Cell Membrane Repair Assay Using a Two-photon Laser Microscope
Summary
Cell membrane wounding via two-photon laser is a widely used method for assessing membrane resealing ability and can be applied to multiple cell types. Here, we describe a protocol for in vitro live-imaging of membrane resealing in dysferlinopathy patient cells following two-photon laser ablation.
Abstract
Numerous pathophysiological insults can cause damage to cell membranes and, when coupled with innate defects in cell membrane repair or integrity, can result in disease. Understanding the underlying molecular mechanisms surrounding cell membrane repair is, therefore, an important objective to the development of novel therapeutic strategies for diseases associated with dysfunctional cell membrane dynamics. Many in vitro and in vivo studies aimed at understanding cell membrane resealing in various disease contexts utilize two-photon laser ablation as a standard for determining functional outcomes following experimental treatments. In this assay, cell membranes are subjected to wounding with a two-photon laser, which causes the cell membrane to rupture and fluorescent dye to infiltrate the cell. The intensity of fluorescence within the cell can then be monitored to quantify the cell’s ability to reseal itself. There are several alternative methods for assessing cell membrane response to injury, as well as great variation in the two-photon laser wounding approach itself, therefore, a single, unified model of cell wounding would beneficially serve to decrease the variation between these methodologies. In this article, we outline a simple two-photon laser wounding protocol for assessing cell membrane repair in vitro in both healthy and dysferlinopathy patient fibroblast cells transfected with or without a full-length dysferlin plasmid.
Introduction
The cell membrane of eukaryotic cells consists of a double-layer of protein-studded phospholipids which defines the intra/extracellular environment of the cell and is essential for maintaining cellular homeostasis and cell survival. Cell membrane injuries arising from mechanical or chemical insults are commonplace in various mammalian cell types, including skeletal and cardiac muscle, stomach, and lung cells1,2,3,4. In addition to injuries consequent to daily physiological function, cell membranes can also be damaged by environmental insults, bacterial toxins, and ischemic reperfusion5. Failure to reseal ruptures in the cell membrane leads to an unregulated influx of extracellular Ca2+– along with other potentially toxic extracellular components – into the cell, triggering downstream signal cascades which can quickly result in cell death1,4,5,6.
To date, there have been several proposed models for membrane repair in cells. Different repair mechanisms may be activated depending on the size and nature of the membrane rupture. For example, it is suggested that cell membranes may utilize lateral flow or protein clogging to repair small disruptions (<1 nm). The lateral fusion model proposes that membrane ruptures are rapidly mended through lateral recruitment of dysferlin-containing membrane7, while the protein clogging model suggests that small perforations are mended through protein aggregations (mostly annexins)8. Conversely, larger membrane lesions trigger Ca2+-dependent, dysferlin-mediated vesicle fusion and formation of a repair patch. In the repair patch model, a rapid Ca2+ influx into the cell triggers the recruitment of multiple proteins (dysferlin, annexins, mitsugumin-53, and EDH proteins) which form a complex or "patch", along with a fusion of intracellular vesicles or lysosomes at the site of membrane damage4,9,10,11,12. It is important to note that these models are not necessarily mutually exclusive and may work in concert to facilitate membrane repair. Failure to properly reseal cell membrane damage is associated with multiple disease states, including muscular dystrophy (dysferlinopathy – including Miyoshi myopathy, limb-girdle muscular dystrophy type IIB, and distal myopathy with anterior tibial onset)13, cardiomyopathy14, and Chediak-Higashi syndrome (CHS)15.
Given that proper cell membrane integrity and resealing plays such an important role in health and disease, a deeper understanding of the underlying molecular mechanisms of cell membrane repair would be beneficial in the search for novel therapeutic strategies. It is, therefore, necessary to possess appropriate experimental techniques to monitor the kinetics and evaluate the ability of cell membrane repair. Several in vitro methods for modeling membrane repair have been designed. One strategy involves mechanical injury, which can be facilitated through cell scraping with a pipette/surgical blade/razor or by rolling glass beads over the cells16. However, this type of mechanical injury generates larger lesions, and creates a high degree of variation in cell injury, both within and between cultures.
