Chondrogenic Pellet Formation from Cord Blood-derived Induced Pluripotent Stem Cells
Summary
Here, we propose a protocol for chondrogenic differentiation from cord blood mononuclear cell-derived human induced pluripotent stem cells.
Abstract
Human articular cartilage lacks the ability to repair itself. Cartilage degeneration is thus treated not by curative but by conservative treatments. Currently, efforts are being made to regenerate damaged cartilage with ex vivo expanded chondrocytes or bone marrow-derived mesenchymal stem cells (BMSCs). However, the restricted viability and instability of these cells limit their application in cartilage reconstruction. Human induced pluripotent stem cells (hiPSCs) have received scientific attention as a new alternative for regenerative applications. With unlimited self-renewal ability and multipotency, hiPSCs have been highlighted as a new replacement cell source for cartilage repair. However, obtaining a high quantity of high-quality chondrogenic pellets is a major challenge to their clinical application. In this study, we used embryoid body (EB)-derived outgrowth cells for chondrogenic differentiation. Successful chondrogenesis was confirmed by PCR and staining with alcian blue, toluidine blue, and antibodies against collagen types I and II (COL1A1 and COL2A1, respectively). We provide a detailed method for the differentiation of cord blood mononuclear cell-derived iPSCs (CBMC-hiPSCs) into chondrogenic pellets.
Introduction
The use of hiPSCs represents a new strategy for drug screening and mechanistic studies of various diseases. From a regenerative perspective, hiPSCs are also a potential source for the replacement of damaged tissues that have limited healing ability, such as articular cartilage1,2.
The regeneration of native articular cartilage has been a challenge for several decades. Articular cartilage is a soft, white tissue that coats the end of bones, protecting them from friction. However, it has limited regenerative ability when damaged, which makes self-repair almost impossible. Therefore, research focused on cartilage regeneration has been ongoing for several decades.
Previously, in vitro differentiation into the chondrogenic lineage was usually performed with BMSCs or native chondrocytes isolated from the knee joint3. Due to their chondrogenic potential, BMSCs and native chondrocytes have numerous merits supporting their use in chondrogenesis. However, because of their limited expansion and unstable phenotype, these cells face several limitations in the reconstruction of articular cartilage defects. Under in vitro culture conditions, these cells tend to lose their own characteristics after 3-4 passages, which eventually affects their differentiation abilities4. Also, in the case of native chondrocytes, additional damage to the knee joint is inevitable when obtaining these cells.
Unlike BMSCs or native chondrocytes, hiPSCs can indefinitely expand in vitro. With the proper culture conditions, hiPSCs have great potential as a replacement source for chondrogenic differentiation. However, it is challenging to change the intrinsic characteristics of hiPSCs5. Moreover, it takes several complicated in vitro steps to direct the fate of hiPSCs to a specific cell type. Despite these complications, the use of hiPSCs is still recommended due to their high self-renewal abilities and their capacity to differentiate into targeted cells, including chondrocytes6.
Chondrogenic differentiation is usually done with three-dimensional culture systems, such as the pellet culture or micromass culture, using MSC-like progenitor cells. If using hiPSCs, the protocol to generate MSC-like progenitor cells differs from the existing protocols. Some groups use monolayer culture of hiPSCs to directly convert the phenotype into MSC-like cells7. However, most studies use EBs to generate outgrowth cells that resemble MSCs8,9,10,11.
Various types of growth factors are used to induce chondrogenesis. Usually, BMP and TGFβ family proteins are used, alone or in combination. Differentiation has also been induced with other factors, such as GDF5, FGF2, and IGF112,13,14,15. TGFβ1 has been shown to stimulate chondrogenesis in a dose-dependent manner in MSCs16. Compared to the other isotype, TGFβ3, TGFβ1 induces chondrogenesis by increasing the pre-cartilage mesenchymal cell condensation.TGFβ3 induces chondrogenesis by significantly increasing the mesenchymal cell proliferation17. However, TGFβ3 is used more frequently by researchers than TGFβ17,10,18,19. BMP2 enhances the expression of genes related to the chondrogenic matrix components in human articular chondrocytes under in vitro conditions20. BMP2 increases the expression of genes critical to cartilage formation in MSCs in combination with TGFβ proteins21. It has also been shown that BMP2 synergistically enhances the effect of TGFβ3 through the Smad and MAPK pathways22.
In this study, CBMC-hiPSCs were aggregated into EBs using EB medium in a low-attachment Petri dish. Outgrowth cells were induced by attaching the EBs to a gelatin-coated dish. Chondrogenic differentiation using outgrowth cells was performed by pellet culture. Treatment with both BMP2 and TGFβ3 successfully condensed the cells and induced extracellular matrix (ECM) protein accumulation for chondrogenic pellet formation. This study suggests a simple yet efficient chondrogenic differentiation protocol using CBMC-hiPSCs.
Protocol
This protocol was approved by the institutional review board of the Catholic University of Korea (KC12TISI0861). CBMCs used for reprogramming were directly obtained from the Cord Blood Bank of the Seoul St. Mary's Hospital.
