Fabricating High-viscosity Droplets using Microfluidic Capillary Device with Phase-inversion Co-flow Structure
Summary
A phase-inversion co-flow device is demonstrated to generate monodisperse high-viscosity droplets above 1 Pas, which is difficult to realize in droplet microfluidics.
Abstract
The generation of monodisperse droplets with high viscosity has always been a challenge in droplet microfluidics. Here, we demonstrate a phase-inversion co-flow device to generate uniform high-viscosity droplets in a low-viscosity fluid. The microfluidic capillary device has a common co-flow structure with its exit connecting to a wider tube. Elongated droplets of the low-viscosity fluid are first encapsulated by the high-viscosity fluid in the co-flow structure. As the elongated low-viscosity droplets flow through the exit, which is treated to be wetted by the low-viscosity fluid, phase inversion is then induced by the adhesion of the low viscosity droplets to the tip of the exit, which results in the subsequent inverse encapsulation of the high-viscosity fluid. The size of the resultant high-viscosity droplets can be adjusted by changing the flow rate ratio of the low-viscosity fluid to the high-viscosity fluid. We demonstrate several typical examples of the generation of high-viscosity droplets with a viscosity up to 11.9 Pas, such as glycerol, honey, starch, and polymer solution. The method provides a simple and straightforward approach to generate monodisperse high-viscosity droplets, which may be used in a variety of droplet-based applications, such as materials synthesis, drug delivery, cell assay, bioengineering, and food engineering.
Introduction
The generation of droplets is becoming a key technology in a variety of applications, such as drug delivery, materials synthesis, 3D bioprinting, cell assays, and food engineering1,2,3,4,5,6. Microfluidic devices with T-junction7,8, co-flow1,9, or flow-focusing10,11 structures are widely used to generate monodisperse single emulsion droplets. Selection of a more viscous continuous phase will facilitate the formation of droplets12, and the viscosities of both the continuous and dispersed fluids are commonly below 0.1 Pas in droplet microfluidics13. However, in many applications, the dispersed phase may have a viscosity several hundred times higher than that of water, such as glycerol14, solutions containing nanoparticles15, proteins16, or polymers17,18,19, while it is difficult to achieve monodisperse droplets directly from high-viscosity fluids in a stable dripping regime11 in microfluidic devices, especially for fluids with a viscosity of η > 1 Pa·s14,17,18,19. Furthermore, it has been reported13,18 that typical microfluidic methods for droplet formation require fluids with a relatively low viscosity and moderate interfacial tension to form uniform droplets in a stable dripping regime.
For a dispersed phase with a viscosity slightly larger than 0.1 Pas, there are several possible approaches to facilitate the droplet formation with typical T-junction, co-flow, or flow-focusing microfluidic devices: (1) decrease the viscosity of the dispersed phase by diluting it in an volatile solvent11,20; (2) decrease the dispersed-to-continuous viscosity ratio by increasing the viscosity of the continuous phase1,11; (3) decrease the flow rate of the dispersed phase to an extremely low value, while keeping a high continuous-to-dispersed flow rate ratio 14,19. However, these approaches are not practical for fluids with much higher viscosity, as they will significantly lower the production rate while dramatically raising the consumption of the volatile solvent or the continuous phase. In addtion, it has been reported that some high viscosity polymer solutions with η > 1 Pa·s still did not break up into droplets with the approaches mentioned above17,19.
There are also several improved designs of microfluidic devices which introduce a third phase of fluid into the system, which facilitates the generation of high-viscosity droplets. Innovations include: bubbles introduced to cut a jetting thread into droplets21, an immiscible chaperoning fluid with moderate viscosity, introduced as the middle phase between the dipsersed phase and the continuous phase18, and microreactors introduced to generate high-viscosity droplets from two low-viscosity precursors21,22,23. However, as one more fluid is involved in the process, the system becomes more complicated, and the devices usually work in a much narrower flow regime than the typical devices for the generation of single emulsion droplets.
To generate monodisperse droplets directly from a high-viscosity fluid with η > 1 Pa·s, surface-controlled phase-inversion methods have been investigated24. As the generation of low-viscosity droplets is much easier than that of high-viscosity droplets12, elongated low-viscosity droplets in a high-viscosity continuous phase are first generated using a typical co-flow structure, and then are broken up due to the change of surface wettability downstream of the co-flow structure. The released low-viscosity fluid inversely encapsulates the downstream high-viscosity fluid into droplets so that phase inversion is completed. According to the phase inversion mechanism, monodisperse high-viscosity droplets can be generated based on a typical co-flow device, while the exit of the co-flow device is treated to be wetted by the low-viscosity fluid, and then connected to a wider tube24,25.
Protocol
1. Producing a Phase-Inversion Co-Flow Capillary Device for Observing the Generation Process of Aqueous, High-Viscosity Droplets with a Diameter of ~500 μm.
NOTE: The square outer tube used here is for taking images of the generation process of the high-viscosity droplets. If there is no need to take images, a simplified version of the device can be made according to protocol step 2.
- Prepare three glass tubes with different sizes for the assembly of the capillary device.
- Take a square glass tube with an inner size of 1.05 mm, and cut a piece of the tube ~4 cm in length. This will be the outer tube of the device.
