A Method for Investigating Change Blindness in Pigeons (Columba Livia)
Summary
Change blindness is a phenomenon of visual attention, whereby changes to a visual display go unnoticed under certain specific circumstances. This protocol describes a variation on the flicker paradigm for investigating change blindness that is appropriate and effective for research with pigeons.
Abstract
Change blindness is a phenomenon of visual attention, whereby changes to a visual display go unnoticed under certain specific circumstances. While many laboratory procedures have been developed that produce change blindness in humans, the flicker paradigm has emerged as a particularly effective method. In the flicker paradigm, two visual displays are presented in alternation with one another. If successive displays are separated by a short inter-stimulus interval (ISI), change detection is impaired. The simplicity of the procedure and the clear, performance-based operational definition of change blindness make the flicker paradigm well-suited to comparative research using nonhuman animals. Indeed, a variant has been developed that can be implemented in operant chambers to study change blindness in pigeons. Results indicate that pigeons, like humans, are worse at detecting the location of a change if two consecutive displays are separated in time by a blank ISI. Furthermore, pigeons' change detection is consistent with an active, location-by-location search process that requires selective attention. The flicker task thus has the potential to contribute to investigations of the dynamics of pigeons' selective spatial attention in comparison to humans. It also illustrates that the phenomenon of change blindness is not exclusive to humans' visual perception, but may instead be a general consequence of selective attention. Finally, while the useful aspects of attention are widely appreciated and understood, it is also important to acknowledge that they may be accompanied by specific imperfections such as change blindness, and that these imperfections have consequences across a wide range of contexts.
Introduction
Cognitive psychology has repeatedly demonstrated striking and often surprising imperfections in our own cognitive processes. Some of the more notable examples include but are not limited to false memories1, suboptimal decision heuristics2, and faulty statistical reasoning3. A more recent addition to this list is the phenomenon of change blindness. Change blindness is a consistent failure of attention, in which one fails to notice even prominent changes to one's environment. In one demonstration4, experimenters had a confederate approach individuals to request directions. During their conversation, workers carrying a door passed between the two, briefly interrupting visual contact and providing an opportunity to swap out the confederate for a different person. After this surreptitious exchange, most individuals failed to notice that their conversation partner was no longer the same person. This failure is surprising because moment-to-moment changes would seem to be signals of potentially important events that ought to draw our attention.
In order to better understand how and why change blindness occurs, researchers have brought it into the lab and developed several ingenious procedures5,6,7,8 for studying it under more controlled conditions. One particularly successful approach has been dubbed "the flicker task"9. In this procedure, the experimenters edited photographs by removing a feature, and then presented the images to participants, rapidly alternating between the original and modified versions. Participants quickly spotted the differences. However, if a brief blank field was inserted between consecutive images (producing a flickering display for which the procedure is named) change detection was much more difficult, resulting in worse accuracy and slower response times. This procedure is appealing because it provides a precise measure of change blindness, and it is easy to manipulate specific aspects of the display such as the size, salience, or timing of a change.
The flicker paradigm has proven to be a powerful tool for learning about perception and attention in humans10. The effect is surprisingly powerful and persistent. Change blindness can occur for changes to the only object in a simple animation11, and when looking directly at a change location12. Even experience with change blindness and awareness of the phenomenon does not eliminate it13. Furthermore, change blindness is quite diverse, and can be induced by a number of different events, such as eye saccades5, mudsplashes14, motion picture cuts7, or visual occlusion4. A parallel phenomenon occurs in auditory15 and tactile16 modalities, indicating that it may not be exclusive to visual stimuli and may be a more general phenomenon of attention.
Humans of course, are not the only animal that makes use of attention. Pigeons, for example, show many of the same attentional abilities that humans do. They can select specific features for preferential processing (as when they use a search image to find specific food targets)17,18. They can direct attention toward specific regions or spatial locations19. They can shift their attention between hierarchical levels of organization20,21. They can also divide attention between different aspects of a stimulus display22,23. It seems then, that pigeons possess many of the same abilities that make attention useful for humans. If change blindness has to do with some of the inherent limitations of attention, we might expect to see a parallel change blindness effect in pigeons (and perhaps other animals). Furthermore, there have recently been multiple successful studies of change detection conducted using pigeons, implementing widely varying methods24,25,26,27,28.