1. Chondrogenic Differentiation from iPSCs
- CBMC-iPSC generation
- Generate CBMC-hiPSCs using the protocol shown in our previous work23.
- Collect the blood cells in a 15 mL conical tube and count them using a hemocytometer.
- Prepare 3 x 105 cells and centrifuge them for 5 min at 515 x g and RT. Discard the supernatant by suction and resuspend the cells in 0.5 mL of blood cell medium.
- Transfer the cells to a well of a non-coated 24-well plate and add the Sendai virus mixture, following the manufacturer's recommendations.
- Centrifuge the plate for 30 min at 1,150 x g and 30 °C.
- After centrifugation, incubate the cells overnight (O/N) at 37 °C in 5% CO2.
- The next day, transfer the transduced cells to a matrix-coated well. Centrifuge the plate at 1,150 x g for 30 min at 30 °C.
- After centrifugation, remove the supernatant, add 1 mL of iPSC medium, and maintain the cells O/N at 37 °C in 5% CO2.
- Maintain the attached cells at 37 °C and 5% CO2. Change the medium daily, replacing it with fresh iPSC medium.
NOTE: Colonies will appear on day 14-21 after transduction.
- EB generation
- Maintain the hiPSCs at 37 °C and 5% CO2. Change the medium daily, replacing it with fresh Essential 8 (E8) medium.
- Prepare 2 x 106 hiPSCs in a vitronectin-coated, 100-mm dish in E8 medium.
- Remove the E8 medium from the culture dish and wash with sterile phosphate-buffered saline (PBS).
- Add 1 mL of 1 mM ethylenediaminetetraacetic acid (EDTA) and incubate at 37 °C and 5% CO2 for 2 min.
- Harvest the cells with 3 mL of E8 medium and transfer them to a new 15-mL conical tube. Centrifuge the cells at 250 x g at room temperature (RT) for 2 min.
- Aspirate the supernatant without disturbing the cell pellet and resuspend the cells in 5 mL of E8 medium.
- Count the cells using a hemocytometer and prepare 2 x 106 cells for each 100 mm Petri dish. Resuspend the prepared cells in a 10-mL mixture of E8 and EB medium (1:1) with 10 µM rho-associated kinase (ROCK) inhibitor.
- Incubate the cells O/N for aggregation at 37 °C and 5% CO2.
- On the following day, harvest the aggregated EBs by pipetting. Centrifuge the cells at 250 x g for 1 min. Remove the supernatant and resuspend the EBs in 10 mL of fresh E8 medium.
- Enlarge the generated EBs for 5 days, performing daily changes with fresh E8 medium. For further maturation, change the culture medium to E7 medium. Maintain the EBs at 37 °C and 5% CO2 for another 5 days, performing daily medium changes with fresh E7 medium.
NOTE: E7 medium is E8 medium without FGF2.
- Outgrowth cell induction from EBs
- Add 6 mL of 1% gelatin to a 100-mm dish and incubate at 37 °C and 5% CO2 for 30 min.
- Remove the gelatin and dry the dish completely for 2-3 h before use.
- Transfer the EBs to a 50-mL conical tube. Allow the EBs to settle to the bottom of the conical tube. Remove the supernatant without disturbing the EBs.
- Resuspend the EBs in 10 mL of Dulbecco's Modified Eagle's Medium (DMEM) with 20% fetal bovine serum (FBS). Transfer the EBs to the gelatin-coated, 100-mm dish. Add 10 µM ROCK inhibitor.
- Incubate and maintain the cells at 37 °C and 5% CO2 for 7 days. Change the medium every other day without adding ROCK inhibitor.
- Chondrogenic pellet formation
- Aspirate the culture medium from the dish and wash the cells three times with PBS.
- Apply 1 mL of 1 mM EDTA and incubate at 37 °C and 5% CO2 for 2 min.
- Harvest the cells using 5 mL of DMEM with 20% FBS and transfer them to a new 15-mL conical tube.
- Centrifuge the cells for 2 min at 250 x g and RT. Discard the supernatant and resuspend the pellet in 10 mL of DMEM with 20% FBS.
- Filter and discard the cell clumps using a 40 µm cell strainer and harvest the single cells. Count the single cells using a hemocytometer.
- Centrifuge the cells for 2 min at 250 x g and RT. Remove the supernatant and seed 3 x 105 cells per pellet in a 15 mL conical tube with 300 µL of chondrogenic differentiation medium (CDM).
- For pellet formation, centrifuge the cells for 5 min at 680 x g and RT.
NOTE: Pellets are maintained in 15 mL conical tubes during the differentiation of the chondrogenic pellets. - Incubate the cells overnight at 37 °C and 5% CO2.
- Change the CDM every 2-3 days. Within 3 days, pellets will exhibit flattened, spheroidal morphologies. Maintain the pellets for 21 days at 37°C and 5% CO2.
2. Chondrogenic Pellet Characterization by Staining
- Chondrogenic embedding
- Before embedding, prepare and melt paraffin at 58 °C.