- Take a round glass tube with an inner diameter (I.D.) of 580 μm and an outer diameter (O.D.) of 1 mm, and cut a piece of the tube ~3 cm in length. This will be the middle tube of the device.
- Take a round glass tube with I.D. = 200 μm and O.D. = 330 μm, and cut a piece of the tube ~2 cm in length. This will be the inner tube of the device.
- Modify the surface wettability of one end of the middle tube to be hydrophobic.
- Take a 1 mL glass vial, and add 0.3 mL of trichloro(octadecyl)silane (OTS) into the glass vial.
- Take the middle tube with I.D. = 580 μm prepared in protocol step 1.1.2, and dip one end of it into the OTS in the glass vial for ~10 s.
- Take out the middle tube, and flush the tube with nitrogen gas from the untreated end.
- Prepare the needles for the inlets of the capillary device.
- Take a 20G blunt tip dispensing needle, and cut a slot with ~0.5 mm x 0.5 mm on the edge of the plastic Luer hub with a blade.
NOTE: This needle will serve as the inlet for the low-viscosity oil phase. - Take another 20G blunt tip dispensing needle, and cut two slots on the edge of the plastic Luer hub. Align the two slots in a line passing the diameter of the Luer hub.
NOTE: One slot has a size of ~0.5 mm x 0.5 mm, while the other slot has a size of ~1.0 mm x 1.0 mm. This needle will serve as the inlet for the high-viscosity aqueous phase. - Take another 20G blunt tip dispensing needle, and cut two slots on the edge of the plastic Luer hub. Align the two slots in a line passing the diameter of the Luer hub.
NOTE: One slot has a size of ~1.5 mm x 1.5 mm; while the other slot has a size of ~1.0 mm x 1.0 mm. This needle will serve as the inlet for cleaning purposes.
- Take a 20G blunt tip dispensing needle, and cut a slot with ~0.5 mm x 0.5 mm on the edge of the plastic Luer hub with a blade.
- Assemble the glass tubes according to Figure 1A.
- Take a regular 7.62 cm x 2.54 cm glass slide as the substrate of the capillary device.
- Put the outer tube with I.D. = 1.05 mm, prepared in protocol step 1.1.1, on the glass slide with ~1 cm extruding the short edge of the glass slide.
- Take the middle tube with I.D. = 580 μm, prepared in protocol step 1.2, and insert the hydrophobic end of the middle tube into the outer tube from the end on the glass slide, and keep ~1 cm of the middle tube outside the outer tube.
- Take the inner tube with I.D. = 200 μm, prepared in protocol step 1.1.3, and insert one end of the inner tube into the middle tube, and keep ~1 cm of the inner tube outside the middle tube.
- Use epoxy glue to fix the three tubes in position along the centerline of the glass slide. Then wait ~5 min or longer for the glue to solidify completely.
- Assemble the inlets on the capillary device.
- Take the inlet needle for the low-viscosity oil phase, prepared in protocol step 1.3.1, and let the Luer hub cover the end of the inner tube on the substrate, and then use epoxy glue to fix the Luer hub on the substrate.
- Take the inlet needle for the high-viscosity aqueous phase, prepared in protocol step 1.3.2, and let the Luer hub cover the junction between the inner tube and the middle tube, and then use epoxy glue to fix the Luer hub onto the substrate.
- Take the inlet needle, prepared in protocol step 1.3.3, and, for cleaning, let the Luer hub cover the junction between the middle tube and the outer tube, and then use epoxy glue to fix the Luer hub on the substrate.
- Wait ~5 min or longer for the glue to solidify completely.
- Use epoxy glue to seal the Luer hubs of the needles on the substrate.
- Wait ~30 min or longer for the glue to solidify completely, and then the device is ready to use.
2. Make a Phase-Inversion, Co-Flow Capillary Device for Fabricating Aqueous High-Viscosity Droplets with a Diameter of ~500 μm.
NOTE: The device made here is a simplified version of the device in protocol step 1.
- Prepare two glass tubes with different sizes for the assembly of the capillary device.
- Take a round glass tube with I.D. = 580 μm and O.D. = 1 mm, and cut a piece of the tube with ~ 3 cm in length. This will be the middle tube of the device.
- Take a round glass tube with I.D. = 200 μm and O.D. = 330 μm, and cut a piece of the tube with ~ 2 cm in length. This will be the inner tube of the device.
- Modify the surface wettability of one end of the middle tube to be hydrophobic.
- Add 0.3 mL of OTS into a 1 mL glass vial.
- Take the middle tube with I.D. = 580 μm, prepared in protocol step 2.1.1, and dip one end of it into the OTS in the glass vial for ~10 s.
- Take out the middle tube, and then flush the tube with nitrogen gas from the untreated end.
- Prepare the needles for the inlets of the capillary device.
- Prepare a 20G blunt tip dispensing needle, which will serve as the inlet for the low-viscosity oil phase. Then, cut a slot of ~0.5 mm x 0.5 mm with a blade on the edge of the plastic Luer hub.
- Take another 20G blunt tip dispensing needle, and cut two slots on the edge of the plastic Luer hub. Align the two slots in a line passing the diameter of the Luer hub.
