crystals become abundant at greater depths, reflecting a change of pyrite crystal habits with depth (Figure 2).
The total CRS content ranges from 0.0 wt.% to 0.98 wt.% (n = 29). Below 50 cmbsf, it exhibits minor fluctuations around the mean value (0.44 wt.%) and two distinct peaks of 0.98 wt.% at 490 cmbsf and 0.78 wt.% at 590 cmbsf6 (Table 1 and Figure 3A). The δ34SCRS values fall between -40.5 and +41.0‰ (n = 28), and the δ34Spy values of hand-picked pyrite range from -37.6 to +52.7‰ (n = 28)6 (Figure 3B). Above 483 cmbsf, both δ34SCRS and δ34Spy values display a similar trend, reflecting an almost linear increase with depth. Further down, two distinctly different groups of δ34SCRS values are observed, one reflecting a notable 34S depletion, with values between -34 and -27‰, and a second group with high δ34SCRS values, ranging from -8 to +41‰. In contrast, all hand-picked pyrite aggregates exhibit exclusively positive δ34S values (> +20‰), revealing 34S enrichment compared to total sulfide sulfur (up to 75‰) (Figure 3B).
Pyrite aggregates from site HS148 display extremely variable in situ δ34S values, between -41.6 and +114.8‰ (n = 81), reflecting an overall range of 156.4‰6 (Table 2 and Figure 3B). These SIMS data reveal a similar down core trend, like the δ34S values of CRS and of pyrite aggregates. CRS and pyrite aggregates are subsumed and referred to as bulk pyrite below. Above 500 cmbsf, both in situ and bulk pyrite δ34S values reflect 34S depletion (as low as -41.6‰). With increasing depth (below 500 cmbsf), the SIMS δ34S values reveal extreme 34S enrichment (up to +114.8‰), while the highest δ34S value of bulk pyrite only reaches +52.7‰. Moreover, all overgrowths and euhedral crystals show higher δ34S values than bulk pyrite, while most of the framboids display lower δ34S values. The δ34S values in different types of pyrite revealed a variability of more than 100‰ in single-pyrite tubes.
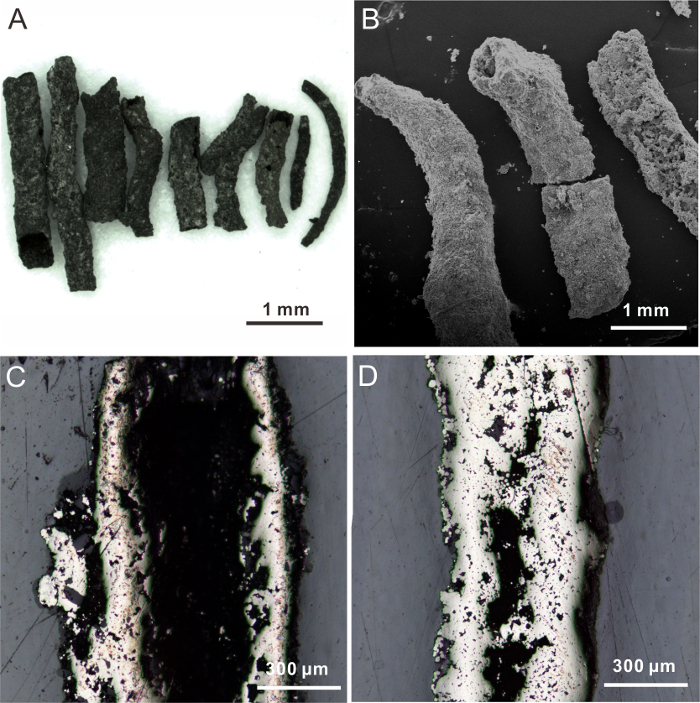
Figure 1. Typical Morphologies of Authigenic Pyrite. (A) Pyrite tubes of various sizes, taken from sediments. (B) Pyrite tubes; SEM micrograph. (C and D) Longitudinal cross-sections of pyrite tubes, with hollow interiors and different wall thicknesses; reflected-light photographs. This figure has been modified from Lin et al.6. Please click here to view a larger version of this figure.