Recent research29,30,31,32 has adapted the flicker paradigm to investigate change blindness in pigeons, and has produced results that parallel human change blindness. The current report describes a simple method for implementing this procedure in an operant chamber. As with human versions of the task, it is easy to isolate and manipulate specific aspects of stimulus presentation in order to investigate their influence over change detection and change blindness. Thus, the procedure should constitute a valuable tool for research on the limitations of avian attention, and the extent to which they are similar to the limitations of human attention.
Protocol
The procedure described here is in accordance with the Office of Laboratory Animal Welfare and with US Public Health Service Policy on Humane Care and Use of Laboratory Animals, and was approved by Whitman College's Institutional Animal Care and Use Committee.
1. Reduce Pigeons' Weights
NOTE: Pigeons' weights are reduced to 80 – 85% of their free-feeding weight33 to ensure that the birds are healthy and adequately motivated to work for food.
- House naïve birds in individual cages with unlimited access to water, grit, and food.
- Weigh each pigeon at approximately the same time each day for 2 to 4 weeks, or until each bird's free-feeding weight has stabilized.
- Calculate a target weight for each pigeon equal to 85% of its stable free-feeding weight.
- Restrict food to gradually reduce each pigeon's weight until the target weight is reached34. Pigeons should still have unrestricted access to water and grit.
NOTE: Published experiments using this protocol29,30,31,32 have yielded significant results using between 4 and 6 pigeons per condition. Similar numbers should be adequate for a direct replication or subtle variation. Variations that reduce the magnitude of the change blindness effect could require a larger sample.
2. Train Pigeons to Peck Stimuli Displayed on the Response Keys in the Operant Chamber and to Eat Grain from the Food Hopper
NOTE: Training and experimental sessions require precise computer control, with temporal resolution of less than 1 ms. Use a flexible programming language (see Table of Materials) to control operant chambers via an I/O relay.
- At the beginning of each day's session, weigh pigeons and place them into operant chambers (see Table of Materials). Naïve pigeons may need time to acclimate to handling, weighing, and transport to and from the operant chamber. Until then, take extra care to handle birds gently and monitor them for signs of stress.
- Run 100 trials per daily session. For each trial:
- Randomly select a visual stimulus element (such as a color or line) and one of the three keys (see Table of Materials) in the operant chamber. Illuminate the selected stimulus element on the relevant key using a compatible stimulus projector (see Table of Materials).
NOTE: The onset and offset times of the incandescent bulbs that are standard in many operant chambers are too slow to be appropriate for this method. Replace any incandescent bulbs in the operant chamber with a faster LED equivalent, and then confirm that the displays appear as intended and that the onset of stimuli is crisp (less than 1 ms from onset to peak brightness). - Wait until a pigeon pecks the key on which the stimulus is displayed. Do not acknowledge pecks to any other keys.
NOTE: Experienced pigeons may immediately know to peck illuminated response keys. Naïve or less experienced birds' pecking can be shaped using handshaping or autoshaping35 procedures as they would be for other laboratory tasks. - Following a single peck to the proper response key, clear the stimulus display and provide access to grain from the food hopper for 2 – 3 seconds.
- Randomly select a visual stimulus element (such as a color or line) and one of the three keys (see Table of Materials) in the operant chamber. Illuminate the selected stimulus element on the relevant key using a compatible stimulus projector (see Table of Materials).
- At the end of each session, remove pigeons from operant chambers and weigh them before returning them to their home cages. Adjust food access time between sessions to maintain birds' individual running weights at 80 – 85% of their free-feeding weights.
- Continue pre-training sessions until pigeons respond quickly and consistently to all of the individual stimulus elements to be included in the experiment, on all three response keys.
3. Train Pigeons to Search for and Peck Changes Presented on Response Keys
- At the beginning of each day's session, weigh pigeons and place them into operant chambers.
- At the beginning of each of 100 trials in a daily session, determine the details of the upcoming stimulus display. Details for each trial can be randomly selected by the experimental control software. A sample program to run a daily experimental session is included as a supplemental file (change.cpp); elements present there could also be used to perform the simpler actions in step 2.
