How to Find Effects of Stimulus Processing on Event Related Brain Potentials of Close Others when Hyperscanning Partners
Summary
This protocol describes key steps involved in assessing the sensitivity of the brain of one person to the stimulus processing of a close other by selecting pairs of partners, recording their electroencephalogram (EEG) simultaneously and computing their event-related brain potentials (ERPs).
Abstract
The partners of each pair must be able to pass the McGill Friendship Questionnaire without communicating. Each partner is then seated in front of a screen in one of two adjacent rooms. These rooms are separated by a glass window through which participants communicate to maintain feelings of togetherness while being fitted with the EEG cap. After checking for adequate EEG signals, the glass is covered by a curtain to prevent visual communication. Then, partners must be silent but are instructed to try to feel in the presence of their partner during the entire experiment. Just before it starts, participants are told that each of them will be presented with one image at a time and that these images will occur at the same time for both of them on their own screen. They are also instructed that, for each trial, the simultaneous images will always be different. However, unbeknownst to them, trials are randomized: only half of them are consistent with this instruction and actually include two different images. These trials form the DSC, that is, the different-stimuli condition. The other half of the trials are inconsistent with the instruction. They include two identical images and form the ISC (identical-stimuli condition). After the experiment, participants are sorted into two groups: those who reported that they felt in the presence of their partner during the majority of the trials and those who reported they did not. The impact of the stimulus processing of the partner is found by subtracting the mean voltages of the ERPs of the ISC (inconsistent with the instructions) from the ERPs of the DSC (consistent with the instructions) in at least two time windows (TWs): firstly, in the 75 to 150 ms TW, where the absolute values of these subtractions are greater, especially at right frontal sites, in those who felt in the presence of their partner than in those who did not; secondly, in the LPP time window (i.e., from 650 to 950 ms post onset), where ERPs are significantly less positive in the DSC than in the ISC in those in whom the raw results of the early (75-150ms) subtractions are negative.
Introduction
EEG indexes the sums of electrical postsynaptic responses of large neuronal populations1 produced during information processing.2 Among these responses, certain patterns are time-locked to sensory, motor or cognitive events. These "event-related" patterns of the EEG are called ERPs.3 An ERP consists of several deflections (e.g. the N300, N400 & P600). Each of these deflections is characterized by its latency relative to the onset of the event, its voltage or amplitude, its positive or negative electrical polarity and its scalp distribution, all of which provide clues about the underlying neural computations3.
ERP studies allow us to obtain information about the basic neural processes underlying higher-order, complex cognitive operations4. The ERP method is primarily used in psychological and neuropsychiatric studies. Some of the advantages associated with ERPs over other neuroimaging modalities, such as functional magnetic resonance imaging (fMRI) and near- infrared spectroscopy (NIRS), include its excellent temporal resolution, which gives researchers the ability to follow the computing activity of the brain down to the millisecond, and its relative cost-effectiveness. This is crucial when testing two participants simultaneously as is the case in our study5,6.
For this experiment, we are mostly interested in the late posterior positivity (LPP), which is an ERP having a late latency (i.e. from 250 to 1000ms post stimulus onset). It is elicited by the presentation of meaningful stimuli, such as words, objects, faces, and scenes. The well-known P3b components belong to the LPP family, which peak around 600 ms post stimulus onset for words and at about 750 ms for face- and scene-stimuli. The greater the amount of new information placed in working memory, and thus in consciousness, and the more vivid, salient and certain this information is, the larger the amplitude of this potential will be7,8 When a stimulus-or any aspect of it, such as its exact time of occurrence-is unexpected, it elicits a larger LPP than when the stimulus and each of its aspects are fully predicted. A very large number of cognitive factors can thus have an impact on the amplitude of the LPP8,9.
Recording the EEG of two participants simultaneously as they are exposed to visual stimuli can help us evaluate whether or not the brain activity of one subject might influence the other's brain electrodynamics when neither sees what his/her partner is being shown.
Given that ERP voltages, scalp distributions and latencies all provide clues as to what neural computations are occurring, they can be measured to test any external impact on the brain and detect differences in the processing of visual stimuli in pairs of closely related individuals. To test the existence of such an impact , we focused on one operational hypothesis: the LPP elicited by a visual stimulus in one person could be affected by the stimulus displayed to his/her partner. This hypothesis is thus based on the idea that if stimulus processing of one person has an impact on the neural activities of another person, this new information arising from the brain of the former might modulate the amplitude of the LPP in the latter.