- Fix the pellets in 1 mL of 4% paraformaldehyde for 2 h at RT in a 1.5 mL tube.
Caution: Paraformaldehyde is highly toxic. Avoid contact with eyes, skin, or mucous membranes. Minimize exposure and avoid inhalation while preparing it. - Place one layer of gauze onto the cassette and transfer the fixed pellets using a pipette. Cover the pellet by folding the gauze and close the cassette lid.
- Initiate dehydration in 100 mL of 70% ethanol (EtOH) twice, 10 min each. Dehydrate the pellets through sequential 10-min washes in 80% and 95% EtOH.
- Transfer the pellets to 100% EtOH for 10 min. Repeat three times with fresh EtOH.
- For "clearing," exchange the solution for a 100 mL, 1:1 mixture of EtOH and xylene, followed by a 1:2 mixture of EtOH and xylene for 10 min each. Clear the remaining EtOH by incubating the pellets twice in 100% xylene, 10 min each.
- For paraffin infiltration, incubate the pellets in sequential xylene and paraffin mixtures. Perform the whole paraffin infiltration process at 58 °C. Incubate the pellets in 100 mL of a 2:1 mixture of xylene and paraffin for 30 min.
- Exchange the solution for 100 mL of a 1:1 mixture of xylene and paraffin and incubate for 30 min.
- Exchange the solution for 100 mL of a 1:2 mixture of xylene and paraffin and incubate for 30 min.
- For the final infiltration, transfer the pellets to the first bath of 100% paraffin and incubate for 2 h.
- Transfer the pellets to the second bath of 100% paraffin and incubate O/N at 58 °C.
- The next day, gently transfer the pellets to a mold using a tweezer. Add paraffin to the mold from the paraffin dispenser. Solidify the paraffin for 30 min at 4 °C.
- Slice the sections at 7 µm and transfer the sections onto the slide. Allow the slides to dry overnight and store the slides at RT until they are ready for use.
- Slide preparation
- Deparaffinize the slides by moving them through 100 mL of 100%, 90%, 80%, and 70% EtOH sequentially for 5 min each.
- Place the slides in a glass jar and rinse with tap water for 5 min.
- Alcian blue staining
- Incubate the slides in 50 mL of 1% alcian blue solution for 30 min.
NOTE: Alcian blue is diluted in 3% acetic acid solution. Adjust the pH to 2.5 using acetic acid. - Place the slides in a glass jar and rinse with tap water for 2 min.
- Rinse the slides in deionized water (DW) and counterstain with nuclear fast red solution for 2 min.
- Place the slides in a glass jar and rinse with tap water for 1 min.
- 2.3.5)Proceed to dehydrate and mount the slides in step 2.6.
- Incubate the slides in 50 mL of 1% alcian blue solution for 30 min.
- Toluidine blue staining
- Incubate slides in 50 mL of toluidine blue solution for 4 min.
- Place the slides in a glass jar and rinse with tap water for 5 min.
- Proceed to dehydrate and mount the slides in step 2.6.
- Immunohistochemical staining
- Incubate the slides in 3% H2O2for 15 min for endogenous peroxidase blocking.
- Apply 200 µL of primary antibody (anti-COL1A1 and -COL2A1) diluted 1:100 in tris-buffered saline (TBS) containing 1% bovine serum albumin (BSA) and 5% normal goat serum (NGS) to the slides and incubate O/N at 4 °C.
- The next day, place the slides in a glass jar and wash them with 50 mL of TBS containing 0.1% polysorbate 20 (TBST) three times for 5 min each.
- Apply 200 µL of secondary antibody (goat anti-rabbit IgG antibody, diluted 1:200 in TBS containing 1% BSA and 5% NGS) to the slides and incubate at RT for 40 min.
- Place the slides in a glass jar and wash them three times with 50 mL, 5 min each.
- For signal amplification, apply HRP-conjugate streptavidin solution to the slides and incubate for 10 min.
- Place the slides in a glass jar and wash them three times with 50 mL, 5 min each.
- Mix the DAB-peroxidase substrate solution, apply 200 µL of solution to each slide, and incubate for 1 min.
- Place the slides in a glass jar and rinse with tap water for 5 min.
- Counterstain with Mayer's hematoxylin for 1 min.
- Wash the slides with DW.
- Proceed to dehydrate and mount the slides in step 2.6.
- Dehydration and mounting
- Dehydrate the slides by moving them sequentially through 100 mL of 70%, 80%, 90%, and 100% EtOH, 30 s each.
- Dip the slides into two changes of xylene for 1 min each.
- Add 50 µL of mounting solution to the coverslips and place the slides on top.
Representative Results
In this study, we generated chondrogenic pellets from CBMC-hiPSCs by inducing outgrowth cells from EBs. Chondrogenic differentiation was induced using CBMC-hiPSCs with confirmed high pluripotency11. A simple scheme of our protocol is shown in Figure 1A. Before differentiation, iPSC colonies were expanded (Figure 1B). The expanded iPSCs were assembled as EBs to initiate differentiation (Figure 1C). The generated EBs were attached to gelatin-coated dishes to induce outgrowth cells (Figure 1D). Then, the outgrowth cells were harvested and used to generate chondrogenic pellets. After 21 days of differentiation, small, bead-like chondrogenic pellets were obtained and used for further characterization (Figure 1E). The quality of the in vitro-generated chondrogenic pellets was confirmed through various assays.