Another method of generating membrane wounds is laser ablation by two-photon microscopy. In contrast to traditional laser confocal microscopy that employs single photon excitation, the two-photon laser simultaneously uses two long-wavelength, low-energy photons to facilitate the excitation of a high-energy electron17. This non-linear process results in excitation exclusively within the focal plane and not along the entire light path17 (Figure 1). This reduced excitation volume helps to minimize photodamage when imaging live cells18,19. Researchers are therefore able to generate precise lesions in the cell membrane and monitor membrane resealing in real time by using fluorescent dyes and observing changes in the fluorescence intensity as the cell membrane ruptures and then reseals.
This approach has been used repeatedly to study membrane wounding in vitro and in vivo and in multiple cell types20,21. For example, membrane repair defects in fibroblasts and myotubes derived from dysferlinopathy patients were assessed using this technique22,23. Also, single muscle fibers isolated from mice were used to monitor repair patch formation13,24,25. The movement of fluorescently tagged proteins can also be observed during membrane repair in single muscle fibers9. Furthermore, the process of sarcolemmal repair following two-photon laser wounding in zebrafish embryos can be observed in real-time in vivo26.
In this article, we outline a methodology for assessing cell membrane repair dynamics in fibroblasts using two-photon laser wounding, although this methodology can be applied to various cell types for the purpose of quantifying plasma membrane resealing ability in vitro. In this method, cells are incubated with FM4-64, a lipophilic, cell-impermeable dye which quickly fluoresces as it binds to negatively charged phospholipids within the cytoplasm upon entering the cell through the membrane lesion (Figure 2&3). Quantification of dye fluorescence adjacent to the membrane lesion allows for monitoring the time that it takes for the cell membrane to reseal itself. To exemplify the utility of this method, we use dysferlinopathy patient fibroblasts transfected with GFP-conjugated full-length dysferlin (DYSF) plasmids to assess the rescue of cell membrane repair.
Protocol
Human fibroblast cells used in this study were used with approval from the Human Ethics Committee of the Faculty of Medicine and Dentistry at the University of Alberta.
1. Transfection of cells with full-length DYSF plasmid
- Culture fibroblast cells in a T225 flask containing 40 mL of growth media – 36 mL Dulbecco's Modified Eagle Medium (DMEM), 4 mL of fetal bovine serum (FBS), and 20 µL of penicillin/streptomycin (P/S) – in a CO2 incubator at 37°C.
NOTE: Cells should be cultured to approximately 70-80% confluency and should not be allowed to become completely confluent. - To prepare cells for seeding, rinse the cells with 40 mL of phosphate buffered saline (PBS) twice, then add 5 mL of 0.05% trypsin to detach the cells. Return the cells to the 37 °C CO2 incubator and incubate the cells for 5 min.
- When the cells are fully detached, add 40 mL of growth media to stop the trypsin reaction.
- Count the cells using a hemocytometer or another cell counting device.
- Seed 2 mL of cells at 1×105 cells/mL into 35 mm collagen-coated glass-bottom dishes. Incubate the cells overnight in a CO2 incubator at 37 °C.
NOTE: Cells should be approximately 50-60% confluent the next day. - Remove the growth media and replace it with 2 mL of serum-deprived media (growth media without FBS).
- Transfect the cells with full-length dysferlin plasmid using lipotransfection.
- Prepare 150 µL of serum-deprived media in a 1.5 mL tube (one tube for each dish to be transfected).
- Add 3 µL of transfection reagent to each 1.5 mL tube containing serum-deprived media. Incubate it at least 5 min at room temperature.
- In a separate 1.5 mL tube, add 175 µL of serum-deprived media and 3.5 µg of plasmid DNA.
NOTE: Multiply the above amounts for each additional dish to be transfected. - Combine 150 µL of media containing plasmid DNA into a tube with 150 µL of serum-deprived media and transfection reagent. Incubate it for 20 min at room temperature.
- Evenly distribute 300 µL of media with plasmid and transfection reagent across the dish. Incubate the dishes for 24 h in a CO2 incubator at 37°C.
- Replace the media with fresh growth media containing serum and incubate it for an additional 24 h in a CO2 incubator at 37 °C.
2. Preparation of cells for two-photon wounding assay
- Remove the media by pipetting and rinse the cells once with 1 mL of Tyrode's solution, then add 1 mL of fresh Tyrode's solution containing 1 µL of 2.5 mM FM 4-64 dye.
NOTE: The final concentration of FM 4-64 dye is 2.5 µM. - If assessment of membrane repair in a calcium-depleted environment is desired, rinse cells with 1 mL of PBS and then add 1 mL of PBS containing 1 mM EGTA.