NOTE: One slot has a size of ~0.5 mm x 0.5 mm, while the other slot has a size of ~1.0 mm x 1.0 mm. This second needle will serve as the inlet for the high-viscosity aqueous phase.
- Assemble the glass tubes according to Figure 1A.
- Take a regular 7.62 cm x 2.54 cm glass slide as the substrate of the capillary device.
- Put the middle tube with I.D. = 580 μm, prepared in protocol step 2.2, on the glass slide with the hydrophobic end extruding ~1 cm over the short edge of the glass slide.
- Take the inner tube with I.D. = 200 μm, prepared in protocol step 2.1.2, and insert one end of the inner tube into the middle tube from the untreated end on the glass slide, and keep ~ 1 cm of the inner tube outside the middle tube.
- Use epoxy glue to fix the two tubes in position along the centerline of the glass slide.
- Wait for ~5 min or longer for the glue to solidify completely.
- Assemble the inlets on the capillary device.
- Take the inlet needle for the low-viscosity oil phase, prepared in protocol step 2.3.1, and let the Luer hub cover the end of the inner tube on the substrate, and then use epoxy glue to fix the Luer hub on the substrate.
- Take the inlet needle for the high-viscosity aqueous phase, prepared in protocol step 2.3.2, and let the Luer hub cover the junction between the inner tube and the middle tube, and then use epoxy glue to fix the Luer hub on the substrate.
NOTE: The other end of the middle tube is the outlet of the device. - Wait ~5 min or longer for the glue to solidify completely.
- Use epoxy glue to seal the Luer hubs of the needles on the substrate.
- Wait ~30 min or longer for the glue to solidify completely.
- Connect the free end of the middle tube with the outlet tubing, i.e., polyethylene tubing with I.D. = 0.86 mm and ~20 mm in length.
NOTE: The slight deformation of the outer tubing will ensure the seal of the connection, so that no glue is needed here. The outlet tubing acts as a wider outer tube for the phase inversion. At this point, the device is ready to use.
3. Make Phase-Inversion Co-Flow Capillary Device for Observing the Generation Process of Aqueous High-Viscosity Droplets with a Diameter of ~200 μm.
NOTE: The device made here is a smaller version of the device of protocol step 1 to make smaller droplets.
- Prepare three glass tubes with different sizes for the assembly of the capillary device.
- Take a square glass tube with I.D. = 400 μm, and cut a piece of the tube ~4 cm in length, which will be the outer tube of the device.
- Take a round glass tube with I.D. = 200 μm and O.D. = 330 μm, and cut a piece of the tube ~3 cm in length, which will be the middle tube of the device.
- Take a round glass tube with I.D. = 100 μm and O.D. = 170 μm, and cut a piece of the tube ~2 cm in length, which will be the inner tube of the device.
- Modify the surface wettability of one end of the middle tube to be hydrophobic.
- Take a 1 mL glass vial, and add 0.3 mL of OTS into the glass vial.
- Take the middle tube with I.D. = 200 μm, prepared in protocol step 3.1.2, and dip one end of it into the OTS in the glass vial for ~10 s.
- Take out the middle tube, and then flush the tube with nitrogen gas from the untreated end.
- Prepare the needles for the inlets of the capillary device.
- Prepare a 20G blunt tip dispensing needle, which will serve as the inlet for the low-viscosity oil phase. Then, with a blade, cut a slot ~0.2 mm x 0.2 mm on the edge of the plastic Luer hub.
- Prepare another 20G blunt tip dispensing needle, and cut two slots on the edge of the plastic Luer hub. Align the two slots in a line passing the diameter of the Luer hub.
NOTE: One slot has a size of ~0.2 mm x 0.2 mm, while the other slot has a size of ~0.4 mm x 0.4 mm. This second needle will serve as the inlet for the high-viscosity aqueous phase. - Take another 20G blunt tip dispensing needle, and cut two slots on the edge of the plastic Luer hub. The two slots are aligned in a line passing the diameter of the Luer hub.
NOTE: One slot has a size of ~0.8 mm x 0.8 mm, while the other slot has a size of ~0.4 mm x 0.4 mm. This third needle will serve as an inlet for cleaning purposes.
- Follow protocol steps 1.4 – 1.6 to finish the device, using the glass tubes prepared in protocol step 3.1 instead of those prepared in protocol step 1.1, and using the needles prepared in protocol step 3.3 instead of those prepared in protocol step 1.3.
4. Observing the Generation of Glycerol Droplets in Liquid Paraffin
NOTE: For taking the images shown in Figures 1B – D, use the device prepared in protocol step 1; for taking images shown in Figure 3, use the device prepared in protocol step 3.
- Prepare solutions to be used in the experiment.
- Use glycerol as the high-viscosity aqueous phase, and add 0.5 w.t.% Toluidine Blue O to dye it blue.
- Use liquid paraffin as the low-viscosity oil phase, and add 1% w.t. Span 80 in it as surfactant.
- Prepare three 1 mL syringes and three syringe pumps.
NOTE: Three syringes for the fluids prepared in protocol step 4.1: one for injecting the high-viscosity glycerol, prepared in protocol step 4.1.1, and the other two for injecting the low-viscosity liquid paraffin, prepared in protocol step 4.1.2, respectively.- Connect the syringe containing glycerol to the inlet to the middle tube.