We histologically evaluated the quality of the chondrogenic pellets on day 21. BMSC chondrogenic pellets were used as the positive control. The accumulation of ECM proteins secreted by the differentiated chondrocytes was confirmed by alcian blue and toluidine blue staining in Figure 2A. The major characteristics of healthy cartilage, such as lacuna and proteoglycan production, increased as the differentiation process continued over 21 days. Chondrogenic pellets generated from CBMC-hiPSCs expressed COL2A1, which is the major ECM component in healthy cartilage (Figure 2B). The COL2A1 expression of CBMC-hiPSC-derived pellets was higher than that of BMSC-derived pellets. The expression of collagen type I, a marker for fibrotic cartilage, was lower in CBMC-hiPSC-derived pellets compared to the expression of COL2A1.
The gene expression of cartilage ECM proteins in day-21 chondrogenic pellets was confirmed by real-time PCR. The aggrecan (ACAN) expression of CBMC-hiPSC-derived pellets was similar to that of BMSC-derived pellets (Figure 3A). The expression of COL2A1 was significantly higher in CBMC-hiPSC-derived pellets (Figure 3B). The expression of sex-determining region Y-box 9 (Sox9), a chondrogenic progenitor marker, was also evaluated. Pellets generated from CBMC-hiPSCs expressed high levels of Sox9 (Figure 3C). We confirmed that these genes were significantly upregulated in CBMC-hiPSC chondrogenic pellets compared to BMSC control pellets on day 21. The expression of a hypertrophic marker, COL1A1, was evaluated (Figure 3D). The expression of COL1A1 in CBMC-hiPSC-derived pellets was reduced compared to that in BMSC-derived pellets. The differentiation efficiency was analyzed by the ratio of COL2A1 to COL1A1 (Figure 3E). The increment of the ratio in CBMC-hiPSC-derived pellets demonstrated the relatively high expression of COL2A1 compared to COL1A1. In conclusion, we have confirmed the chondrogenic differentiation capacity of CBMC-hiPSCs. The quality of the generated CBMC-hiPSC-derived chondrogenic pellets had a quality compatible with that of BMSCs.
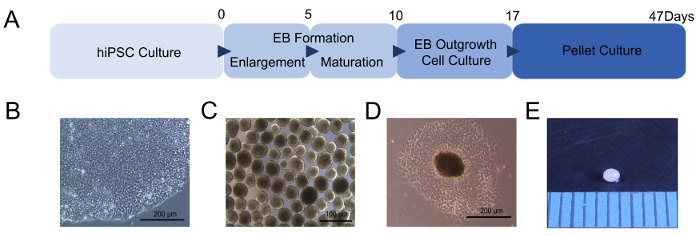
Figure 1: Chondrogenic differentiation of hiPSCs. (A) Scheme of chondrogenic pellet generation from hiPSCs. (B) Morphology of the generated CBMC-hiPSC. (C) Morphology of generated EBs. (D) Outgrowth cells derived from EBs attached to a gelatin-coated culture dish. (E) Image of the chondrogenic pellet after 21 days of differentiation. The units of the intervals are shown in millimeters. Scale bars = 200 µm. Please click here to view a larger version of this figure.
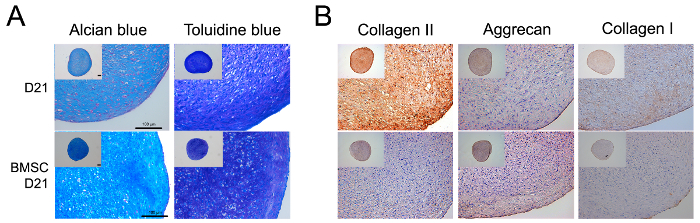
Figure 2: Histological analysis of the chondrogenic pellet. (A) Histological evaluation of chondrogenic pellets on day 21 using alcian blue and toluidine blue staining. (B) Immunohistochemistry image of chondrogenic pellets stained with COL1A1 and COL2A1 antibodies. Scale bars = 100 µm. Please click here to view a larger version of this figure.