NOTE: As PBS will cause the cells to detach over time, the wounding assay should be completed as quickly as possible.
3. Two-photon laser wounding assay
- Using an inverted confocal microscope and associated program software, create a 0.2 µm x 2 µm target and place it at the edge of the cell membrane so that the target line overlaps and lies perpendicular to the direction of the cell membrane (Figure 2) .
- Use a 543-nm HeNe laser to excite FM dye signal and set the detection range for 600-760 nm. Use a 488-nm Argon laser to excite GFP signal (Figure 4) and set the detection range for 500-550 nm.
- Create a 5 min time series of sequential image scans, imaging cells every 5 s.
- To generate a membrane lesion, bleach cells using a two-photon laser set to 820-nm, using 15% laser power with 10 iterations and bleach cells 25 s after the beginning of the time series.
- To quantify FM 4-64 dye fluorescence, use the software to draw a 6 µm x 6 µm region of interest (ROI) and place it adjacent to the wounding location and within the cell (Figure 3).
NOTE: Avoid sampling areas of pixel oversaturation by using the "Range Indicator" function in the software – in this application, red pixels represent oversaturated pixels. Export raw fluorescence values into an electronic spreadsheet for statistical analysis. - Calculate relative fluorescence values for each time point by subtracting the background value (mean fluorescence value of timepoints prior to membrane wounding) and dividing the net increase by the fluorescence value at t = 0 (Figure 5, 6).
Representative Results
Healthy human fibroblasts, non-treated dysferlinopathy patient fibroblasts, and patient fibroblasts transfected with a plasmid containing the full-length dysferlin sequence were subjected to two-photon laser wounding to assess membrane resealing ability in real time. Healthy human fibroblast cells displayed low levels of FM 4-64 fluorescence activation following laser wounding and non-treated patient fibroblasts exhibited a high degree of relative fluorescence intensity following injury (Figure 5&6). Patient cells that were transfected with full-length dysferlin plasmid showed reduced relative FM 4-64 fluorescence intensity compared with non-treated patient controls and were comparable with fluorescence values observed in the healthy control (Figure 5&6).
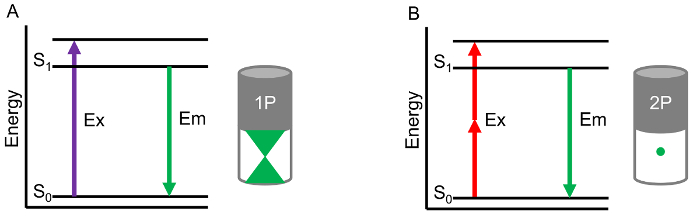
Figure 1. One-photon versus two-photon microscopy. In a traditional one-photon microscope system, a single high energy photon (UV or visible spectrum) is used to excite a fluorophore (A). In a two-photon system, two lower-energy photons (near infrared) are used to excite a fluorophore (B). Depicted within the cylinders is the fluorescence signal generated throughout the focal plane, demonstrating how the two-photon laser system can focus excitation light to within a specific region (B) and exclude light from out-of-focus planes (A). Please click here to view a larger version of this figure.
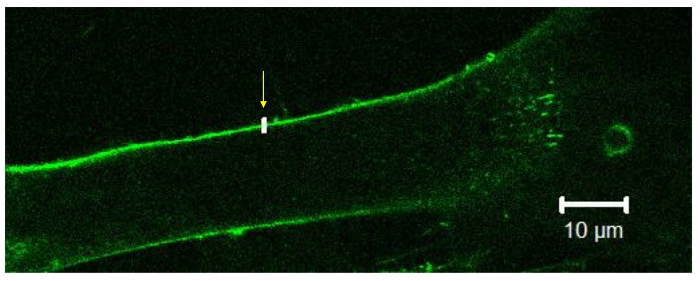
Figure 2. Placing a target at the cell membrane. Using software, a 0.2 µm x 2 µm target is drawn and placed overlapping and perpendicular to the cell membrane.The arrow indicates the target. Please click here to view a larger version of this figure.