- Connect one syringe containing liquid paraffin to the inlet of the inner tube, while connecting the other to the inlet for cleaning purposes.
- Place the device prepared in protocol step 1 on an inverted microscope, and place a piece of a Kimwipe under the outlet of the outer tube to absorb the leaked fluid.
CAUTION: Do not let the fluid leak outside the Kimwipe area. - Set the flow rates of the syringe pumps.
NOTE: Use the syringe pump connected to the outer tube for cleaning purposes when there are trapped bubbles or droplets around the exit of the middle tube. Otherwise, just leave the pump stopped.- Set the flow rate of glycerol injecting to the middle tube of Qw = 10 μL/min.
- Set the flow rate of liquid paraffin injecting to the inner tube of Qo = 30 μL/min.
- Run the two pumps to generate glycerol droplets.
- Wait for ~0.5 min until the flows are stabilized and the glycerol droplets are uniformly generated at the exits of the middle tube. Then, take videos or images of the droplet generation process.
NOTE: Images in Figures 1B- C can be taken using the device prepared in protocol step 1, while images in Figure 3A can be taken using the device prepared in protocol step 3. Stop all the pumps as soon as videos or images are taken, and take the device off the microscope right away. - Prepare for collecting the high-viscosity droplets.
- Place the device in a vertical plane with the outlet pointed down, and put a petri dish under the outlet. Use tape to fix the device with the outlet ~2 mm above the bottom of the petri dish.
- Pour some liquid paraffin prepared in protocol step 4.1.2 into the petri dish, and just immerse the outlet of the device.
- Run the two syringe pumps again at Qw = 10 μL/min and Qo = 30 μL/min, and collect the glycerol droplets in the petri dish.
NOTE: Wait for ~1 min until the flows are stabilized and the glycerol droplets are uniformly generated at the exits of the outer tube, the image of the droplets in the petri dish can be taken, as shown in Figure 1D for the device prepared in protocol 1, or Figure 3B for the device prepared in protocol step 3.
5. Generating and Collecting the Glycerol Droplets in Liquid Paraffin with the Simplified Device Prepared in Step 2.
NOTE: This is for taking images of the glycerol droplets generated under different flow rate ratio of Qo/Qw, and measuring the corresponding size variation of the droplets for the data points in Figure 2.
- Prepare solutions to be used in the experiment by following protocol step 4.1.
- Prepare two 1 mL syringes and two syringe pumps.
NOTE: Two syringes for the fluids prepared in protocol step 4.1: One for injecting the high-viscosity glycerol, prepared in protocol step 4.1.1, and the other for injecting the low-viscosity liquid paraffin, prepared in protocol step 4.1.2, respectively.- Connect the syringe containing 0.8 mL glycerol to the inlet to the middle tube.
- Connect the syringe containing 0.8 mL liquid paraffin to the inlet of the inner tube.
- Prepare for collecting the high-viscosity droplets.
- Place the device in a vertical plane with the outlet pointed down, and put a 35 mm Petri dish under the outlet. Use tape to fix the device with the outlet ~2 mm above the bottom of the petri dish.
- Pour some liquid paraffin prepared in protocol step 4.1.2 into the petri dish, and just immerse the outlet of the device.
- Set the flow rates of the syringe pumps.
NOTE: For each flow rate ratio in Figure 2, fix the flow rate of glycerol Qw = 2 μL/min, while increasing the flow rate of liquid paraffin Qo to different values according to the required flow rate ratios of Qo/Qw. For each flow rate ratio, wait ~1 min until the flows are stabilized and uniform glycerol droplets are collected in the petri dish, then take images of the droplets.- Set the flow rate of glycerol injected into the middle tube of Qw = 2 μL/min.
- Set the flow rate of liquid paraffin injected into the inner tube of Qo = 6 μL/min.
- Run the two pumps to generate glycerol droplets.
NOTE: The generation process of the droplets can be directly observed with a cellphone camera, or a digital camera mounted on a tripod.
- Wait ~1 min until the flows are stabilized, and change a new petri dish for collecting uniform glycerol droplets.
6. Generate Other High-Viscosity Droplets in Liquid Paraffin Using the Phase-Inversion Co-Flow Device.
NOTE: This is for the images in Figure 4. All the low-viscosity oil phase used in the experiments is the same as used in protocol step 4.1.2.
- Use pure honey as the high-viscosity aqueous phase for Figure 4A.
- Prepare 6 w.t.% starch solution for Figure 4B.
CAUTION: Use a proper high-temperature glass media bottle and a high-temperature cap. Wear heat resistant gloves.- Add 47 g of water in a 100 mL glass media bottle and put a stir bar in the bottle.
- Put the bottle in a water bath and set the temperature to 100 °C.
- Add 3 g of starch powder into the hot water after the water bath reaches 100 °C.
- Cover the cap of the bottle and keep stirring for ~4 h until the solution is clear.
- Wait until the solution cools down to room temperature before use.
- Prepare 10 w.t.% PVA-124 solution for Figure 4C.