- Randomly choose an Inter-Stimulus Interval (ISI) of either 250 ms or 0 ms (with p = 0.5 for each).
- Randomly determine the number of change repetitions to present, either 1, 2, 4, 8, or 16 (with p = 0.2 for each).
- Define an original stimulus display consisting of one or more elements (the colors or lines presented during pretraining) on each response key.
- Change the original stimulus display to define a modified display by adding, deleting, or changing one element on one key. See Figure 1 for examples of original and modified stimulus displays.
- Ensure that pigeons will not see all possible stimulus displays during training. If necessary, designate a subset of displays for transfer (step 4) and refrain from presenting them during training.
- Present a 5 s Inter-Trial Interval (ITI), with the houselight on and all response keys dark to separate each trial from the immediately preceding trial.
- Present a stimulus display using the values determined for the current trial in step 3.2.
- Illuminate the stimulus elements that compose the original display for 250 ms.
- Clear the stimulus display and wait for an ISI of either 0 or 250 ms.
- Illuminate the stimulus elements that compose the modified display for 250 ms.
- Clear the stimulus display and wait for an ISI of either 0 or 250 ms.
- Repeat steps 3.4.1 to 3.4.4 until completion of the number of repetitions previously determined for the current trial. Present all repetitions in their entirety and ignore any keypecks during stimulus presentation.
- Illuminate all three response keys with white light and wait until a pigeon pecks one of the three response keys. Consider the first peck on any response key after stimulus presentation is complete to be the response for that trial. See Figure 2 for a schematic description of trials with or without an ISI.
- Clear all keys and conclude the trial with either reinforcement or an error signal:
- If a bird's response was on the key that displayed a change, provide access to grain from the food hopper for 2 – 3 s.
- If a bird's response was not on the key that changed, switch the houselight between on and off every 0.5 s for 10 seconds to indicate an incorrect response.
- At the end of each session, remove pigeons from operant chambers and weigh them before returning them to their home cages. Adjust food access time between sessions to maintain birds' individual running weights at 80 – 85% of their free-feeding weights.
- Continue daily training sessions until the accuracy of pigeons' responses is stable, and reliably better than chance accuracy of 33%. A sample file is provided (change.xlsx) that analyzes raw data to show the effects of ISI presence and number of repetitions.
4. Present Novel Transfer Trials Within Daily Sessions
- Follow the procedure exactly as outlined in step 3, but without any potential displays excluded (see step 3.2.5).
NOTE: With large numbers of potential stimulus displays, it is not necessary to exclude potential displays during training and introduce them later. In those cases, simply continue training as normal, as never-before-seen displays will occur naturally. - Analyze accuracy on novel, never-before-seen stimulus displays, excluding any displays pigeons have previously encountered. Better than chance accuracy will confirm that pigeons have learned a general change detection rule and are not relying on memorization of familiar stimulus displays.
Representative Results
The primary result of interest is the difference in accuracy between trials with and without an ISI. In particular, the operational definition of change blindness in the flicker paradigm is significantly reduced change-detection accuracy on trials with an ISI relative to trials without an ISI. This effect can be seen in Figure 3, which shows previously published data29. In that experiment, pigeons detected changes to stimuli consisting of color elements (the type depicted on the left side of Figure 1). As shown in the figure, pigeons saw 10 kinds of stimuli, differing based on the number of change repetitions (2, 4, 8, 16, or 32 on the x-axis) and presence of a 250 ms ISI (separate lines). Average accuracy on trials with an ISI (M = 76.4%) was worse than on trials without an ISI (M = 79.6%), F(1, 3) = 11.338, p = .043, partial η2 = .791. This figure also shows that this difference in accuracy indicative of change blindness was present for each number of repetitions tested.
Figure 3 also shows a secondary result of interest: change detection accuracy is influenced by the number of repetitions presented. In particular, change detection accuracy increased along with the number of repetitions, F(4, 12) = 11.104, p = .001 partial η2 = .787. This pattern is consistent with the interpretation that pigeons engaged in a serial search, much the way humans do in similar change detection tasks9, and supports the possibility that change detection requires an active search process requiring attention. Additional repetitions afford additional opportunities to consider more potential change locations, and attention gets directed to different spatial locations accordingly. Lower accuracy on trials with few repetitions may largely be a consequence of not having sufficient time to consider the actual change location before stimulus presentation was complete.