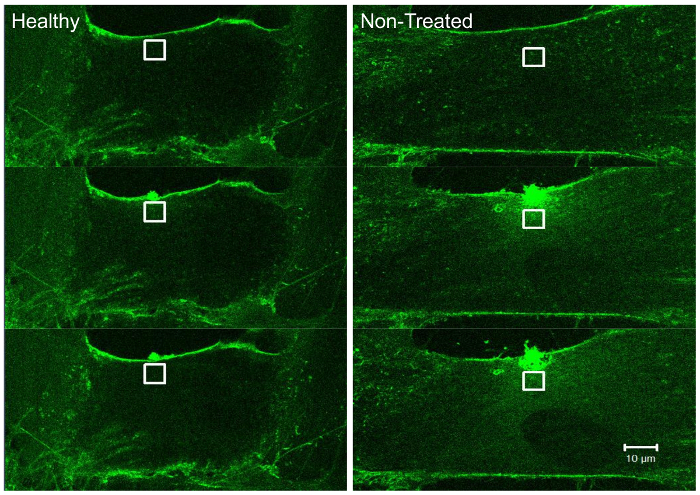
Figure 3. Fluorescence signal from FM dye as it infiltrates the cell through membrane rupture. As the cell membrane ruptures, lipophilic FM 4-64 dye infiltrates the cell and fluoresces as it binds negatively-charged phospholipids within the cytoplasm. Using software, a region of interest (ROI) can be drawn (white box) and fluorescence values within the box can be used to calculate relative fluorescence changes within the cell. Please click here to view a larger version of this figure.
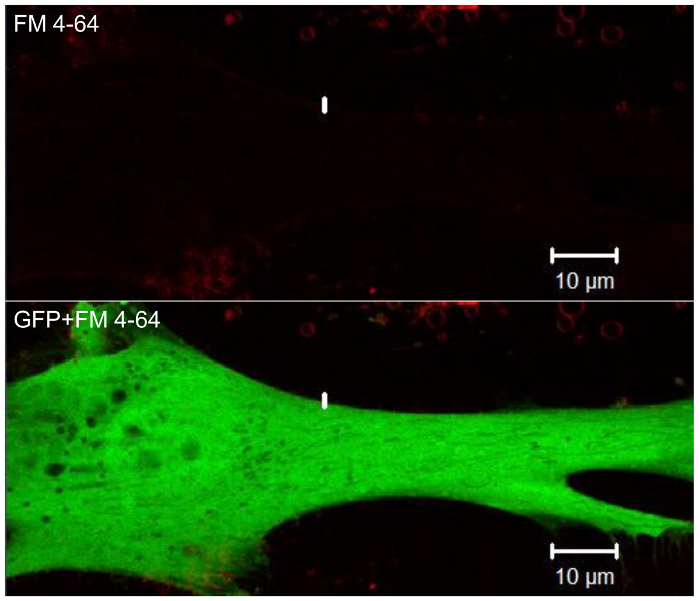
Figure 4. Fluorescence signal from GFP-conjugated full-length DYSF plasmid. Following plasmid transfection, patient fibroblast cells express full-length DYSF protein. Please click here to view a larger version of this figure.
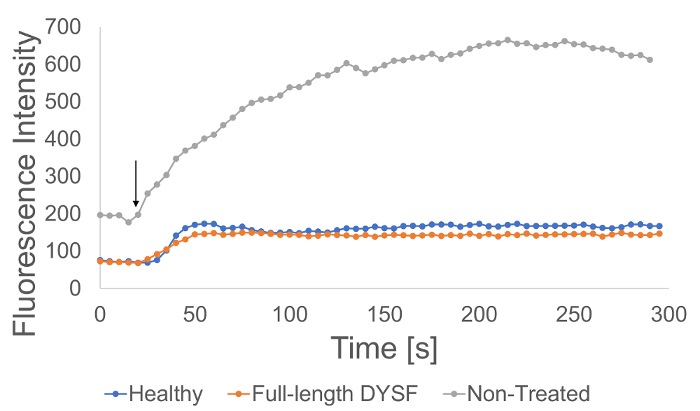
Figure 5. Quantification of fluorescence over time. Fluorescence values calculated by computer software from within the ROI can be used to determine relative fluorescence changes over time for each treatment group. The arrow indicates the time of laser ablation. Please click here to view a larger version of this figure.