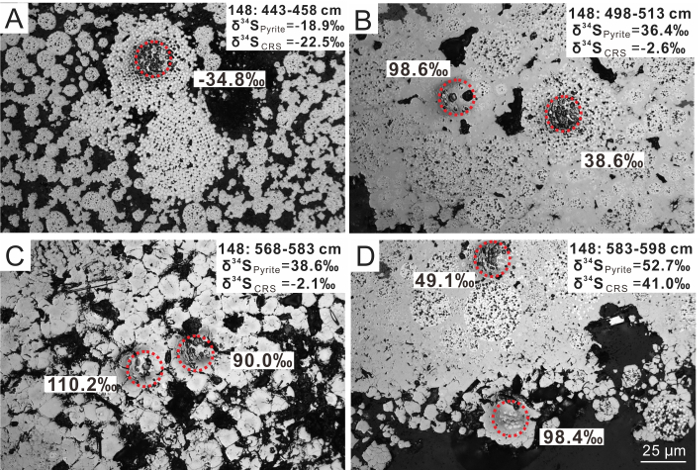
Figure 2. Sulfur Isotopic Composition in Pyrite Aggregates. The δ34S values in ‰ versus V-CDT were analyzed by secondary ion mass spectrometry; corresponding spot locations are indicated on the reflected-light photomicrographs (see the red circles). (A-D) Typical pyrite aggregates, from shallow to deep sediments. Most of the pyrite in (A) is framboidal and depleted in 34S, while overgrowths and euhedral crystals enriched in 34S are abundant in (B-D). The scale provided in (D) is the same for all micrographs. This figure has been modified from Lin et al.6. Please click here to view a larger version of this figure.
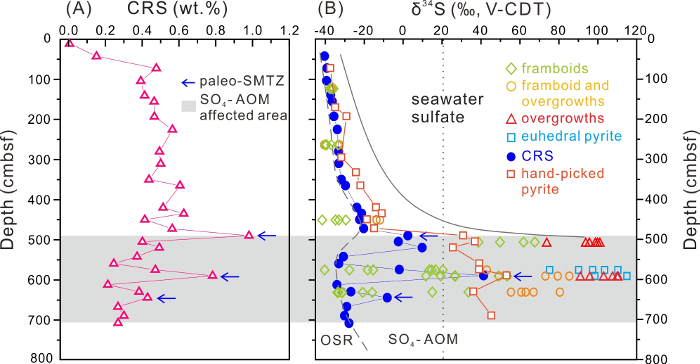
Figure 3. The Content and Sulfur Isotopic Compositions of Sulfide Minerals at Site HS148. (A) CRS content; (B) SIMS δ34S values of the three types of pyrite and δ34S values of CRS and hand-picked pyrite aggregates. The dashed line separates a zone to the left, suggested to be dominated by OSR, and a zone to the right, suggested to be dominated by SO4-AOM. The shaded area refers to the zone affected by SO4-AOM. This figure has been modified from Lin et al.6. Please click here to view a larger version of this figure.
| Depth (cmbsf) | CRS (wt.%) | δ34SCRS (‰ V-CDT) | δ34Spyrite (‰ V-CDT) |
| 0-20 | 0 | – | – |
| 35-50 | 0.15 | -40.5 | – |
| 65-80 | 0.48 | -39.3 | -37.6 |
| 95-113 | 0.39 | -39.4 | – |
| 113-133 | – | – | – |
| 133-148 | 0.41 | -37.3 | – |
| 148-163 | 0.46 | -36.5 | – |
| 163-178 | – | – | -35.2 |
| 183-203 | 0.47 | -35.6 | -29.4 |
| 218-233 | 0.56 | -33.9 | – |
| 253-273 | – | – | – |
| 273-288 | 0.49 | -33.2 | – |
| 288-303 | – | – | -32 |
| 303-318 | 0.5 | -33.2 | – |
| 323-343 | – | – | -24.5 |
| 343-358 | 0.44 | -31.8 | – |
| 358-373 | 0.6 | -29.7 | -22.2 |
| 393-413 | – | – | – |
| 413-428 | 0.51 | -23.9 | -14.2 |
| 428-443 | 0.63 | -21.5 | -11.3 |
| 443-458 | 0.41 | -22.5 | -18.9 |
| 463-483 | 0.56 | -20.4 | -15.2 |
| 483-498 | 0.98 | 2.2 | 30.4 |
| 498-513 | 0.4 | -2.6 | 36.4 |
| 513-528 | 0.49 | 9.5 | 25.2 |
| 533-553 | 0.37 | -30.7 | – |
| 553-568 | 0.24 | -33.2 | 38.7 |
| 568-583 | 0.47 | -2.1 | 38.6 |
| 583-598 | 0.78 | 41 | 52.7 |
| 603-623 | 0.21 | -34.1 | – |
| 623-638 | 0.38 | -26.9 | 35.6 |
| 638-653 | 0.43 | -8.3 | – |
| 653-668 | 0.27 | -29.1 | – |
| 683-698 | 0.3 | -30.2 | 44.9 |
| 699-719 | 0.27 | -28 | – |
Table 1. Content of the CRS and Sulfur Isotopic Composition. Content of the CRS and sulfur isotopic composition of CRS and pooled, hand-picked samples of pyrite at site HS1486.