Figure 4 summarizes in a parallel fashion additional previously published results from the same lab30. The experiment presented pigeons with stimuli consisting of line segments (the type depicted in the right side of Figure 1), again for a fixed number of repetitions (1, 2, 4, 8, or 16) and with or without an ISI. Note that the condition depicted in the bottom panel shows both of the effects described above in Figure 3. Accuracy on trials with an ISI (M = 45.6%) was worse than on trials with no ISI (M = 75.8%), F(1, 3) = 60.571, p = .004 partial η2 = .953. Furthermore, accuracy increased along with the number of repetitions, F(1, 3) = 23.452, p < .001 partial η2 = .887. Thus, the same pattern of findings relevant to change blindness were obtained using the same general procedure, but using different kinds of stimulus elements.
The top panel of Figure 4 shows results from a different condition of the same experiment, in which the timing of change displays was manipulated. This condition revealed no change blindness effect. That is, there was no difference in accuracy between trials with (M = 66.1%) and without (M = 67.4%) an ISI, F(1, 3) = 0.189, p = .693 partial η2 = .059. This negative result suggests that change blindness can be dependent on the particular details of stimulus presentation, and that investigation of factors such as timing and salience may be informative in the future.
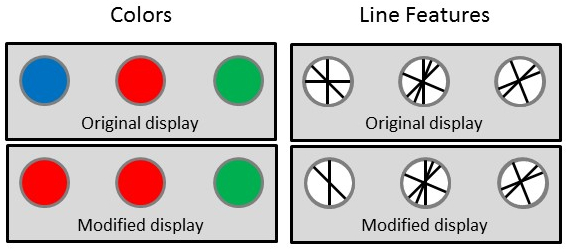
Figure 1: Examples of paired (original and modified) stimulus displays that make up change detection displays. Displays on the left consist of color elements (representative of displays from Herbranson & Jeffers, 201729). Displays on the right consist of line segments (representative of displays from Herbranson & Davis, 201630). In both examples, the change is on the leftmost key.
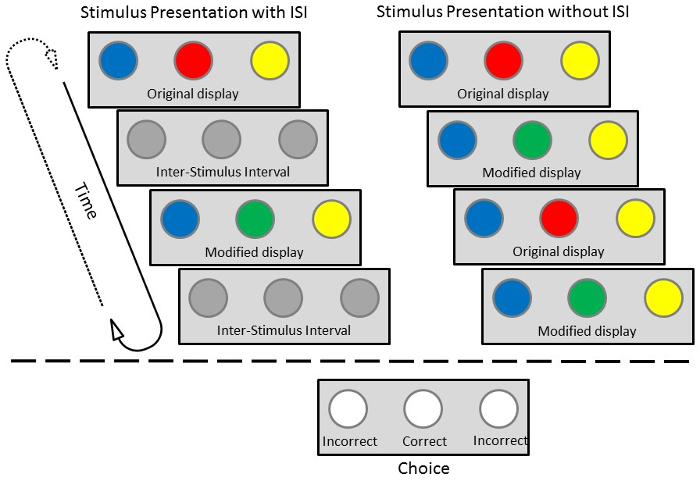
Figure 2: Schematic depiction of a trial with (left) or without (right) an ISI. The top section represents the stimulus presentation portion of a trial. The sequence on the left includes an ISI between each consecutive display. The sequence on the right does not. In both sequences, the change is on the center key (red-green). The bottom panel illustrates the subsequent choice portion of a trial. The correct response is the center key, corresponding to the location of the change. Please click here to view a larger version of this figure.