A more precise hypothesis was built from a complementary idea. The impact of the processing of the stimulus on the brain activity of a close other should be prevented when the stimulus is known to be different from the one seen by the close other. In effect, in that situation, this impact constitutes an irrelevant interference. To create that knowledge, the two participants of each pair were told that they would be presented with different stimuli. Nevertheless, only half of the trials of the experiment were consistent with this instruction. They thus constitute the different-stimulus condition, the DCS. The other half of the trials were inconsistent with this instruction. There, the stimuli simultaneously presented to the two subjects of each pair were the same and thus made up the identical-stimulus condition, the ISC. This latter condition was used in order to have a control condition in which the inhibition should not develop, as it would pertain to information corresponding to the stimulus actually presented to the close other. Our prediction was that, in the absence of such an inhibition, more information should enter the content of working memory, which could be responsible larger LPPs in the ISC than in the DSC. Moreover, finding such ERPs differences would confirm the possibility of an effect of stimulus processing on the ERPs of a close other given that subjects cannot see the actual stimulus their partners is presented with.
These predictions were confirmed in two previous experiments, which also showed that the two participants of each pair had to be socially close and not strangers.10,11 Nevertheless, in these experiments, the two participants of these pairs were not acoustically and visually separated. Despite the extreme unlikeliness that the ERP effects observed could be due to classical visual and/or acoustic communications between partners and given the importance the results would have for social cognition, we decided to introduce a glass- and a curtain-separation between partners to verify that the ERP differences persist.
However, we were aware that by doing this, the participants might no longer feel together during the experiment and that this could have an effect. Therefore, we felt important to remind the participants to try to feel the presence of their partner during the entire experiment, and at the debriefing session we asked them if they managed to do so.
Additionally, in the first two experiments assessing the effects of stimulus processing on the ERP of close others 10,11, the DSC and the ISC were corresponding to different blocks of trials, To prevent fatigue, strategy bias and other confounds, the experimental conditions for this experiment now correspond to trials randomized within blocks.
In this new experiment, the two participants (A and B) are each seated in front of their own computer screen in two adjacent rooms. The wall that separates them contains an 86-by-178-cm glass window that is covered on both sides by a curtain. Thus, the participants are seated side-by-side but can neither see nor hear each other during the actual experiment. However, right before the experiment, when they are being fitted with the EEG caps, the curtains are open and the participants can see each other and maintain a feeling of closeness. Once they are fitted with an EEG cap to record their brain activity and the EEG signals are checked for quality, the curtains are closed. However, most importantly, participants are instructed to try to continue feeling the presence of their partner during the entire experiment. The on-screen directives instruct each participant to try and memorize the images that will be flashing simultaneously, each on their respective screens, and to avoid excessive blinking and facial movements.
Their belief in the nature of the two images is experimentally controlled via the on-screen directives that clearly inform them that they will always be exposed to different visual stimuli than what will be presented to their partner. However, as mentioned, each participant sees 200 images, 100 of which are actually different from the ones presented to their partner and form the consistent condition or DSC (i.e., the different-stimulus condition) and 100 of which are actually the same as the ones presented to their partner. They constitute the inconsistent condition or ISC (i.e., the identical stimulus condition). Thus, during an inconsistent ISC trial, both participants are simultaneously presented with an identical image. During a consistent DSC trial, both participants are simultaneously presented with a different image. The order of these trials is randomized.
We systematically explored the ERPs in time windows earlier than that of the LPPs to detect indexes of the inhibition that the different-stimuli-instruction should trigger when the stimuli of the trial actually differ. We found that between 75-150ms post image onset, the absolute value of the subtraction of the mean voltages of the ERPs of the DSC trials from those of the ISC-trails were greater in participants who felt together during the experiment than in those who did not. This was observed at right frontal electrode sites, especially at F8, and thus over the ventro-lateral prefrontal cortex. Based on our previous works on inhibition and negative ERP-components16,17,18, we selected, among the participants who felt together, those in whom ERPs to DSC-trials were more negative than those to ISC-trials and thus those in whom the inhibition might have occurred. As expected, these particular participants had significantly smaller LPPs for the consistent DSC-trials than for the inconsistent ISC-trials (see Figure 4). These results suggest that a greater amount of information entered the content of working memory in ICS trials, with this information potentially becoming more salient and/or vivid, and/or being integrated with more confidence. Moreover, they prove the existence of an effect of stimulus processing on ERPs of close others given the impossibility for participants to see the image actually presented to their partners and the impossibility to communicate.