CAUTION: Use a proper high-temperature glass media bottle and a high-temperature cap. Wear heat resistant gloves.- Add 45 g of water in a 100 mL glass media bottle and put a stir bar in the bottle.
- Put the bottle in a water bath and set the temperature to 70 °C.
- Add 5 g of PVA-124 powder into the bottle after the water bath reaches 70 °C.
- Cover the cap of the bottle and keep stirring for ~1 h until the solution is clear.
- Wait until the solution cools down to room temperature before use.
- Generate high-viscosity droplets in liquid paraffin.
- Follow protocol step 5 using the high-viscosity fluids prepared in step 6.1 – 6.3, instead of the glycerol in protocol step 5.
- Use the flow rate settings of Qw = 1 μL/min and Qo = 5 μL/min for Figure 4.
Representative Results
A microfluidic capillary device with a phase-inversion, co-flow structure was designed to generate monodisperse aqueous high-viscosity droplets, as shown in Figure 1A. In Figure 1, the high-viscosity aqueous phase was glycerol, which has a viscosity of ηw = 1.4 Pas; the low-viscosity oil phase was liquid paraffin, which has a viscosity of ηo = 0.029 Pas; the surface tension between the two phases was γ = 27.7 mN/m. In the middle tube, elongated oil droplets can be encapsulated by glycerol in a well-controlled dripping mode9,13, because the viscosity of the glycerol is much higher than that of liquid paraffin, and the capillary numbers, Ca, of both phases are as low as 10-4– 10-2, where Ca = ηU/γ, U = Q/A is the average velocity of the fluid, and A is the area of the cross section of the channel. As the elongated oil droplets flowed out of the exit of the middle tube into a wider outer tube, as shown in Figure 1B, the oil droplets broke at the hydrophobic tip of the middle tube, and inversely encapsulated the downstream cap of glycerol, so that high-viscosity glycerol droplets were obtained, as shown in Figure 1C. As long as the droplet size and the distance between any two adjacent oil droplets are kept unchanged, the formed glycerol droplets will be monodisperse24,25. The images in Figure 1B-C were obtained using the device of Step 1, and following experimental protocol step 4. The glycerol droplets generated at Qo = 30 uL/min and Qw = 10 uL/min are shown in Figure 1D, wherein the droplets had an average diameter of 521 μm, and the coefficient of variation (CV) of the droplet size, defined as the standard deviation divided by the average droplet size, was CV = 0.9%, which indicates the droplets were monodisperse.
The size of the high-viscosity droplets can be adjusted by altering the flow rate ratios Qo/Qw with a fixed Qw. A set of typical experimental results, obtained from the experiments following protocol step 5 with the device of protocol step 2, is shown in Figure 2. As Qw was fixed, the droplet size decreased with the increase of Qo. Further increasing the flow rate ratio might have yield smaller droplets, however the volume fraction of the droplets would have decreased accordingly, and there would have been a dramatic increase of the total drag and inner pressure inside the devices. Therefore, within the range of flow rate ratio in Figure 2, the droplet size was comparable to the inner diameter of the middle tube.
The droplet size can be further decreased when a device with smaller tubes is used. The typical experimental results, following protocol step 4 with the device from protocol step 3, are shown in Figure 3, where the middle tube had an I.D. = 200 μm.
The same device from protocol step 2 can be used to generate monodisperse droplets from various high-viscosity fluids, which have viscosities higher than glycerol. Typical results of monodisperse droplets of honey (11 Pas), starch solution (8.5 Pas), and polymer solution (2.5 Pas) are shown in Figure 4. The preparation of the fluids in Figure 4 is detailed in protocol step 6.
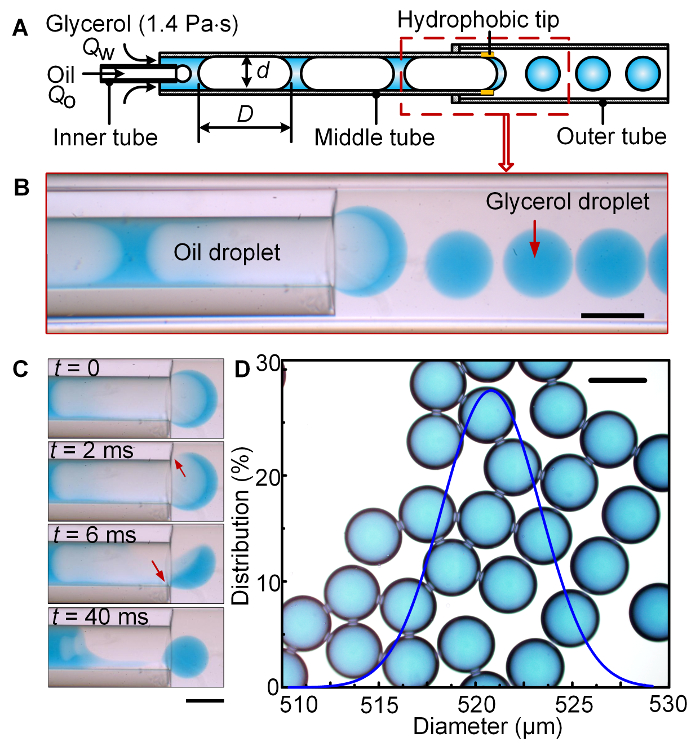
Figure 1: Generation of high-viscosity glycerol droplets in low-viscosity liquid paraffin using phase-inversion co-flow device. (A) Schematic of the phase-inversion co-flow devices. (B) The observation of the generation of the glycerol droplets from oil-in-glycerol slug flow in the middle tube to the glycerol-in-oil single emulsion in the outer tube. (C) Time sequence images of the phase-inversion process. (D) The monodisperse glycerol droplets and the size distribution of the droplets. The average diameter of the droplets is 521 μm, and CV = 0.9%. Reprinted with permission from [24]. Copyright 2017 American Chemical Society. The scale bars are 500 μm. Please click here to view a larger version of this figure.