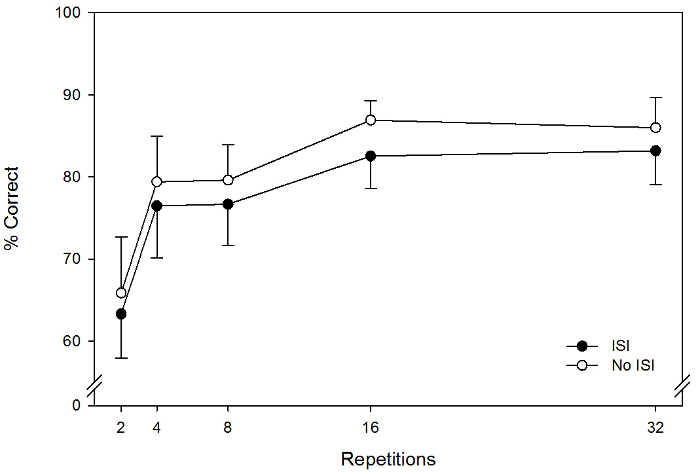
Figure 3: Rates of accurate change detection from Experiment 1 of Herbranson and Jeffers, 201729. This experiment used 250 ms display times and ISIs of either 250 ms or 0 ms). Error bars represent one standard error. The operational definition of change blindness is worse change detection on trials with an ISI (filled circles) relative to trials without an ISI (open circles). Note also that accuracy improves with the number of repetitions, indicating a serial search. Please click here to view a larger version of this figure.
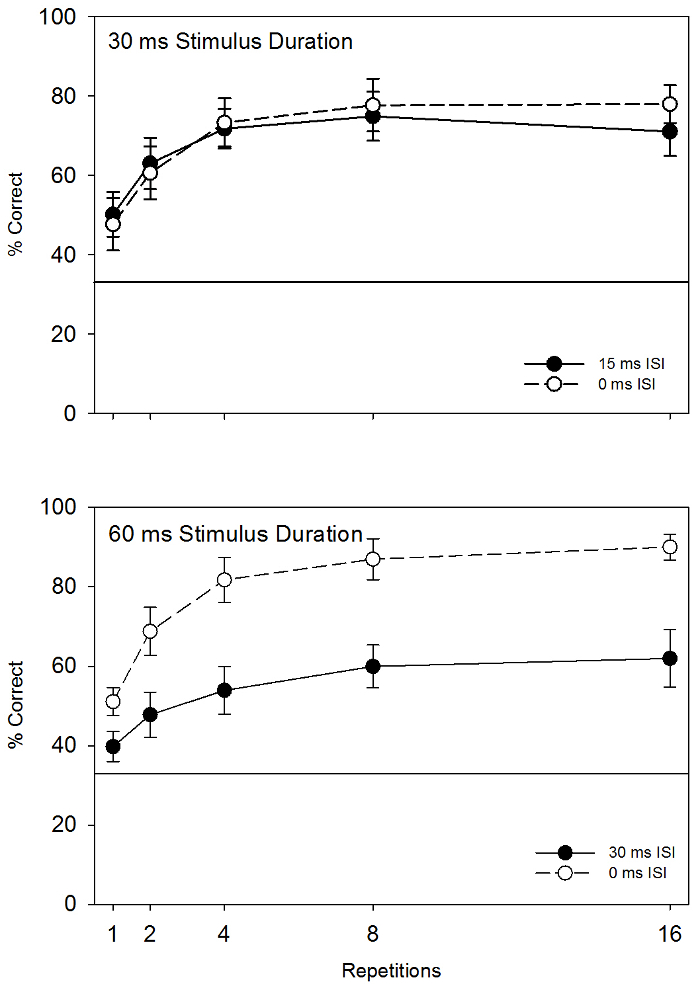
Figure 4: Rates of accurate change detection in two conditions from Experiment 2 of Herbranson and Davis, 201630. Error bars represent one standard error. The condition represented in the bottom panel produced a significant change blindness effect (better accuracy on trials without an ISI), whereas the condition represented in the top panel did not. Please click here to view a larger version of this figure.
Discussion
The method presented here is inspired by the so-called "flicker paradigm" commonly used by cognitive psychologists to study change blindness in humans9. In this human research, change blindness is operationally defined as the impairment in change detection produced by the presence of an ISI that interrupts transitions between stimulus displays. The same is true for the pigeon implementation described here. Furthermore, humans tend to approach the flicker task using a serial search strategy, considering possible locations one at a time and at each location either successfully identifying the change or rejecting that location and moving along to another. The method presented here produces results consistent with the same interpretation. Pigeons' change detection accuracy improves with additional repetitions32, as one would expect from a serial search that progresses from one possible change location to the next. Note however, that while the primary results are congruent with a serial search process, it is not the only logical possibility. Alternate interpretations (such as limited-capacity parallel search) should be considered, and may even motivate variations on the procedure. For example, if pigeons do engage in a location-by-location search process, they should be able to take advantage of base rate differences by selectively initiating their search at high-probability locations (enhancing accuracy at those high probability locations while reducing accuracy at others). A parallel search process would not make such a prediction.