Protocol
All methods described herein were pre-approved by the Douglas Institute Research and Ethics Board.
1. Participant recruitment, in-lab greeting and questionnaires
- Recruit pairs of participants (close friends/siblings/spouses ages 18-35) and inform them they will have to separately complete a Friendship Eligibility Questionnaire upon arrival in the lab to ensure only close others are included in the experiment[see Annex 1 for an example advertisement].
- Make sure they meet all the other inclusion criteria (i.e. right-handedness, university level education, perfect or corrected to perfect vision, no contact lenses, no dreads, no drug abuse, no psychiatric disorder, no use or psychotropic medications). Schedule their visit to the lab if they are eligible.
- Greet the pair of participants upon their arrival at the lab. Obtain informed consent, separate them and have each participant fill out the Friendship Eligibility Questionnaire and McGill Friendship Questionnaire alone.
- This is used to assess their attitude about their relationship and to exclude partners who are not close enough and do not reach the minimum score of 13 correct answers.
- Once their answers have been sent to the lab's database, check for at least 13 correct answers from each participant.
- Escort the partners into the EEG recording rooms. Turn on the stimulus presentation computer and the EEG acquisition computer. Start the EEG acquisition application [Photo 1] and set the status of the EEG channels to " impedance check" [Photo 2].
- Have each participant sit at a designated computer desk in the adjacent rooms separated by a glass window. Keep the curtains open so that each participant can see his or her partner. Encourage them to talk (e.g., about their answers at the Friendship Eligibility Questionnaire) in order to maintain the feeling of the presence of the other.
2. Electrode cap placement (see Gu et al., 2014)
- Measure the size of the head of the participant and use the pencil to mark Fp1 and Fp2 electrode sites and select the cap of appropriate size.
- Clean the forehead and earlobes of each participant with an alcohol swab.
- Insert two frontal sticky sponge discs in the EEG electrode cap on Fp1 and Fp2.
- Place the sticky ends of the discs against the forehead of the participant at the marked Fp1 and Fp2 locations. Ask the participant to press them firmly and pull the cap over the head to snugly fit the cranium. Check whether the cap is fitted symmetrically over the head (both from right vs. left and forward vs. backward perspectives) and then connect the EEG outlet to the amplifier's plug.
- Using a 10mL blunt needle tip syringe, gently but firmly touch the scalp of the participant and move the needle sideways to tease the hair apart. Insert conductive gel (~0.5mL) from that position on the scalp and up to create a column of gel in the ground electrode first. Then, insert gel into both ear electrodes and attach them to the earlobes. Plug the left ear electrode into the top channel and the right one, which will be used as the reference, below it in its amplifier box.
- Using a sterilized blunt needle tip previously fitted to the syringe, move the hair strands apart by wiggling the syringe in all the other electrode sites, ensuring that the tip is in contact with the scalp. Then, start inserting conductive gel in each of the other electrode placements with a slow upward motion in order to build a column of gel that will go from the scalp to the metal of the electrode.
- Use a sterile sharp needle to cautiously and gently scratch the surface of the scalp through each electrode, starting with the ground and the ears, to remove dead skin and increase electrical conductance by having the gel make contact with the living cells of the scalp and earlobes.
- Check for proper impedance while scratching the scalp. The lights corresponding to the electrode channels on the amplifier boxes will change color from orange to green as the impedance for each channel drops below 5 kΩ [see Photo 3].
- Note: if a particular electrode is not functioning properly, add more gel and scratch a bit more with the needle. If the problem persists, use a shortcut wire by plugging it inside the slot for the faulty electrode on the amplifiers and hooking up the other end to the electrode placement in the EEG cap.
3. EEG/ERP data recording 4. EEG/ERP data recording
- Just prior to the experiment, instruct the participants to try to feel the presence of their partner during the entire testing period. Then, draw the curtains on both sides of the double-glass window, dim the lights, and close the door of each participant's room.
- Type in the appropriate command for the given stimulus sequence in order to run the stimulus presentation software. Then, start recording the EEG of both participants while they are presented with the visual stimuli simultaneously.
- Once the stimulus presentation sequence is complete, stop recording the EEG data.
- At the end of the experiment, carefully remove the EEG caps and assist the participants in washing and drying their hair.
- After the participants have cleaned their hair, have them complete a debriefing questionnaire in which they report the degree to which they felt the presence of their partner, specifically during which part of the experiment and for how long they felt this way.