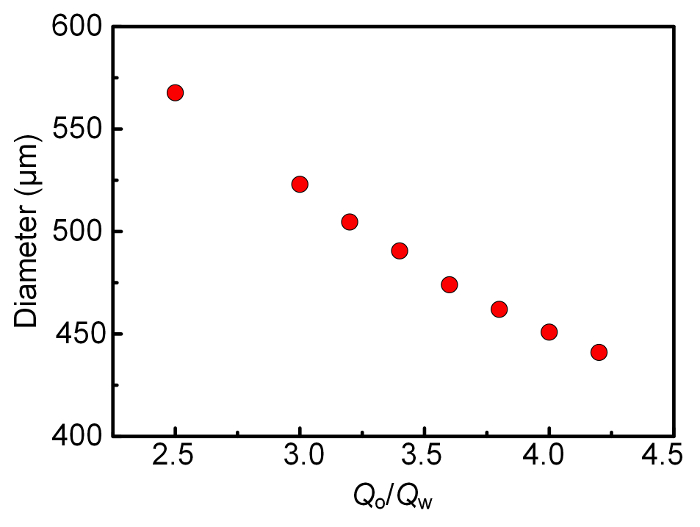
Figure 2: Variation of the droplet size with the change of the flow rate ratio QO/QW while QW = 2 μL/min. For each data point, 30 droplets are measured, and the average diameter is reported. As the error bars of the standard deviation are smaller than the symbol used in the plot, they are not shown here. Reprinted with permission from [24]. Copyright 2017 American Chemical Society. Please click here to view a larger version of this figure.
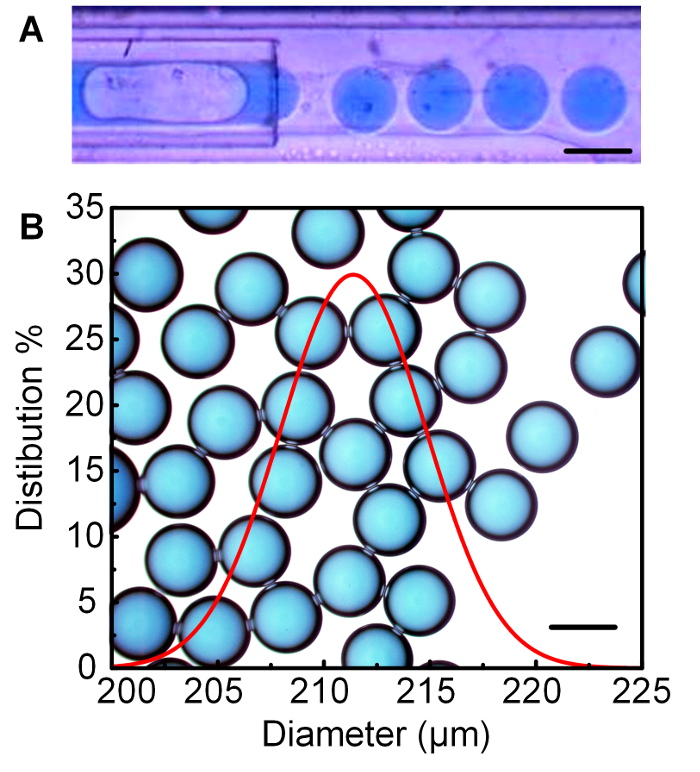
Figure 3: Smaller glycerol droplets generated from the device with the middle tube of I.D. = 200 μm. (A) The observation of the generation of glycerol droplets in the outer tube of the device. (B) The resultant monodisperse glycerol droplets with an average diameter of 212 μm and CV = 1.9%. Reprinted with permission from [24]. Copyright 2017 American Chemical Society. The scale bars are 200 μm. Please click here to view a larger version of this figure.
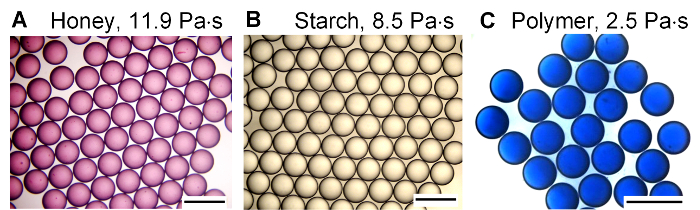
Figure 4: Typical examples of high-viscosity droplets generated from different solutions. Reprinted with permission from [24]. Copyright 2017 American Chemical Society. (A) Honey droplets with an average diameter of 612 μm and CV = 0.7%. (B) Starch droplets with an average diameter of 600 μm and CV = 0.9%, (C) PVA-124 polymer droplets with an average diameter of 773 μm and CV = 0.7%. All the scale bars are 1.0 mm. Please click here to view a larger version of this figure.