| Depth (cmbsf) | δ34S (‰ V-CDT) | 2SD | Pyrite type |
| 113-133 | -35.9 | 0.1 | F |
| 113-133 | -37.4 | 0.1 | F+O |
| 113-133 | -36 | 0.13 | F |
| 113-133 | -37.3 | 0.07 | F+O |
| 113-133 | -36.6 | 0.11 | F |
| 113-133 | -35.9 | 0.11 | F |
| 113-133 | -36.8 | 0.1 | F |
| 113-133 | -37.7 | 0.13 | F |
| 113-133 | -36.1 | 0.02 | F+O |
| 113-133 | -35.9 | 0.11 | F |
| 113-133 | -36 | 0.1 | F+O |
| 113-133 | -35.8 | 0.12 | F |
| 253-273 | -39.4 | 0.12 | F |
| 253-273 | -40.6 | 0.03 | F+O |
| 253-273 | -33.6 | 0.09 | F |
| 253-273 | -40.1 | 0.14 | F |
| 253-273 | -32.7 | 0.17 | F+O |
| 253-273 | -33 | 0.08 | F+O |
| 253-273 | -36.9 | 0.08 | F |
| 443-458 | -33 | 0.11 | F |
| 443-458 | -34.8 | 0.1 | F |
| 443-458 | -41.6 | 0.05 | F |
| 443-458 | -34.8 | 0.15 | F |
| 443-458 | -11.9 | 0.25 | F+O |
| 443-458 | -29.5 | 0.05 | F |
| 443-458 | -13.8 | 0.23 | F+O |
| 498-513 | 38.6 | 0.35 | F |
| 498-513 | 98.6 | 0.26 | O |
| 498-513 | 67.5 | 0.09 | F |
| 498-513 | 99.6 | 0.15 | O |
| 498-513 | 93.6 | 0.25 | O |
| 498-513 | 95.5 | 0.02 | O |
| 498-513 | 49.6 | 0.19 | F |
| 498-513 | 100.8 | 0.06 | O |
| 498-513 | 61.7 | 0.08 | F |
| 498-513 | 73.6 | 0.2 | O |
| 568-583 | 110.2 | 0.04 | E |
| 568-583 | 90 | 0.19 | E |
| 568-583 | 97.1 | 0.27 | E |
| 568-583 | 75.1 | 0.2 | E |
| 568-583 | -27.7 | 0.07 | F |
| 568-583 | 42.8 | 0.23 | O |
| 568-583 | 103.3 | 0.16 | E |
| 568-583 | 13.3 | 0.32 | F |
| 568-583 | -15.5 | 0.18 | F |
| 568-583 | -40.5 | 0.02 | F |
| 568-583 | 20.3 | 0.15 | F |
| 568-583 | -18.4 | 0.06 | F |
| 568-583 | 16.5 | 0.1 | F |
| 568-583 | 14.5 | 0.18 | F |
| 583-598 | 107.4 | 0.16 | O |
| 583-598 | 26.8 | 0.17 | F |
| 583-598 | 100.6 | 0.14 | O |
| 583-598 | 95.8 | 0.14 | O |
| 583-598 | 90.9 | 0.02 | O |
| 583-598 | 85.2 | 0.46 | F+O |
| 583-598 | 79.2 | 0.07 | F+O |
| 583-598 | 103.8 | 0.3 | O |
| 583-598 | 53.3 | 0.26 | F |
| 583-598 | 48.9 | 0.15 | F |
| 583-598 | 114.8 | 0.32 | E |
| 583-598 | 109.9 | 0.45 | O |
| 583-598 | 72.9 | 0.23 | F+O |
| 583-598 | 11.6 | 0.1 | F |
| 583-598 | 19.2 | 0.3 | F |
| 583-598 | 49.1 | 0.18 | F+O |
| 583-598 | 26.4 | 0.45 | F |
| 583-598 | 110.3 | 0.4 | O |
| 623-638 | 66.9 | 0.23 | F+O |
| 623-638 | 80.3 | 0.03 | F+O |
| 623-638 | 55.3 | 0.24 | F+O |
| 623-638 | 14.9 | 0.24 | F |
| 623-638 | 33.5 | 0.12 | F |
| 623-638 | 62.8 | 0.04 | F+O |
| 623-638 | 60.5 | 0.29 | F+O |
| 623-638 | -31.4 | 0.04 | F |
| 623-638 | -33.7 | 0.03 | F |
| 623-638 | -20.6 | 0.05 | F |
| 623-638 | -15.9 | 0.03 | F |
| 623-638 | -31.5 | 0.12 | F |
| 623-638 | -33.2 | 0.12 | F |
| Uncertainty for δ34S is 2SD (standard deviation). | |||
Table 2. In Situ Sulfur Isotopic Composition of Different Types of Pyrite. In situ sulfur isotopic composition of different types of pyrite, analyzed by secondary ion mass spectrometry at site HS148. F = framboid, O = overgrowth, E = euhedral crystal, F+O = mixture of framboid and overgrowth6.
Discussion