- Detach the cap and ear electrodes from the amplifiers, remove the disposable sponge discs, and clean the cap and ear electrodes under running water. Use a mild soap and a toothpick to clear the gel from the electrodes, Rinse thoroughly and allow the EEG cap to air dry.
- Save the recorded data on USB drive by inserting the USB drive into one of the USB ports on the EEG data acquisition computer and dragging the data file into the USB directory. Then, transfer the data to another computer for data processing.
4. Data processing
Note: all data processing is done using EEGLab.15
- Open the data processing software [see Table of Materials] and then, EEGLab by typing "eeglab" in the command interface [see Screenshot 1 & 2].
- Import the data file. For this step, first, click "File" on the EEGLAB GUI, select "Import data", select "Using EEGLAB functions and plugins", and click "From EDF/EDF+/GDF files (BIOSIG toolbox)" [see Screenshot 3]. Choose the desired data file.
- Create and EEG event list, which consists of a list of entries that correspond to the difference types of visual stimuli that were used during experiment (i.e. the same image believed to be different labeled "S-BD" and the different image believed to be different labeled "D-BD"). To do this, click on "ERPLAB" on the EEGLAB GUI, select "EventList," and click "Create EEG EVENTLIST" [see Screenshot 4]. In the new window, enter the relevant information under "Event info" and "Bin info (optional)" for the S-BD category and click "Update Line". Repeat this process for the D-BD category. Click "APPLY" [see Screenshot 5].
- Extract the bin-based epochs, each epoch (or trial) consisting of a single ERP waveform that spans 1,204 ms from -204ms to 1000ms, where 0 corresponds to the onset of the visual stimulus. For this step, select "ERPLAB" on the EEGLAB GUI and click "Extract bin-based epochs" [see Screenshot 6]. In the new window under "Bin-based epoch time range (ms)," write "-204 -4". Click "RUN" [see Screenshot 7].
- Perform artifact detection on the epochs. This step removes all the trials that have altered been by amplifier saturation or A/D clipping. Epochs with segments that are inferior to -100 microvolts and/or superior to +100 microvolts will be eliminated for the 4 frontal EEG electrodes (Fp1, Fp2, F7, and F8). Similarly, epochs with segments that are inferior to -75 microvolts and/or superior to +75 microvolts will be deleted for the remaining 24 non-frontal electrodes. Additionally, epochs containing segments that include flat lines that persist for more than 100ms will be cut out in all 28 electrodes. To eliminate the extreme voltages, first click "ERPLAB" on the EEGLAB GUI, then select "Artifact detection in epoched data" and click "Simple voltage threshold" [see Screenshot 8]. In the new window under "Test period (start end) [ms]," write "-204 1000"; under "Voltage limits[uV] (e.g. -100 100):" write "-100 100"; under "Channel(s)," write "1:4" (to select only the 4 frontal electrodes). Select "ACCEPT" [see Screenshot 9]. Repeat this process for the remaining 24 electrodes, making the appropriate changes where necessary (i.e. writing "-75 75" instead of "-100 100" for the voltage limits; writing "5:28" instead of "1:4" to select the remaining 24 electrodes). Next, to remove the flat lines, click "ERPLAB" on the EEGLAB GUI, select "Artifact detection in epoched data", and click "Blocking & flat line" [see Screenshot 10]. In the new window under "Test period (start end) [ms]", write "-204 1000"; under "Amplitude tolerance (single value, e.g. 2):", write "-1e-07 1e- 07"; under "Duration [ms]", write "100"; under "Channel(s)", write "1:28" (to select all 28 electrodes). Select "ACCEPT" [see Screenshot 11].
- Compute the averaged ERPs of each participant for each condition (consistent vs. inconsistent). To do this, click "ERPLAB" on the EEGLAB GUI and select "Compute averaged ERPs" [see Screenshot 12].
- Compute the grand averages for the ERP sets in each condition (consistent vs. inconsistent) and plot the resulting ERP waveforms. For this step, click "ERPLAB" on the EEGLAB GUI and select "Average across ERPsets (Grand Average)" [see Screenshot 13]. In the new window, add the relevant ERP sets by clicking "Add Erpset" and then, click "RUN" [see Screenshot 14]. To plot the ERP waveforms, click "ERPLAB" on the EEGLAB GUI, click "Plot ERP", and select "Plot ERP waveforms" [see Screenshot 15]. In the new window under "Time range (min max, in ms)", write "-204.0 1000.0" and click the button "positive is up" (this will change the label of the button to "negative is up" so that negative y-values are displayed above the x-axis); under "Style", select "Topographic" and change the "w" and "h" values to "0.1". Click "PLOT" [see Screenshot 16].