Discussion
The phase-inversion co-flow device provides a simple and straight forward method to generate monodisperse high-viscosity droplets. This device has a similar structure to common co-flow devices, as the basic co-flow structure consists of an inner tube inserted in the middle tube, the exit of which is connected to outlet tubing. However, there are two main differences between the phase-inversion co-flow device and common co-flow device for the generation of high-viscosity droplets with a viscosity of η > 1 Pa·s.
First, in common co-flow devices, an inner tube with a tapered tip is used, while a straight inner tube can be used in the phase-inversion co-flow devices. The tapered tip typically has I.D. = 20 μm and O.D. = 30 μm1,8, so that a pipette puller is usually necessary to make the tapered tip. In the phase-inversion co-flow device, a round glass tube with I.D. = 100 – 200 μm can be used directly without being tapered.
Second, in common co-flow devices, a high-viscosity fluid is injected into the inner tube to be encapsulated by low-viscosity fluid; while in phase-inversion co-flow devices, a low-viscosity fluid is injected into the inner tube to be encapsulated by a high-viscosity fluid, which is much easier to realize. For example, when we use a common co-flow device to generate glycerol droplets in liquid paraffin, the flow rate ratio Qo/Qw should be at least 25 to realize a well-controlled dripping mode, which results in a volume fraction of the glycerol droplets no higher than 4%. On the contrary, in the phase-inversion co-flow devices, the flow rate ratio Qo/Qw can be as low as 2.5 to realize a well-controlled dripping mode, therefore, a volume fraction of 28% of glycerol droplets can be realized.
In the phase-inversion co-flow device, the phase inversion is induced by the breakup of the elongated oil droplet at the hydrophobic exit of the middle tube. Therefore, the treatment of surface wettability of the exit of the middle tube is a critical step for the phase-inversion method, where the exit of the middle tube must be treated to be wetted by the low-viscosity phase to induce the phase inversion. In addition, there is a critical flow rate above which the elongated oil droplets will not breakup and the phase inversion will subsequently not occur24. When the oil droplets cannot breakup at the hydrophobic exit, lower the flow rates of both fluids with a fixed flow rate ratio, until the elongated oil droplets break and induce phase inversion. Moreover, if both the high-viscosity fluid and the low-viscosity fluid have similar wettability on the treated surface, then the phase-inversion method would be invalid.
Although we only provide the protocols and examples for the generation of aqueous high-viscosity droplets in this work, the phase-inversion co-flow device can also be used to generate high-viscosity oil droplets in low-viscosity aqueous solutions23. In such a device, the upstream of the middle tube needs to be treated to be wetted by the high-viscosity phase, while the exit of the middle tube needs to be treated to be wetted by the low-viscosity phase. The phase-inversion co-flow device provides a straightforward method to encapsulate high viscosity fluids in a well-controlled manner in rapidly developing droplet-based applications.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 51420105006 and 51322501). We thank Daniel for his helpful discussion on the high viscosity ideas.
Materials
| VitroTubes Glass Tubing | VitroCom | 8240 | Square – Miniature Hollow Glass Tubing, I.D.=0.4mm, OD=0.8mm |
| VitroTubes Glass Tubing | VitroCom | CV2033 | Round – Miniature Hollow Glass Tubing, I.D.=0.2mm, O.D.=0.33mm |
| VitroTubes Glass Tubing | VitroCom | CV1017 | Round – Miniature Hollow Glass Tubing, I.D.=0.1mm, O.D.=0.17mm |
| VitroTubes Glass Tubing | VitroCom | Q14606 | Square – Miniature Hollow Glass Tubing, I.D.=1.05mm+0.1/-0, OD=1.5mm |
| Standard Glass Capillaries | WPI | 1B100-6 | Round – Glass Tubing, I.D.=0.58mm, O.D.=1.00mm |
| Glycerol | Sinopharm Chemical Reagent Beijing | 10010618 | |
| Paraffin Liquid | Sinopharm Chemical Reagent Beijing | 30139828 | |
| Poly(vinyl alcohol), PVA-124 | Sinopharm Chemical Reagent Beijing | 30153084 | |
| Span 80 | Sigma-Aldrich | 85548 | |
| Starch | Sigma-Aldrich | S9765 | |
| Trichloro(octadecyl)silane | Sigma-Aldrich | 104817 | |
| Toluidine Blue O | Sigma-Aldrich | T3260 | |
| Honey | Chaste tree honey, common food product purchased from supermarket | ||
| DEVCON 5 Minute Epoxy | ITW | Epoxy glue | |
| Blunt Tip Stainless Steel Dispensing Needles (Luer Lock) | Suzhou Lanbo Needle, China | LTA820050 | 20G x 1/2" |
| Tungsten/Carbide Scriber | Ullman | 1830 | For cutting glass tubing |
| Microscope Slides | Sail Brand | 7101 | 76.2 mm x 25.4 mm, Thickness 1 – 1.2 mm |
| Polyethylene Tubing | Scientific Commodities | BB31695-PE/5 | I.D. = 0.86 mm, O.D. = 1.32 mm |
| Syringe Pumps | Longer Pump, China | LSP01-1A | 3 pumps needed for the experiments |
References
- Shah, R. K., Shum, H. C., Rowat, A. C., Lee, D., Agresti, J. J., Utada, A. S., Chu, L. Y., Kim, J. W., Fernandez-Nieves, A., Martinez, C. J., Weitz, D. A. Designer emulsions using microfluidics. Mater. Today. 11, 18-27 (2008).