The sulfur isotope analysis of pyrite is a useful approach and can help in identifying the biogeochemical processes that impact pyritization. However, if bulk sulfur isotope analysis is applied, the obtained sulfur isotope signatures commonly represent mixed signals, as sedimentary pyrite aggregates typically consist of multiple, closely interfingering generations. Here, we present a method (i.e., SIMS analysis) for analyzing the in situ sulfur isotopic compositions of various pyrite generations on the micro-scale. The critical steps within this protocol include: (1) the selection of well-characterized pyrite generations from different sediment depths (e.g., framboids, overgrowths, and euhedral grains); (2) the identification of pyrite aggregates large enough (> 20 µm) for SIMS analysis, to avoid the mixing of different paragenetic phases; and (3) the analysis of a sufficient number of spots (i.e., at least 10 spots per sample, if possible), to ensure that the obtained isotope patterns are representative, reflecting the environmental conditions during pyrite formation.
In this study, we applied SIMS to analyze the in situ δ34S values of various pyrite generations with different morphologies, including framboids, overgrowths, and euhedral grains. In addition, the bulk δ34S values of the total CRS and hand-picked pyrite aggregates (> 0.063 mm) in the sediment were also determined for comparison. It is obvious that the SIMS δ34S values cover a much wider range (from -41.6 to +114.8‰) than those of bulk pyrite. From the SIMS analysis, it becomes apparent that 34S-depleted framboids are particularly abundant in the shallow sediment (i.e., above 483 cmbsf), which is also recorded by the 34S depletion of bulk pyrite. Such sulfur isotope patterns indicate that OSR is the dominant diagenetic process in the shallow sediment in the study area6,9.
With increasing depth (i.e., below 483 cmbsf), both in situ and bulk pyrite analyses yielded high δ34S values, including extremely high SIMS δ34S values (as high as +114.8‰). It is interesting to note that some individual zones, with the synchronous increase of the CRS content and δ34SCRS value, can be identified throughout the sediment column (see the arrows in Figure 3). Such a synchronous increase is attributed to the formation of progressively 34S-enriched hydrogen sulfide during SO4-AOM in a paleo-SMTZ6,8,23. Moreover, below 483 cmbsf, the SIMS δ34S values of radial overgrowths and euhedral crystals are systematically higher than those of framboids. The increase in δ34S values along a transect from the framboidal core to the overgrowth layers and euhedral crystals within individual pyrite aggregates is best explained by the subsequent growth of later pyrite generations derived from SO4-AOM over initial framboids derived from OSR at shallower depths6. Such great variability in the δ34S values of different pyrite generations reveals a complex diagenetic history of pyritization, which cannot be resolved by traditional bulk sulfur isotope analysis.
SIMS is a versatile technique for in situ isotope analysis, but some factors still limit its wider application. For example, it is challenging, if not impossible, to apply SIMS to minerals with a crystal diameter that is smaller than the spatial resolution of this method (~10 µm)15,16. Also, SIMS can only be used to analyze the isotopic composition of a mineral if a corresponding mineral standard (the same mineral with a known isotopic composition) is available14,15.