Representative Results
Three figures have been presented herein. Each part of these figures (28 parts in total) represents a single EEG channel with its own label (i.e. Fp1, Fp2, F7, F8, etc.). Figure 1 shows a typical example of "good" results, depicting ERP waveforms obtained from a single participant. The black lines correspond to the consistent condition and the red lines correspond to the inconsistent condition. In contrast, Figure 2 depicts "poor" results due to a problematic session for which the waveforms portray either unintelligible ERP components, flat lining, or noise. These were also obtained from one participant. The black lines correspond to the consistent condition and the red lines correspond to inconsistent condition. Figure 3 shows a grand average of 27 ERP sets from the participants who felt together during more than 50% of the experiment. The black lines correspond to the control-consistent category and the red lines correspond to the critical-inconsistent category. Figure 4 is a depiction of the average of ERP's from the 13 individuals who felt together for more than 50% of the trials and for whom the inconsistent condition was more positive at the F8 electrode site for the 75-150ms time window. The inconsistent condition is more positive than the consistent condition for most electrodes.
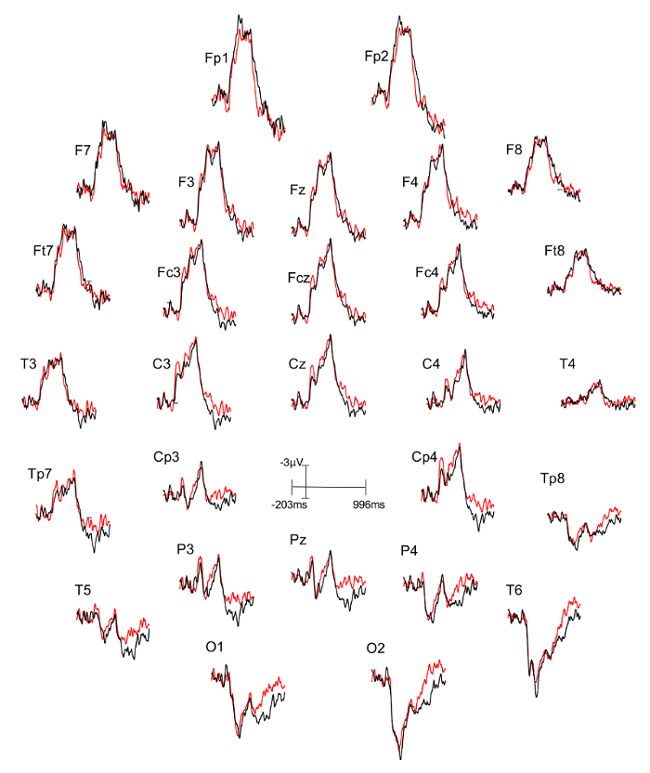
Figure 1: Typical "good" results representing ERPs from one participant. Each part (28 parts in total) represents a single EEG channel with its own label (i.e. Fp1, Fp2, F7, F8, etc.). The ERP components are well defined in the waveforms. The black lines correspond to the consistent condition (different stimulus condition, or DSC) and the red lines correspond to the inconsistent condition (identical stimulus condition, or ISC). Please click here to view a larger version of this figure.
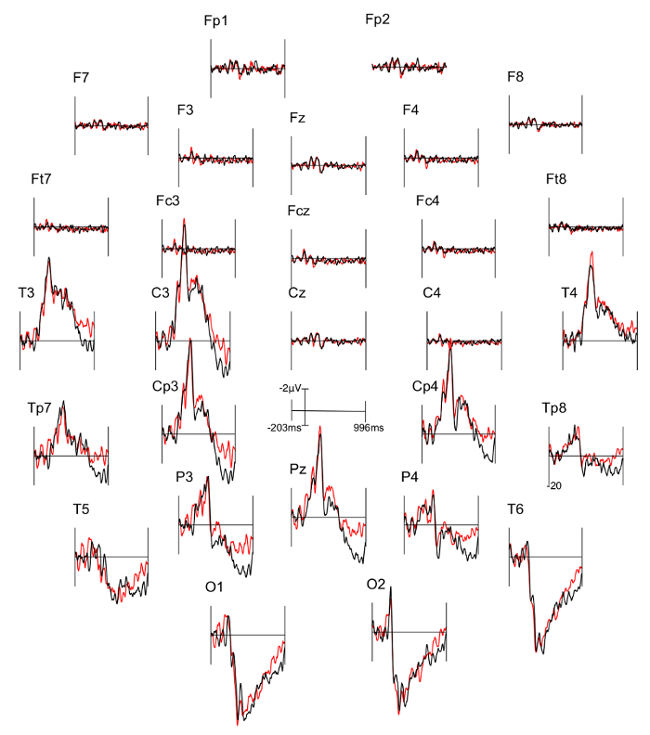
Figure 2: Typical "Poor" results representing ERPs from one participant. Each part (28 parts in total) represents a single EEG channel with its own label (i.e. Fp1, Fp2, F7, F8, etc.). The black lines correspond to the consistent condition (DSC) and the red lines correspond to inconsistent condition (ISC).