- Park, J. I., Saffari, A., Kumar, S., Günther, A., Kumacheva, E. Microfluidic synthesis of polymer and inorganic particulate materials. Annu. Rev. Mater. Res. 40, 415-443 (2010).
- Heath, J. R., Ribas, A., Mischel, P. S. Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug Discovery. 15, 204-216 (2016).
- Murphy, S. V., Atala, A. 3D Bioprinting of tissues and organs. Nat. Biotechnol. 32, 773-785 (2014).
- Du, G., Fang, Q., den Toonder, J. M. Microfluidics for cell-based high throughput screening platforms-a review. Anal. Chim. Acta. 903, 36-50 (2016).
- Ushikubo, F. Y., Oliveira, D. R. B., Michelon, M., Cunha, R. L. Designing food structure using microfluidics. Food Eng. Rev. 7, 393-416 (2015).
- Xu, J. H., Li, S. W., Tan, J., Wang, Y. J., Luo, G. S. Preparation of highly monodisperse droplet in a T-junction microfluidic device. AIChE Journal. 52, 3005-3010 (2006).
- van Steijn, V., Kleijn, C. R., Kreutzer, M. T. Flows around confined bubbles and their importance in triggering pinch-off. Phys. Rev. Lett. 103, 214501 (2009).
- Utada, A. S., Fernandez-Nieves, A., Stone, H. A., Weitz, D. A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 99, 094502 (2007).
- Anna, S. L., Bontoux, N., Stone, H. A. Formation of dispersions using "flow focusing" in microchannels. Appl. phys. lett. 82, 364-366 (2003).
- Utada, A. S., Lorenceau, E., Link, D. R., Kaplan, P. D., Stone, H. A., Weitz, D. A. Monodisperse double emulsions generated from a microcapillary device. Science. 308, 537-541 (2005).
- Teh, S. Y., Lin, R., Hung, L. H., Lee, A. P. Droplet microfluidics. Lab Chip. 8, 198-220 (2008).
- Nunes, J. K., Tsai, S. S. H., Wan, J., Stone, H. A. Dripping and jetting in microfluidic multiphase flows applied to particle and fiber synthesis. J. Phys. D: Appl. Phys. 46, 114002 (2013).
- Cubaud, T., Mason, T. G. Capillary threads and viscous droplets in square microchannels. Phys. Fluids. 20, 053302 (2008).
- Shestopalov, I., Tice, J. D., Ismagilov, R. F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip. 4, 316-321 (2004).
- Zheng, B., Roach, L. S., Ismagilov, R. F. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J. Am. Chem. Soc. 125, 11170-11171 (2003).
- Nie, Z. H., Xu, S. Q., Seo, M., Lewis, P. C., Kumacheva, E. Microfluidic production of biopolymer microcapsules with controlled morphology. J. Am. Chem. Soc. 127, 8058-8063 (2005).
- Abate, A. R., Kutsovsky, M., Seiffert, S., Windbergs, M., Pinto, L. F., Rotem, A., Utada, A. S., Weitz, D. A. Synthesis of monodisperse microparticles from non-Newtonian polymer solutions with microfluidic devices. Adv. Mater. 23, 1757-1760 (2011).
- Seo, M., Nie, Z., Xu, S., Mok, M., Lewis, P. C., Graham, R., Kumacheva, E. Continuous microfluidic reactors for polymer particles. Langmuir. 21, 11614-11622 (2005).
- Duncanson, W. J., Lin, T., Abate, A. R., Seiffert, S., Shah, R. K., Weitz, D. A. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip. 12, 2135-2145 (2012).
- Song, H., Chen, D. L., Ismagilov, R. F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 45, 7336-7356 (2006).
- Chen, H., Zhao, Y., Li, J., Guo, M., Wan, J., Weitz, D. A., Stone, H. A. Reactions in double emulsions by flow-controlled coalescence of encapsulated drops. Lab Chip. 11, 2312-2315 (2011).
- Wang, P., Li, J., Nunes, J., Hao, S., Liu, B., Chen, H. Droplet micro-reactor for internal gelation to fabricate ZrO2 ceramic microspheres. J. Am. Ceram. Soc. 100, 41-48 (2017).
- Chen, H., Man, J., Li, Z., Li, J. Microfluidic generation of high-viscosity droplets by surface-controlled breakup of segment flow. ACS Appl. Mater. Interfaces. 9, 21059-21064 (2017).
- Man, J., Li, Z., Li, J., Chen, H. Phase inversion of slug flow on step surface to form high viscosity droplets in microchannel. Appl. Phys. Lett. 110, 181601 (2017).