In our study of authigenic pyrite from methane-bearing sediments, high-spatial resolution SIMS analysis proved its potential to distinguish the effects of OSR and SO4-AOM on pyritization. This analytical approach can serve as a sensitive tool for reconstructing the pyritization sequence that developed during diagenesis in marine sediments. Future applications of this protocol should also target ancient sedimentary sequences, aiming to resolve the effects of different biogeochemical processes on mineral formation when pore water data is lacking.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was jointly funded and supported by the Natural Science Foundation of China (No. 91128101, 41273054, and 41373007), the China Geological Survey Project for South China Sea Gas Hydrate Resource Exploration (No. DD20160211), Fundamental Research Funds for the Central Universities (No. 16lgjc11), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (No. 2011). Zhiyong Lin acknowledges the financial support provided by the China Scholarship Council (No. 201506380046). Yang Lu thanks the Guangzhou Elite Project (No. JY201223) and the China Postdoctoral Science Foundation (No. 2016M592565). We are grateful to Dr. Shengxiong Yang, Guangxue Zhang, and Dr. Jinqiang Liang of the Guangzhou Marine Geological Survey for providing samples and valuable suggestions. We thank Dr. Xianhua Li and Dr. Lei Chen of the Institute of Geology and Geophysics (Beijing), Chinese Academy of Sciences, for help with the SIMS analysis. Dr. Xiaoping Xia is thanked for making available the SIMS Lab of the Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, for the filming of this article. The manuscript benefited from comments from Dr. Alisha Dsouza, review editor of JoVE, and two anonymous referees.
Materials
| secondary ion mass spectroscopy | Cameca | IMS-1280 | |
| thermal field emission scanning electron microscopy | Quanta | Quanta 400F | |
| elemental analyser – isotope ratio mass spectrometry | ThermoFinnigan | ThermoFinnigan Delta Plus | |
| binocular microscope | any | NA | |
| reflected light microscope | Carl Zeiss | 3519001617 | |
| polishing machicine | Struers | 60210535 | |
| cutting machicine | Struers | 50110202 | |
| carbon/gold coating machicine | any | NA | |
| ethanol | any | NA | |
| acetic acid | any | NA | |
| zinc acetate solution (3%) | any | NA | |
| HCl solution (25%) | any | NA | |
| 1 M CrCl2 solution | any | NA | |
| 0.1 M AgNO3 solution | any | NA | |
| V2O5 powder | any | NA | |
| pure nitrogen | any | NA | |
| syringe | any | NA | |
| filter(<0.45 µm) | any | NA | |
| tin cups | any | NA | |
| round bottom flasks | any | NA | |
| epoxy | Struers | 41000004 | |
References
- Judd, A. G. The global importance and context of methane escape from the seabed. Geo-Mar Lett. 23 (3), 147-154 (2003).
- Suess, E. Marine cold seeps and their manifestations: geological control, biogeochemical criteria and environmental conditions. Int J Earth Sci. 103 (7), 1889-1916 (2014).
- Boetius, A., et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 407 (6804), 623-626 (2000).
- Orphan, V. J., House, C. H., Hinrichs, K. -. U., McKeegan, K. D., DeLong, E. F. Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science. 293 (5529), 484-487 (2001).
- Jørgensen, B. B. Mineralization of organic matter in the seabed – the role of sulfate reduction. Nature. 296, 643-645 (1982).
- Lin, Z. Y., et al. How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chem Geol. 440, 26-41 (2016).
- Jørgensen, B. B., Böttcher, M. E., Lüschen, H., Neretin, L. N., Volkov, I. I. Anaerobic methane oxidation and a deep H2S sink generate isotopically heavy sulfides in Black Sea sediments. Geochim Cosmochim Ac. 68 (9), 2095-2118 (2004).
- Borowski, W. S., Rodriguez, N. M., Paull, C. K., Ussler, III, W. Are 34S-enriched authigenic sulfide minerals a proxy for elevated methane flux and gas hydrates in the geologic record?. Mar Petrol Geol. 43, 381-395 (2013).
- Canfield, D. E. Isotope fractionation by natural populations of sulfate-reducing bacteria. Geochim Cosmochim Ac. 65 (7), 1117-1124 (2001).