Despite these important similarities, there are also some notable differences that must be acknowledged. Whereas human research often utilizes complex visual stimuli such as photographic images, the method presented here relies on simpler elements that can be projected onto the response keys in an operant chamber. One consequence of this difference is that the number of possible change locations in a standard operant chamber is limited to three (the number of response keys), and this number is much smaller than the innumerable possible change locations in a photographic display. So while both pigeons and humans may use a serial search strategy to locate changes, accurate performance on the procedure described here could be supported by a much simpler search process. Second, human research often measures response times to assess change detection. More difficult-to-find changes (such as those obscured by an ISI) require additional search time and produce longer response times. This pigeon method uses accuracy as a stand-in for response times because it was virtually impossible to prevent pigeons from pecking the stimulus displays during presentation (presumably before they had a chance to identify any change). Counting these stimulus pecks as responses led to consistently fast but meaningless response times and unacceptably low change detection accuracy. Nevertheless, the logic for interpreting accuracy as a measure of performance is similar to that for interpreting response times. Easy-to-find changes can be identified in a short time (producing good accuracy after even a small number of repetitions), whereas more difficult changes take longer (only reaching high levels of accuracy after many repetitions). Because the method requires that all repetitions be presented in their entirety before a response is recorded, there is no way to pinpoint precisely when, during stimulus presentation, a pigeon identified the change. Presumably some changes are identified well before the recorded response whereas others are identified only just before. This undermines the usefulness of any resulting response times, but not the usefulness of analyses based on accuracy.
There are several practical matters to consider when implementing this task. While the method has shown itself to be replicable, some scenarios have produced no change blindness effect despite good overall accuracy (see Figure 4, top panel). Thus, stimulus details should be chosen carefully to maximize the likelihood of producing the hypothesized effects. In particular, it appears that very short ISI durations may weaken or even eliminate change blindness30. The recommended 250 ms ISI specified in the protocol should be effective, but note that shorter durations can be used as well; a change blindness effect has been confirmed with an ISI as short as 7 ms32. Note also that the difficulty of ISI trials increases with the duration of the ISI. Thus, if animals struggle to learn the procedure, shortening the duration of the ISI may be an easy way to increase accuracy, especially early in training. Conversely, lengthening the ISI (even beyond the recommended 250 ms) may be a way to increase the effect size. One of the most powerful factors may be the salience of a change, and this is true for both humans36 and pigeons29,31. Larger or more prominent changes are easier to detect, but the effect is larger on the more difficult ISI trials than on no-ISI trials. Finally, one should consider the number of stimuli that can be generated given the available equipment. With large numbers of change detection stimuli, pigeons will likely see novel, never-before-seen stimuli in every session, and so a general change detection rule is the only strategy that can produce consistently good accuracy. On the other hand, with smaller numbers of stimuli available, memorization becomes a feasible approach, as the same stimuli may be presented repeatedly across and even within sessions. In such cases, it is especially important to reserve stimuli for transfer, in order to demonstrate that the results are due to rule learning and not memorization29. Note that even a small number of available stimulus elements can combine to produce a relatively large set of possible displays. For example, Herbranson and Jeffers29 (Experiment 1), used just four different color elements, yielding 43 = 64 unique original displays. Each original display could then be paired with one of nine unique modified displays, yielding a total training set of 576 paired change displays. With more elements come even more possible displays. Herbranson and colleagues32 used combinations of eight-line orientation elements on each of three keys to produce a set of over 400 million unique paired displays.