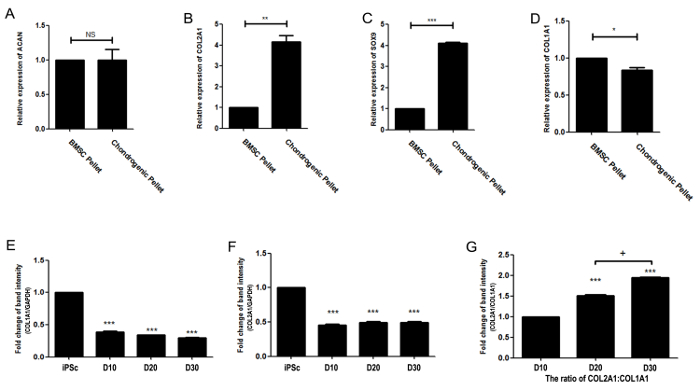
Figure 3: Gene expression of the chondrogenic pellet. (A) Relative gene expression of ACAN. (B) Relative gene expression of COL2A1. (C) Relative gene expression of SOX9. (D) Relative gene expression of COL1A1. (E) Gene expression of COL1A1. (F) Gene expression of COL10A1. (G) The ratio of COL2A1:COL1A1 gene expression. Pellets were harvested and analyzed after 21 days of differentiation. Data were obtained using real-time PCR and displayed as the mean standard error of triplicate experiments per sample (n = 3). The gene expression was normalized to GAPDH as an internal control. (*p <0.05, **p <0.01, ***p <0.001). Please click here to view a larger version of this figure.
| Cell number | Outgrowth cell yield | Pellet yield | |
| hiPSCs | 2 x 106 | 2-5 x 107 | 70-150 |
| MSC | 2 x 106 | – | 6 |
Table 1: Yield comparison of MSC and hiPSCs.
| Target Name | Direction | Primer Sequence | Size |
| SOX9 | Forward | TTCCGCGACGTGGACAT | 77 bp |
| Reverse | TCAAACTCGTTGACATCGAAGGT | ||
| ACAN | Forward | AGCCTGCGCTCCAATGACT | 107 bp |
| Reverse | TAATGGAACACGATGCCTTTCA | ||
| COL2A1 | Forward | GGCAATAGCAGGTTCACGTACA | 79 bp |
| Reverse | CGATAACAGTCTTGCCCCACTTA | ||
| COL1A1 | Forward | CCCCTGGAAAGAATGGAGATG | 148 bp |
| Reverse | TCCAAACCACTGAAACCTCTG |
Table 2: Sequences of primers against chondrogenic markers in real-time PCR.
Discussion
This protocol successfully generated hiPSCs from CBMCs. We reprogrammed CBMCs to hiPSCs using a Sendai viral vector containing Yamanaka factors24. Three cases were used in differentiation, and all experiments successfully generated chondrogenic pellets using this protocol. Numerous studies have reported protocols for the differentiation of hiPSCs into chondrocytes25,26,27,28. However, additional research is required to confirm the use of CBMC-hiPSCs a candidate for cartilage regeneration and recovery.
Chondrogenesis was confirmed using hiPSCs generated from various somatic cell types11,25,26,27,29. Many reports demonstrate that the origin of the somatic cell used in reprogramming can affect the differentiation outcome of the hiPSCs30,31,32,33. Cord blood is a source enriched with HSCs and MSCs. There is no report on the difference between cord blood-derived cells, such as blood cells or MSCs. However, previous studies have shown that articular chondrocyte-derived hiPSCs are more likely to go through chondrogenesis than hiPSCs generated from cord blood or skin fibroblasts29. Results from endothelial differentiation using hiPSCs generated from fibroblasts, cardiac progenitor cells, and endothelial cells demonstrated that the tissue-of-origin can reflect the tissue-specific somatic memory in terminally differentiated iPSC derivation, especially at early passages, between 10 and 2034. In early passages of hiPSCs, endothelial cell-derived hiPSCs differentiated more efficiently into the endothelial lineage. However, the significant difference between these cell lines disappeared in hiPSCs over 20 passages. Therefore, it is important to carefully consider the somatic memory carried by the hiPSCs in early passages when they are used in clinical research.
Recently, various procedures have been developed to repair defected articular cartilage. Microfracture is done by drilling multiple holes in the bone to induce the influx of bone marrow for natural repair35. Knee replacement, also known as knee arthroplasty, is used to replace the damaged cartilage. However, microfracture is not useful for severe articular cartilage damage, and the instrument used for knee replacements must be changed every 10-15 years.
These days, cell-based therapies are a promising alternative method for cartilage repair. Autologous chondrocyte implantation (ACI) is widely used for cell-based cartilage recovery. ACI is done by directly injecting autologous chondrocytes into the defect. However, autologous chondrocytes turn into fibroblast-like cells under in vitro cultivation conditions. Additional damage is also inevitable during the harvesting process of autologous chondrocytes. MSCs have been suggested as a cell source for cartilage recovery. Cord blood is a useful and accessible cell source of MSCs for engineering cartilage36. The success rate for isolating cord-blood MSCs, however, is controversial37,38. Numerous studies report the successful isolation of cord-blood MSCs. Several studies have shown that cord blood volume is a critical parameter that can affect the yield rate of MSC isolation36,39. To acquire high-quality MSCs, the passage of the cells is also critical. Previous studies indicate that MSCs have a restricted life span after a certain cell division number. By entering senescence, MSCs are characterized by decreased proliferation, as with any normal somatic cell40. Therefore, cord blood-derived MSCs must be used before the sixth passage to avoid cell senescence and chromosomal abnormalities41.