- McKibben, M. A., Eldridge, C. S. Micron-scale isotopic zoning in minerals; a record of large-scale geologic processes. Mineral Mag. 58A, 587-588 (1994).
- Peevler, J., Fayek, M., Misra, K. C., Riciputi, L. R. Sulfur isotope microanalysis of sphalerite by SIMS: constraints on the genesis of Mississippi valley-type mineralization, from the Mascot-Jefferson City district, East Tennessee. J Geochem Explor. 80 (2-3), 277-296 (2003).
- Ferrini, V., Fayek, M., De Vito, C., Mignardi, S., Pignatti, J. Extreme sulphur isotope fractionation in the deep Cretaceous biosphere. J Geol Soc. 167, 1009-1018 (2010).
- Ireland, T. R., et al. Charge-mode electrometer measurements of S-isotopic compositions on SHRIMP-SI. Int J Mass Spectrom. 359, 26-37 (2014).
- Pimminger, A., Grasserbauer, M., Schroll, E., Cerny, I. Microanalysis in galena by Secondary Ion Mass Spectrometry for determination of sulfur isotopes. Anal Chem. 56 (3), 407-411 (1984).
- Eldridge, C. S., Compston, W., Williams, I. S., Walshe, J. L., Both, R. A. In situ microanalysis for 34S/32S ratios using the ion microprobe SHRIMP. Int J Mass Spectrom Ion Processes. 76 (1), 65-83 (1987).
- Kozdon, R., Kita, N. T., Huberty, J. M., Fournelle, J. H., Johnson, C. A., Valley, J. W. In situ sulfur isotope analysis of sulfide minerals by SIMS: precision and accuracy, with application to thermometry of 3.5 Ga Pilbara cherts. Chem Geol. 275 (3-4), 243-253 (2010).
- Farquhar, J., et al. Pathways for Neoarchean pyrite formation constrained by mass-independent sulfur isotopes. Proc Natl Acad Sci USA. 110 (44), 17638-17643 (2013).
- Whitehouse, M. Multiple sulfur isotope determination by SIMS: evaluation of reference sulfides for Δ33S with observations and a case study on the determination of Δ36S. Geostand Geoanal Res. 37 (1), 19-33 (2013).
- Chen, L., et al. Extreme variation of sulfur isotopic compositions in pyrite from the Qiuling sediment-hosted gold deposit, West Qinling orogen, central China: an in situ SIMS study with implications for the source of sulfur. Miner Depos. 50 (6), 643-656 (2015).
- LaFlamme, C., et al. In situ multiple sulfur isotope analysis by SIMS of pyrite, chalcopyrite, pyrrhotite, and pentlandite to refine magmatic ore genetic models. Chem Geol. 444, 1-15 (2016).
- Peckmann, J., et al. Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar Geol. 177 (1-2), 129-150 (2001).
- Zhang, M., et al. Morphology and formation mechanism of pyrite induced by the anaerobic oxidation of methane from the continental slope of the NE South China Sea. J Asian Earth Sci. 92, 293-301 (2014).
- Lin, Z. Y., et al. Stable isotope patterns of coexisting pyrite and gypsum indicating variable methane flow at a seep site of the Shenhu area, South China Sea. J Asian Earth Sci. 123, 213-223 (2016).
- Virtaslo, J. J., et al. Pyritic and baritic burrows and microbial filaments in postglacial lacustrine clays in the northern Baltic Sea. J Geol Soc London. 167 (6), 1185-1198 (2010).
- Kohn, M. J., Riciputi, L. R., Stakes, D., Orange, D. L. Sulfur isotope variability in biogenic pyrite: Reflections of heterogeneous bacterial colonization?. Am Mineral. 83 (11-12 Pt 2), (1998).
- Canfield, D. E., Raiswell, R., Westrich, J. T., Reaves, C. M., Berner, R. A. The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chem Geol. 54 (1-2), 149-155 (1986).
- Rice, C. A., Tuttle, M. L., Reynolds, R. L. The analysis of forms of sulfur in ancient sediments and sedimentary rocks: comments and cautions. Chem Geol. 107 (1-2), 83-95 (1993).
- Kita, N. T., Huberty, J. M., Kozdon, R., Beard, B. L., Valley, J. W. High-precision SIMS oxygen, sulfur and iron stable isotope analyses of geological materials: accuracy, surface topography and crystal orientation. Surf Interface Anal. 43 (1-2), 427-431 (2011).