To conclude, the flicker paradigm is exciting because it produces a robust effect that highlights a striking failure of selective attention. The protocol presented here can be implemented using readily available operant chambers to produce results in a nonhuman animal that parallel those previously demonstrated in humans. Given that operant equipment is widely available and not specifically limited to pigeons, the method may prove to be just as useful for the myriad other species of animal that can exert control over attentional processes. While research on attention in animals is not new37, much of it has (quite understandably) focused on the successful or adaptive aspects of attention. Change blindness highlights a consistent and systematic failure of attention that deserves the same level of scrutiny. Thus, change blindness is more than a fascinating quirk of human visual attention. It implies for example, that much of our visual experience relies on representations that are less detailed than we might expect10. The existence of a parallel effect in pigeons further suggests that this sparseness of representation may be evolutionarily quite old and widespread. Change blindness thus may be a general consequence of selective attention that can provide some valuable insights into the origins of attention and the reasons for its limitations and imperfections.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The author extends thanks to members of the Whitman College Comparative Psychology Lab for their help in data collection, including Mark Arand, Michael Barker, Eva Davis, Kuba Jeffers, Brett Lambert, Tara Mah, Theo Pratt, Tvan Trinh, Lyla Wadia, and Patricia Xi.
Materials
| Small Environment Cublicle | BRS/LVE | SEC-002 | |
| Pigeon Intelligence Panel | BRS/LVE | PIP-016 | |
| Grain Feeder | BRS/LVE | GFM-001 | |
| Pigeon Pecking Key | BRS/LVE | PPK-001 | |
| Stimulus projector | BRS/LVE | IC-901 | |
| LED Lamp | Martek Industries, Cherry Hill NJ | 1820 | |
| I/O module | Acces IO | USB-IDIO-8 | |
| Personal Computer | Dell | Optiplex 3040 | |
| Visual C++ | Microsoft | ||
| White Carneau pigeons | Double-T Farm |
References
- Loftus, E. F., Pickrell, J. E. The formation of false memories. Psychiat. Ann. 25 (12), 720-725 (1995).
- Kahneman, D., Tversky, A. Prospect theory: An analysis of decision under risk. Econometrica. 47, 263-291 (1979).
- Granberg, D., Brown, T. A. The Monty Hall dilemma. Pers. Soc. Psychol. B. 21, 711-723 (1995).
- Simons, D. J., Levin, D. T. Failure to detect changes to people during a real-world interaction. Psychon. Bull. Rev. 5, 644-649 (1998).
- Grimes, J., Akins, K. A. On the failure to detect changes in scenes across saccades. Perception. , 89-110 (1996).
- Simons, D. J. In sight, out of mind: when object representations fail. Psychol. Sci. 7, 301-305 (1996).
- Levin, D. T., Simons, D. J. Failure to detect changes to attended objects in motion pictures. Psychon. Bull. Rev. 4, 501-506 (1997).
- Divita, J., Obermayer, R., Nugent, W., Linville, J. M. Verification of the change blindness phenomenon while managing critical events on a combat information display. Hum. Factors. 46, 205-218 (2004).
- Rensink, R. A., O’Regan, J. K., Clark, J. J. To see or not to see: The need for attention to perceive changes in scenes. Psychol. Sci. 8 (5), 368-373 (1997).
- Rensink, R. A. Change detection. Ann. Rev. Psychol. 53, 245-277 (2002).
- Williams, P., Simons, D. J. Detecting changes in novel, complex three-dimensional objects. Visual Cogn. 7, 297-322 (2000).
- Regan, J. K., Deubel, H., Clark, J. J., Rensink, R. A. Picture changes during blinks: looking without seeing and seeing without looking. Visual Cogn. 7, 191-211 (2000).
- Levin, D. T., Momen, N., Drivdahl, S. B., Simons, D. J. Change blindness blindness: The metacognitive error of overestimating change-detection ability. Visual Cogn. 7, 397-412 (2000).
- Regan, J. K., Rensink, R. A., Clark, J. J. Change-blindness as a result of ”mudsplashes. Nature. 398, 34 (1999).
- Eramudugolla, R., Irvine, D. R. F., McAnally, K. I., Martin, R. L., Mattingley, J. B. Directed attention eliminates ‘Change deafness’ in complex auditory scenes. Curr. Biol. 15, 1108-1113 (2005).