The ERP components are not well defined in the waveforms and many are marked by a flat-line (i.e. F8, Fc4). Please click here to view a larger version of this figure.
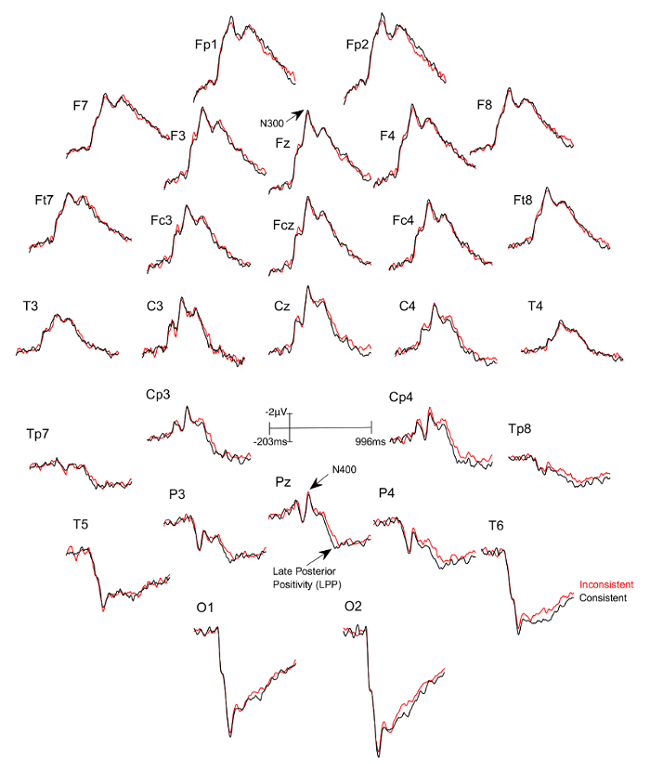
Figure 3: Grand averages of ERPs of the 27 participants who felt together.
Each part (28 parts in total) represents a single EEG channel with its own label (i.e. Fp1, Fp2, F7, F8, etc.). The black lines correspond to the consistent condition (DSC) and the red lines correspond to the inconsistent condition (ISC). Please click here to view a larger version of this figure.
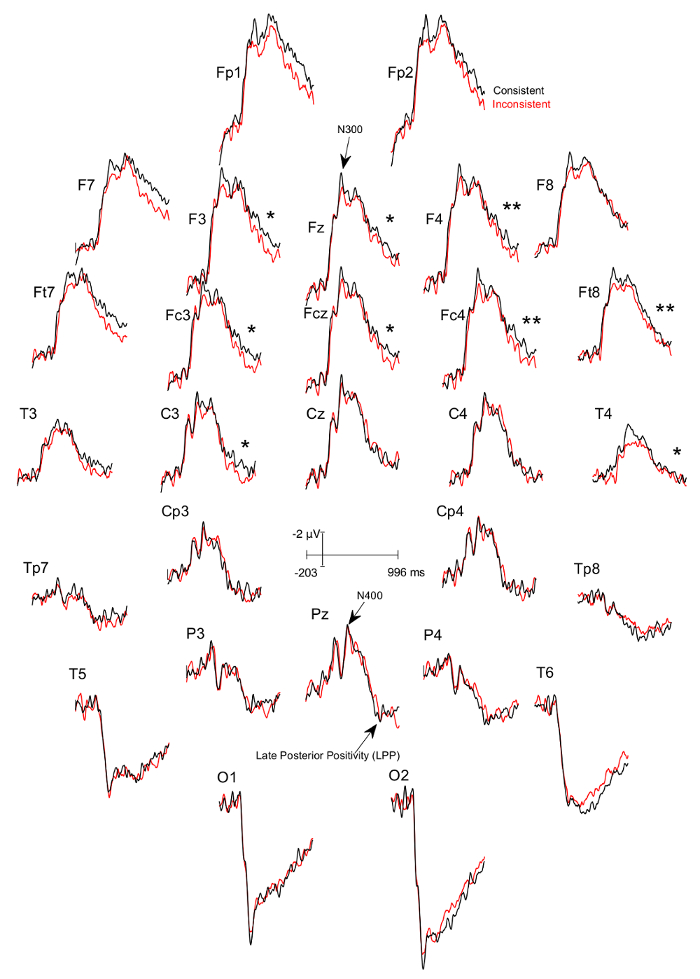
Figure 4: Grand averages of ERPs of the 13 participants who felt together and for whom the ERPs to the consistent DSC-trials was more negative at the F8 electrode site between 75-150ms than the ERPs to the inconsistent ISC-trials. Each part (28 parts in total) represents a single EEG channel with its own label (i.e. Fp1, Fp2, F7, F8, etc.). The black lines correspond to the consistent condition and the red lines correspond to the inconsistent condition. There is a significant difference in the 600 – 900 ms time window between the consistent and inconsistent condition at F3 (p=0.024), F4 (p=0.001), Fz (p=0.024), Fc3 (p=0.041), Fcz (p=0.022), Fc4 (p=0.002), Ft8 (p=0.004), C3 (p=0.022), and T4 (p=0.039), with the inconsistent condition being more positive. Please click here to view a larger version of this figure.
Supplemental File 1 Please click here to download this file.
Supplemental File 2 Please click here to download this file.
Supplemental File 3 Please click here to download this file.
Supplemental File 4 Please click here to download this file.
Discussion
In our investigation about the possibility that the brain of an individual is sensitive to the stimulus processing of another, we recorded the EEG of pairs of participants as they were each presented a set of images.
We manipulated the sameness of the images that were shown to both participants. Each person was instructed via on-screen directives that what he/she would be seeing would always differ from what his/her partner would. Half of the time, participants were shown different images (i.e. consistent condition) and half of the time, the same image (i.e. inconsistent condition). Trials were randomized between inconsistent and consistent conditions.
If the stimulus processing of one can influence the electrodynamics of the other’s brain, and vice-versa, then the mean voltages of the LPP component for the inconsistent trials could be different from those of the consistent ones across sessions. Indeed, our preliminary results are in agreement with our hypothesis: the LPP values for the critical session are different from those of the control session as a function of consistency. This effect occurred in the absence of block bias and of any possible covert detection of inconsistencies due to noise from the partner, such as changes of breathing induced by shocking visual stimuli.