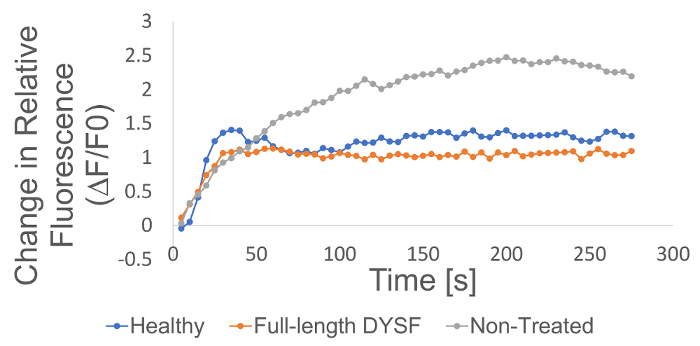
Figure 6. Change in relative fluorescence over time. Relative fluorescence (RF) values for each time point are calculated by subtracting the background fluorescence value (calculated as the mean of the fluorescence values from the timepoints prior to membrane wounding) from the given fluorescence values (Signal Intensity – Background = ΔF) and dividing the net increase (ΔF) by the fluorescence value at t = 0 (F0). Therefore, RF = ΔF/F0. Please click here to view a larger version of this figure.
Discussion
Two-photon laser wounding of the cell membrane is a precise and versatile technique for assessing the dynamics of membrane resealing in vitro. In this article, we described a protocol for determining cell membrane resealing ability in dysferlinopathy patient cells using the two-photon laser wounding assay. Our results show that dysferlinopathy patient cells are defective in cell membrane resealing, which is consistent with findings by other researchers and which further highlights the striking similarities in cell membrane resealing dynamics between muscle and non-muscle cell types3,11,15,22. We also observed that patient cells transfected with a plasmid containing the full-length dysferlin sequence were rescued from their defective membrane resealing phenotype, demonstrating that full-length dysferlin plasmid can rescue phenotype in non-muscle cell types as well as in muscle27.
Together, our results reinforce the feasibility and reliability of the two-photon membrane wounding assay as a gold-standard in measuring cell membrane resealing ability in vitro. However, the cost and availability associated with two-photon microscopy could be a potential limiting factor for some research groups seeking to utilize the two-photon membrane wounding assay. Furthermore, our setup utilizes an inverted confocal microscope, which is well-suited to in vitro work but may be less appropriate for in vivo imaging.
It is important to note that the precision of the two-photon laser alignment is critical to the success of the technique. In our experience, troubleshooting of laser alignment was the most common issue encountered. Other issues which could affect the ability to score successful hits during laser wounding include the levelling of the glass-bottom dish on the stage, as well as the orientation of the cells in solution.
The assay is applicable to current and future studies investigating disease states in which cell membrane integrity or resealing is perturbed, and the efficacy of therapies, such as gene therapy and antisense oligonucleotide-based therapy, which would benefit from having an effective assay for characterizing membrane dynamics so that novel therapeutic options can be explored28,29.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by University of Alberta Faculty of Medicine and Dentistry, The Friends of Garrett Cumming Research Chair Fund, HM Toupin Neurological Science Research Chair Fund, Muscular Dystrophy Canada, Canada Foundation for Innovation (CFI), Alberta Advanced Education and Technology (AET), Canadian Institutes of Health Research (CIHR), Jesse’s Journey – The Foundation for Gene and Cell Therapy, the Women and Children’s Health Research Institute (WCHRI), and Alberta Innovates Health Solutions (AIHS).
We would like to thank Dr. Steven Laval for supplying us with the full-length dysferlin plasmid. We would also like to thank Dr. Katsuya Miyake for technical advice.
Materials
| Dulbecco's Modified Eagle Medium (DMEM) | Thermo Fisher | 11320033 | |
| Fetal Bovine Serum | Sigma-Aldrich | F1051 | |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher | 15140122 | |
| Trypsin-EDTA (0.05%), phenol red | Thermo Fisher | 25300062 | |
| Dulbecco’s Phosphate Buffered Saline | Sigma-Aldrich | D8537 | |
| 35mm collagen-coated glass-bottom dishes | MatTek | P53GCOL-1.5-14-C | |
| Serum-deprived media | Thermo Fisher | 31985070 | |
| Transfection reagent | Thermo Fisher | 15338100 | |
| FM 4-64 Dye | Invitrogen | T13320 | |
| Tyrode’s Salts Solution | Sigma-Aldrich | T2397 | |
| Confocal laser scanning microscope | Carl Zeiss | NA | |
| Chameleon Two-photon laser | Coherent | NA |
References
- Cong, X., Hubmayr, R. D., Li, C., Zhao, X. Plasma membrane wounding and repair in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 312 (3), L371-L391 (2017).