- Gallace, A., Tan, H. Z., Spence, C. The failure to detect tactile change: a tactile analogue of visual change blindness. Psychon. Bull. Rev. 13, 300-303 (2006).
- Reid, P. J., Shettleworth, S. J. Detection of cryptic prey: search image or search rate?. J. Exp. Psychol. Anim. B. 18 (3), 273-286 (1992).
- Cook, R. G. Dimensional organization and texture discrimination in pigeons. J. Exp. Psychol. Anim. B. 18, 354-363 (1992).
- Shimp, C. P., Friedrich, F. J. Behavioral and computational models of spatial attention. J. Exp. Psychol. Anim. B. 19 (1), 26 (1993).
- Fremouw, T., Herbranson, W. T., Shimp, C. P. Priming of attention to local or global levels of visual analysis. J. Exp. Psychol. Anim. B. 24 (3), 278 (1998).
- Cavoto, K. K., Cook, R. G. Cognitive precedence for local information in hierarchical stimulus processing by pigeons. J. Exp. Psychol. Anim. B. 27, 3-16 (2001).
- Maki, W. S., Leith, C. R. Shared attention in pigeons. J. Exp. Anal. Behav. 19 (2), 345-349 (1973).
- Herbranson, W. T., Fremouw, T., Shimp, C. P. The randomization procedure in the study of categorization of multidimensional stimuli by pigeons. J. Exp. Psychol. Anim. B. 25, 113-134 (1999).
- Wright, A. A., Katz, J. S., Magnotti, J., Elmore, L. C., Babb, S. Testing pigeon memory in a change detection task. Psychon. Bull. Rev. 17 (2), 243-249 (2010).
- Gibson, B., Wasserman, E., Luck, S. J. Qualitative similarities in the visual short-term memory of pigeons and people. Psychon. Bull. Rev. 18 (5), 979 (2011).
- Elmore, L. C., Magnotti, J. F., Katz, J. S., Wright, A. A. Change detection by rhesus monkeys (Macaca mulatta) and pigeons (Columba livia). J. Comp. Psychol. 126 (3), 203-212 (2012).
- Hagmann, C. E., Cook, R. G. Active change detection by pigeons and humans. J. Exp. Psychol. Anim. B. 39 (4), 383-389 (2013).
- Leising, K. J., Elmore, L. C., Rivera, J. J., Magnotti, J. F., Katz, J. S., Wright, A. A. Testing visual short-term memory of pigeons (Columba livia) and a rhesus monkey (Macaca mulatta) with a location change detection task. Anim. Cognition. 16 (5), 839-844 (2013).
- Herbranson, W. T., Jeffers, J. S. Pigeons (Columba livia) show change blindness in a color change detection task. Anim. Cognition. 20 (4), 725-737 (2017).
- Herbranson, W. T., Davis, E. T. The effect of display timing on change blindness in pigeons (Columba livia). J. Exp. Anal. Behav. 105 (1), 85-99 (2016).
- Herbranson, W. T. Change blindness in pigeons (Columba livia): The effects of change salience and timing. Front. Psychol. 6, 1109 (2015).
- Herbranson, W. T., Trinh, Y. T., Xi, P. M., Arand, M. P., Barker, M. S. K., Pratt, T. H. Change detection and change blindness in pigeons (Columba livia). J. Comp. Psychol. 128 (2), 181-187 (2014).
- Poling, A., Nickel, M., Alling, K. Free birds aren’t fat: Weight gain in captured wild pigeons maintained under laboratory conditions. J. Exp. Anal. Behav. 53 (3), 423-424 (1990).
- National Institute of Mental Health. . Methods and welfare considerations in behavioral research with animals: Report of a National Institutes of Health workshop (NIH Publication No. 02-5083). , (2002).
- Brown, P. L., Jenkins, H. J. Autoshaping of the pigeon’s keypeck. J. Exp. Anal. Behav. 11, 1-8 (1968).
- Smilek, D., Eastwood, J. D., Merikle, P. M. Does unattended information facilitate change detection?. J. Exp. Psychol. Hum. Percept. Perform. 26, 480-487 (2000).
- Herbranson, W. T., Call, J. Selective and divided attention in comparative psychology. APA handbook of comparative psychology. , 183-201 (2017).