The objective of this article was to introduce a novel paradigm involving EEG for testing pairs of participants simultaneously. Regarding the actual EEG recording, it is important to belabor a few points. First, it is crucial that the cap is snugly fit. A cap that is too large can affect the quality of the recording by having instable gel columns and thus varying the impedance19. Second, it is also important that the participants understand that they must avoid excessive movements, blinking or flexing of facial and cervical muscles, as these will potentially skew the EEG traces, rendering the data very difficult to interpret20. After the experiment, the equipment must be properly cleaned to ensure that the electrodes are not electrically insulated by dry gel residue, which could affect future signal collection. Third, if there are problems in the signal, such as noise or flat lines, ensure that both the ground and reference electrodes are properly connected. Reducing the impedance of all electrodes reduces noise as it prevents them from acting as antennae that capture ambient electromagnetic noise. Therefore, if there are problems in the connectivity, the gel must be re-applied and and the scalp underneath the electrodes re-scratched. If there are myograms on the EEG, we must allow the subject to relax, reminding him/her to relax his/her facial and cervical muscles before proceeding with the experiment.
At the outset of any EEG experiment, it is important to bear in mind the limitations associated with this technique. For example, its suboptimal spatial resolution may be something to consider. Another consideration is the EEG’s sensitivity to eye blinking, muscle activity and bodily movements, which introduce artifacts in the recording21. Overall, these limitations may be addressed with alternative neuroimaging methods such as fMRI and NIRS or by combining EEG with these other alternatives. Even so, with respect to alternative brain imaging techniques, EEG has its own advantages, the most obvious one being its remarkable temporal resolution, allowing researches to probe neural activity on the order of milliseconds. It is also a non-invasive and pain free tool, posing no risk to the participant. In addition, EEG is relatively cheap when compared to other neuroimaging techniques. As such, it was the obvious choice of monitoring technique in the novel pair-testing approach presented in this article.
Divulgations
The authors have nothing to disclose.