To avoid these limitations, human iPSCs opened a new possibility for personalized, cell-based therapy with high productivity11. Established hiPSCs can theoretically proliferate limitlessly. Also, with the same immune identity as the donor, they can avoid rejection and lower side effects when implanted in vivo. The transition of cord blood banks into hiPSC banks for allogeneic medical treatment has tremendous possibility and potential42. The screening of homozygous HLA-typed CBMCs before reprogramming can widely utilize the allogeneic iPSC lines for clinical treatment. Homozygous iPSC lines can avoid immunological reactions after allograft transplantation. This concept can also be applied to cartilage regeneration by generating HLA-homozygous cartilage11.
Usually, chondrogenic differentiation is performed through two critical steps: the induction of EB-derived outgrowth cells and pellet formation. The initial differentiation of hiPSCs into EB-derived outgrowth cells is critical to increase their chondrogenic differentiation potential. Outgrowth cells differentiated from hiPSCs are functionally and molecularly similar to native BMSCs7,43. Previous studies used monolayer culture or EB culture as a pre-differentiation step to induce mesenchymal-like cells10,43. However, direct differentiation by monolayer culture is time-consuming compared to the use of EBs, and chondrogenic pellets tend to differentiate into fibrocartilage with hypertrophic characteristics. It was reported that cell morphology and the chondrogenic differentiation potential of generated mesenchymal-like progenitor cells differ greatly according to the cell density of the cell monolayer9.
We used EBs to induce outgrowth cells, which is a relatively fast and simple method compared to the monolayer culture. Using this protocol, we were able to generate many pellets using a relatively small number of hiPSCs (Table 1). The size and number of EBs were critical for successful chondrogenic pellet mass production. For that reason, we enlarged the aggregated EBs in E8 medium, until more than half of the generated EBs were larger than 100 µm. After EB enlargement, we maintained the EBs in E8 medium without FGF2. Previously, researchers maintained generated EBs without FGF2 for mesodermal lineage induction9.
Even though we attempted to maintain the generated outgrowth cells at a high density for better quality, several limitations still remain. While we have confirmed that the generated outgrowth cells share a similar morphology, the cells may be still heterogenous. Previous studies have attempted to sort for a specific cell type; however, this procedure has resulted in the collection of a low number of cells. A new attempt to isolate homogenous outgrowth cells on a larger scale will be required to generate a large quantity of chondrogenic pellets with enhanced characteristics.
Various groups are inducing progenitor cells from hiPSCs by using monolayer or EB culture. The induction of progenitor cells with a high similarity to MSCs can be the key to obtaining chondrogenic pellets of higher quality. These progenitor cells derived from EBs have characteristics similar to those of MSCs. However, this might be because of their fibroblastic nature. Therefore, a detailed validation study on the outgrowth cells is required.
In this study, we suggest a protocol that can generate a relatively large quantity of chondrogenic pellets. We confirmed that the cartilage regenerated using CBMC-hiPSCs showed a healthy phenotype and can be used as material for tissue regeneration. However, an improved method with a shorter differentiation timeline is required for further application. The further development of quality-control standards to validate cartilage with a higher level of hyaline is also required for future applications of CBMC-hiPSCs as cell material for cartilage regeneration. The protocol is simple yet effective and does not require any additional sorting processes before pellet formation. In conclusion, using this protocol, high-quality chondrogenic pellets can be generated for studies on disease modeling, drug screening, and regenerative medicine to further our understanding of the nature of cartilage.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the Korea Healthcare Technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI16C2177).
Materials
| Plasticware | |||
| 100mm Dish | TPP | 93100 | |
| 6-well Plate | TPP | 92006 | |
| 50 mL Cornical Tube | SPL | 50050 | |
| 15 mL Cornical Tube | SPL | 50015 | |
| 10 mL Disposable Pipette | Falcon | 7551 | |
| 5 mL Disposable Pipette | Falcon | 7543 | |