- Demonbreun, A. R., McNally, E. M. Plasma Membrane Repair in Health and Disease. Curr Top Membr. 77, 67-96 (2016).
- McNeil, P. L., Ito, S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 96 (5 Pt 1), 1238-1248 (1989).
- McNeil, P. L., Kirchhausen, T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 6 (6), 499-505 (2005).
- Cooper, S. T., McNeil, P. L. Membrane Repair: Mechanisms and Pathophysiology. Physiol Rev. 95 (4), 1205-1240 (2015).
- Geeraerts, M. D., Ronveaux-Dupal, M. F., Lemasters, J. J., Herman, B. Cytosolic free Ca2+ and proteolysis in lethal oxidative injury in endothelial cells. Am J Physiol. 261 (5 Pt 1), C889-C896 (1991).
- McDade, J. R., Archambeau, A., Michele, D. E. Rapid actin-cytoskeleton-dependent recruitment of plasma membrane-derived dysferlin at wounds is critical for muscle membrane repair. FASEB J. 28 (8), 3660-3670 (2014).
- Benninger, R. K., Piston, D. W. Two-photon excitation microscopy for the study of living cells and tissues. Curr Protoc Cell Biol. Chapter 4 (Unit 4), 11-24 (2013).
- Demonbreun, A. R., et al. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J Cell Biol. 213 (6), 705-718 (2016).
- McNeil, P. L., Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 140 (5), 1097-1109 (1992).
- Rodriguez, A., Webster, P., Ortego, J., Andrews, N. W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 137 (1), 93-104 (1997).
- Reddy, A., Caler, E. V., Andrews, N. W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 106 (2), 157-169 (2001).
- Bansal, D., et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 423 (6936), 168-172 (2003).
- Han, R., et al. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 117 (7), 1805-1813 (2007).
- Huynh, C., Roth, D., Ward, D. M., Kaplan, J., Andrews, N. W. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci U S A. 101 (48), 16795-16800 (2004).
- Corrotte, M., Castro-Gomes, T., Koushik, A. B., Andrews, N. W. Approaches for plasma membrane wounding and assessment of lysosome-mediated repair responses. Methods Cell Biol. 126, 139-158 (2015).
- Denk, W., Strickler, J. H., Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science. 248 (4951), 73-76 (1990).
- Jontes, J. D., Buchanan, J., Smith, J. S. Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat Neurosci. 3 (3), 231-237 (2000).
- Galbraith, J. A., Terasaki, M. Controlled damage in thick specimens by multiphoton excitation. Mol Biol Cell. 14 (5), 1808-1817 (2003).
- McNeil, P. L., Miyake, K., Vogel, S. S. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci U S A. 100 (8), 4592-4597 (2003).
- Weisleder, N., et al. Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp. (52), (2011).
- Matsuda, C., Kiyosue, K., Nishino, I., Goto, Y., Hayashi, Y. K. Dysferlinopathy Fibroblasts Are Defective in Plasma Membrane Repair. PLoS Curr. 7, (2015).
- Philippi, S., et al. Dysferlin-deficient immortalized human myoblasts and myotubes as a useful tool to study dysferlinopathy. PLoS Curr. 4, RRN1298 (2012).
- Cai, C., et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 11 (1), 56-64 (2009).
- Swaggart, K. A., et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc Natl Acad Sci U S A. 111 (16), 6004-6009 (2014).
- Roostalu, U., Strahle, U. In vivo imaging of molecular interactions at damaged sarcolemma. Dev Cell. 22 (3), 515-529 (2012).
- Azakir, B. A., et al. Modular dispensability of dysferlin C2 domains reveals rational design for mini-dysferlin molecules. J Biol Chem. 287 (33), 27629-27636 (2012).
- Lee, J., Yokota, T., Takeda, S., Miyagoe-Suzuki, Y., Mori-Yoshimura, M. Ch 6. Translational Research in Muscular Dystrophy. , 87-102 (2016).
- Barthelemy, F., Wein, N., Krahn, M., Levy, N., Bartoli, M. Translational research and therapeutic perspectives in dysferlinopathies. Mol Med. 17 (9-10), 875-882 (2011).