Materials
| EEG acquisition software | Psychlab | http://www.psychlab.com/softw_general.html | |
| 8 Digital EEG Amplifiers (NuAmp) | Neuro Scan Labs | ||
| 2 computers | |||
| Matlab | The MathWorks, Inc | http://www.mathworks.com/products/matlab/ | |
| EEGLab Matlab toolbox | http://sccn.ucsd.edu/eeglab/ | ||
| ERPLAB Toolbox | http://erpinfo.org/erplab | ||
| Stimulus generation software | E-Prime | ||
| ECI Electrode cap | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/caps/ | |
| Special Head Measuring Tape (4 Color ribbon) | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| Disposable Sponge Disks | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| Cap straps | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| Electro-gel | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| Blunt needle (BD Vacutainer PrecisionGlide Multiple Sample Needle) | Becton, Dickinson and Company | ||
| 2 Syringes | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| 4 Ear Electrodes | Electro-cap International, Inc | http://www.electro-cap.com/index.cfm/supplies/ | |
| Alcohol wipes | |||
| 2 Red pencils | |||
| Facilities and supplies for participants to wash their hair after the experiment- sink, shampoo, comb, towels, hair dryer |
References
- Bressler, S. L., Arbib, M. A. . The Handbook of Brain Theory and Neural Networks. , 412-415 (2002).
- Peterson, N. N., Schroeder, C. E., Arezzo, J. C. Neural generators of early cortical somatosensory evoked potentials in the awake monkey. Electroencephalogr Clin Neurophysiol. 96 (3), 248-260 (1995).
- Bressler, S. L., Mingzhou, D. Event-Related Potentials. Wiley Encyclopedia of Biomedical Engineering. , 1-8 (2006).
- Blackwood, D. H., Muir, W. J. Cognitive brain potentials and their application. The British Journal of Psychiatry Supplement. 9, 96-100 (1990).
- Di Russo, F., Pitzalis, S. EEG-fMRI Combination for the Study of Visual Perception and Spatial Attention. Cognitive Electrophysiology of Attention. , 58-70 (2014).
- Strait, M., Scheutz, M. What we can and cannot (yet) do with functional near infrared spectroscopy. Front Neurosci. 8 (117), 1-12 (2014).
- Gratton, G., Bosco, C. M., Kramer, A. F., et al. Event-related brain potentials as indices of information extraction and response priming. Electroencephalogr Clin Neurophysiol. 75 (5), 419-432 (1990).
- Donchin, E., Coles, M. G. H. Is the P300 a manifestation of context up-dating. Behav Brain Sci. 11 (3), 357-374 (1988).
- Brower, H., Fitz, H., Hoeks, J. Getting real about semantic illusion: rethinking the functional role of the P600 in language comprehension. Brain Res. 1446, 127-143 (2012).
- Bouten, S., Pantecouteau, H., Debruille, J. B. Finding indexes of spontaneous brain-to-brain communications when looking for a cause of the similarity of qualia assumed across individuals. F1000Research. 3, 316 (2015).
- Haffar, M., Pantecouteau, H., Bouten, S., Debruille, J. B. Further data for a potential cause for the similarity of percepts assumed across individuals. Journal of Cognitive Neuroscience. (H39), 232 (2016).
- Gu, V., Mohamed Ali, O., L’Abbée Lacas, K., Debruille, J. B. Investigating the Effects of Antipsychotics and Schizotypy on the N400 Using Event-Related Potentials and Semantic Categorization. J. Vis. Exp. (93), e52082 (2014).
- . Regents of the University of California Available from: https://sccn.ucsd.edu/eeglab/downloadtoolbox.php (2017)
- Touzel, M., Snidal, C., Segal, J., Debruille, J. B. The fronto-central N1 event-related potential could index an early inhibition of the actions systematically activated by objects. , (2018).
- Debruille, J. B., Brodeur, M. B., Porras, C. F. N300 and social affordances: a study with a real person and a dummy as stimuli. PLoS One. 7 (10), e47922 (2012).
- Debruille, J. B., et al. The N400 potential could index a semantic inhibition. Brain Research Reviews. 56 (2), 472-477 (2007).
- Light, G. A., et al. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr Protoc Neuro. , 21-24 (2010).
- Luck, S. J. . An Introduction to the Event-Related Potential Technique. , (2005).
- Giacino, J. T., Fins, J. J., Laureys, S., Schiff, N. D. Disorders of consciousness after acquired brains injury: the state of the science. Nat Rev Neurol. , 1-16 (2014).