| 12-well Plate | TPP | 92012 | |
| Name | Company | Catalog Number | Description |
| E8 Medium Materials | |||
| DMEM/F12, HEPES | Life Technologies | 11330-057 | E8 Medium (500 mL) |
| Sodium Bicarbonate | Life Technologies | 25080-094 | E8 Medium (Conc.: 543 μg/mL) |
| Sodium Selenite | Sigma Aldrich | S5261 | E8 Medium (Conc.: 14 ng/mL) |
| Human Transfferin | Sigma Aldrich | T3705 | E8 Medium (Conc.: 10.7 μg/mL) |
| Basic FGF2 | Peprotech | 100-18B | E8 Medium (Conc.: 100 ng/mL) |
| Human Insulin | Life Technologies | 12585-014 | E8 Medium (Conc.: 20 μg/mL) |
| Human TGFβ1 | Peprotech | 100-21 | E8 Medium (Conc.: 2 ng/mL) |
| Ascorbic Acid | Sigma Aldrich | A8960 | E8 Medium (Conc.: 64 μg/mL) |
| DPBS | Life Technologies | 14190-144 | |
| Vitronectin | Life Technologies | A14700 | |
| ROCK Inhibitor | Sigma Aldrich | Y0503 | |
| Name | Company | Catalog Number | Description |
| Quality Control Materials | |||
| 18 mm Cover Glass | Superior | HSU-0111580 | |
| 4% Paraformaldyhyde | Tech & Innovation | BPP-9004 | |
| Triton X-100 | BIOSESANG | 9002-93-1 | |
| Bovine Serum Albumin | Vector Lab | SP-5050 | |
| Anti-SSEA4 Antibody | Millipore | MAB4304 | |
| Anti-Oct4 Antibody | Santa Cruz | SC9081 | |
| Anti-TRA-1-60 Antibody | Millipore | MAB4360 | |
| Anti-Sox2 Antibody | Biolegend | 630801 | |
| Anti-TRA-1-81 Antibody | Millipore | MAB4381 | |
| Anti-Klf4 Antibody | Abcam | ab151733 | |
| Alexa Fluor 488 goat anti-mouse IgG (H+L) antibody | Molecular Probe | A11029 | |
| Alexa Fluor 594 goat anti-rabbit IgG (H+L) antibody | Molecular Probe | A11037 | |
| DAPI | Molecular Probe | D1306 | |
| Prolong gold antifade reagent | Invitrogen | P36934 | |
| 4% Paraformaldyhyde | Tech & Innovation | BPP-9004 | |
| Tween 20 | BIOSESANG | T1027 | |
| Bovine Serum Albumin | Vector Lab | SP-5050 | |
| Anti-Collagen II antibody | abcam | ab34712 | 1:100 |
| Alcian blue | Sigma Aldrich | A3157-10G | |
| Fast Green FCF | Sigma Aldrich | F7252-25G | |
| Safranin O | Sigma Aldrich | 090m0039v | |
| Nuclear fast red | Americanmastertech | STNFR100 | |
| xylene | Duksan | 115 | |
| Ethanol | Duksan | 64-17-5 | |
| Mayer's hematoxylin solution | wako pure chemical industries | LAK7534 | |
| DAP | VECTOR LABORATORIES | SK-4100 | |
| Slide Glass, Coated | Hyun Il Lab-Mate | HMA-S9914 | |
| Trizol | Invitrogen | 15596-018 | |
| Chloroform | Sigma Aldrich | 366919 | |
| Isoprypylalcohol | Millipore | 109634 | |
| Ethanol | Duksan | 64-17-5 | |
| RevertAid First Strand cDNA Synthesis kit | Thermo Scientfic | K1622 | |
| Name | Company | Catalog Number | Description |
| Chondrogenic Differentiation Materials | |||
| DMEM | Life Technologies | 11885 | Chondrogenic media component (500 mL) |
| Penicilin Streptomycin | Life Technologies | P4333 | Chondrogenic media component (Conc.: 1 %) |
| Ascorbic Acid | Sigma Aldrich | A8960 | Chondrogenic media component (Conc.: 64 μg/mL) |
| MEM Non-Essential Amino Acids Solution (100X) | Life Technologies | 11140-050 | Chondrogenic media component (Conc.: 100 mM) |
| rhBMP-2 | R&D | 355-BM-050 | Chondrogenic media component (Conc.:100ng/ml) |
| Recombinant Hman TGF-beta3 | R&D | 243-B3-002 | Chondrogenic media component (Conc.:10ng/ml) |
| KnockOut Serum Replacement | Life Technologies | 10828-028 | Chondrogenic media component (Conc.: 1 %) |
| ITS+ Premix | BD | 354352 | Chondrogenic media component (Conc.: 1 %) |
| Dexamethasone-Water Soluble | Sigma Aldrich | D2915-100MG | Chondrogenic media component (Conc.:10-7 M) |
| GlutaMAX Supplement | Life Technologies | 35050-061 | Chondrogenic media component (Conc.: 1 %) |
| Sodium pyruvate solution | Sigma Aldrich | S8636 | Chondrogenic media component (Conc.: 1 %) |
| L-Proline | Sigma Aldrich | P5607-25G | Chondrogenic media component (40μg/ml) |
References
- van Osch, G. J., et al. Cartilage repair: past and future–lessons for regenerative medicine. J Cell Mol Med. 13 (5), 792-810 (2009).
- Ahmed, T. A., Hincke, M. T. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 16 (3), 305-329 (2010).
- Diekman, B. O., et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA. 109 (47), 19172-19177 (2012).
- Solchaga, L. A., Penick, K., Goldberg, V. M., Caplan, A. I., Welter, J. F. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 16 (3), 1009-1019 (2010).
- Guzzo, R. M., Drissi, H. Differentiation of Human Induced Pluripotent Stem Cells to Chondrocytes. Methods Mol Biol. 1340, 79-95 (2015).
- Drissi, H., Gibson, J. D., Guzzo, R. M., Xu, R. H. Derivation and Chondrogenic Commitment of Human Embryonic Stem Cell-Derived Mesenchymal Progenitors. Methods Mol Biol. 1340, 65-78 (2015).
- Nejadnik, H., et al. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev. 11 (2), 242-253 (2015).
- Teramura, T., et al. Induction of mesenchymal progenitor cells with chondrogenic property from mouse-induced pluripotent stem cells. Cell Reprogram. 12 (3), 249-261 (2010).
- Koyama, N., et al. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells Dev. 22 (1), 102-113 (2013).
- Ko, J. Y., Kim, K. I., Park, S., Im, G. I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 35 (11), 3571-3581 (2014).
- Nam, Y., Rim, Y. A., Jung, S. M., Ju, J. H. Cord blood cell-derived iPSCs as a new candidate for chondrogenic differentiation and cartilage regeneration. Stem Cell Res Ther. 8 (1), 16 (2017).
- Hotten, G. C., et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 13 (1-2), 65-74 (1996).
- Murphy, M. K., Huey, D. J., Hu, J. C., Athanasiou, K. A. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells. 33 (3), 762-773 (2015).
- Shintani, N., Siebenrock, K. A., Hunziker, E. B. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS One. 8 (1), e53086 (2013).
- Fukumoto, T., et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 11 (1), 55-64 (2003).
- Worster, A. A., Nixon, A. J., Brower-Toland, B. D., Williams, J. Effect of transforming growth factor beta1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res. 61 (9), 1003-1010 (2000).
- Knippenberg, M., et al. Differential effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of collagen-modifying enzymes in human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 15 (8), 2213-2225 (2009).
- Jang, Y., et al. Centrifugal gravity-induced BMP4 induces chondrogenic differentiation of adipose-derived stem cells via SOX9 upregulation. Stem Cell Res Ther. 7 (1), 184 (2016).
- Kang, R., et al. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res Ther. 6, 144 (2015).
- Tao, H., et al. Biological evaluation of human degenerated nucleus pulposus cells in functionalized self-assembling peptide nanofiber hydrogel scaffold. Tissue Eng Part A. 20 (11-12), 1621-1631 (2014).
- Sekiya, I., Larson, B. L., Vuoristo, J. T., Reger, R. L., Prockop, D. J. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 320 (2), 269-276 (2005).
- Shen, B., Wei, A., Tao, H., Diwan, A. D., Ma, D. D. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 15 (6), 1311-1320 (2009).
- Rim, Y. A., Nam, Y., Ju, J. H. Induced Pluripotent Stem Cell Generation from Blood Cells Using Sendai Virus and Centrifugation. J Vis Exp. (118), (2016).
- Kim, Y., et al. The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells Int. 2016, 1329459 (2016).
- Oldershaw, R. A., et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 28 (11), 1187-1194 (2010).
- Toh, W. S., et al. Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. J Cell Mol Med. 13 (9B), 3570-3590 (2009).
- Hwang, N. S., Varghese, S., Elisseeff, J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One. 3 (6), e2498 (2008).
- Nakagawa, T., Lee, S. Y., Reddi, A. H. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum. 60 (12), 3686-3692 (2009).
- Guzzo, R. M., Scanlon, V., Sanjay, A., Xu, R. H., Drissi, H. Establishment of human cell type-specific iPS cells with enhanced chondrogenic potential. Stem Cell Rev. 10 (6), 820-829 (2014).
- Rim, Y. A., Park, N., Nam, Y., Ju, J. H. Generation of Induced-pluripotent Stem Cells Using Fibroblast-like Synoviocytes Isolated from Joints of Rheumatoid Arthritis Patients. J Vis Exp. (116), (2016).
- Pfaff, N., et al. Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells. Stem Cells Dev. 21 (5), 689-701 (2012).
- Xu, H., et al. Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Res. 22 (1), 142-154 (2012).
- Lee, S. B., et al. Contribution of hepatic lineage stage-specific donor memory to the differential potential of induced mouse pluripotent stem cells. Stem Cells. 30 (5), 997-1007 (2012).
- Hu, S., et al. Effects of cellular origin on differentiation of human induced pluripotent stem cell-derived endothelial cells. JCI Insight. 1 (8), 1-12 (2016).
- Vonk, L. A., de Windt, T. S., Slaper-Cortenbach, I. C., Saris, D. B. Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res Ther. 6 (94), 1-11 (2015).
- Gomez-Leduc, T., et al. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci Rep. 6, 32786 (2016).
- Mareschi, K., et al. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 86 (10), 1099-1100 (2001).
- Wexler, S. A., et al. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 121 (2), 368-374 (2003).
- Zhang, X., et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 112 (4), 1206-1218 (2011).
- Wagner, W., et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 3 (5), e2213 (2008).
- Tarte, K., et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 115 (8), 1549-1553 (2010).
- Chen, W. C., et al. Prediction of poor survival by cyclooxygenase-2 in patients with T4 nasopharyngeal cancer treated by radiation therapy: clinical and in vitro studies. Head Neck. 27 (6), 503-512 (2005).
- Guzzo, R. M., Gibson, J., Xu, R. H., Lee, F. Y., Drissi, H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 114 (2), 480-490 (2013).

