Engineering Transplantation-suitable Retinal Pigment Epithelium Tissue Derived from Human Embryonic Stem Cells
Summary
We describe a method to engineer a retinal tissue composed of retinal pigment epithelial cells derived from human pluripotent stem cells cultured on top of human amniotic membranes and its preparation for grafting in animal models.
Abstract
Several pathological conditions of the eye affect the functionality and/or the survival of the retinal pigment epithelium (RPE). These include some forms of retinitis pigmentosa (RP) and age-related macular degeneration (AMD). Cell therapy is one of the most promising therapeutic strategies proposed to cure these diseases, with already encouraging preliminary results in humans. However, the method of preparation of the graft has a significant impact on its functional outcomes in vivo. Indeed, RPE cells grafted as a cell suspension are less functional than the same cells transplanted as a retinal tissue. Herein, we describe a simple and reproducible method to engineer RPE tissue and its preparation for an in vivo implantation. RPE cells derived from human pluripotent stem cells are seeded on a biological support, the human amniotic membrane (hAM). Compared to artificial scaffolds, this support has the advantage of having a basement membrane that is close to the Bruch's membrane where endogenous RPE cells are attached. However, its manipulation is not easy, and we developed several strategies for its proper culturing and preparation for grafting in vivo.
Introduction
RPE is crucial for the survival and homeostasis of the photoreceptors with which it is tightly associated1. Several pathological conditions alter its functionality and/or survival, including RP and AMD.
RP is a group of inherited monogenic mutations that affect the functions of photoreceptors or RPE cells or both2,3. It is estimated that mutations that affect specifically the RPE cells account for 5% of RP2. AMD is another condition where the RPE layer is altered, leading ultimately to central vision loss. AMD is caused by the complex interactions of genetic and environmental factors and affects the elderly4,5,6. According to projections, AMD will be a concern for 196 million patients worldwide by 20207. For these disorders, no effective cure exists, and one of the strategies proposed is the transplantation of new RPE cells in order to compensate for dead/nonfunctional preexisting RPE cells8.
The mode of formulation of the final product to be grafted is essential to ensure the best functional outcomes. RPE cells injected as a cell suspension, despite being an easy and straightforward method of delivery, raise concerns regarding their survival, integration, and functionality9,10,11,12,13. Scientists are now developing more complex formulations to deliver engineered retinal tissue9,13,14,15,16. In this context, we developed an original method to generate in vitro RPE tissue that could be used for transplantation9.
RPE cell banks derived from human embryonic stem (ES) cells are used in this protocol. However, alternative RPE cell banks from different cell sources (human-induced pluripotent stem cells, primary RPE cells, etc.) and differentiated with a different method are also suitable for this protocol. It includes directed differentiation protocols using cytokines and/or small molecules17,18,19,20,21,22.
To be transplanted, the engineered tissue should be prepared on a scaffold. In the past few years, different scaffolds were developed based on a polymer or on a matrix of biological origin13,23,24. Here, the biological substrate used is the hAM, but other substrates, like denuded Bruch membranes, could be implemented. The method described herein has the advantage of using a biological scaffold that is more relevant to the RPE native environment.
Human ES cell-derived RPE cells are cultured for at least 4 weeks in order to be fully organized as a cobblestone monolayer. At that stage, the epithelium obtained is functional and polarized9. Finally, as this tissue wrinkles easily, it is embedded in a thin layer of a hydrogel carrier to give it more rigidity and elasticity and to protect it during the injection procedure. This product is then stored at 4 °C until grafting.
Protocol
All human materials used in this protocol were used in accordance with European Union regulations. The human ES cell line used in this study was derived from a unique embryo. The couple who had donated the embryo was fully informed and gave their consent for an anonymous donation. A clinical-grade human ES cell line was derived from this embryo, banked, qualified, and properly documented by Roslin Cells (UK). hAMs were procured under sterile conditions during a cesarean section in mothers who signed an informed consent for placenta donation according to hospital guidelines (APHP, Hôpital Saint Louis).
1. Preparation of Culture Media and Reagents
- Preparation of the RPE cell culture medium
- To prepare the RPE cell culture medium, add 4% serum substitute (20 mL for a final volume of 500 mL), 1,000x diluted 2-mercaptoethanol (500 µL for a final volume of 500 mL), and 1% Eagle′s minimum essential medium (MEM) non-essential amino acids solution (5 mL for a final volume of 500 mL) to Dulbecco's modified Eagle's medium (DMEM) high glucose (475 mL for a final volume of 500 mL).
- Preparation of the transport/conservation medium
- To prepare the transport/conservation medium, add 1% of penicillin-streptomycin (5 mL for a final volume of 500 mL) to CO2-independent medium (495 mL for a final volume of 500 mL) and keep it at 4 °C.
- Resuspension of the thermolysin enzyme
- Preparation of a stock solution of thermolysin
- To prepare a stock solution of thermolysin, thaw the thermolysin powder, which was stored at -20 °C, at room temperature.
- As the enzymatic activity may be variable from batch to batch, calculate this activity based on the certificate of analysis provided with the thermolysin powder of the batch to be used. Divide the "Activity Neutral Protease calculated = ANPC" (indicated in the Certificate of Analysis) by 181 (which is tyrosine's molecular weight). The result obtained corresponds to the total enzymatic activity of the supplied thermolysin powder (U/vial).
- Prepare a stock solution at 200 U/mL by adding the volume of water corresponding to the total enzymatic activity (U/vial) divided by 200 (U/mL).
- Dissolve the enzyme by pipetting the water that was added up and down. Vortex the solution for 30 s and check whether the powder has completely been suspended. More pipetting may be required for a full dissolution.
- Aliquot the stock solution and store it at -20 °C.
- Preparation of a working solution of thermolysin
NOTE: The thermolysin solution must be prepared on the day of the hAM treatment.- Thaw the stock aliquots of thermolysin at 200 U/mL stored at -20 °C. Dilute the stock solution 200x in phosphate-buffered saline (PBS) in order to obtain a final enzymatic activity of 1 U/mL. Prepare 40 mL to treat 1–4 hAM patches.
- Filter the thermolysin solution through a 0.2 µm filter prior to use.
- Preparation of a stock solution of thermolysin
- Resuspension of gelatin
- Preparation of 20% gelatin solution and block
NOTE: The 20% gelatin solution and block may be prepared up to 1 week before use.- Warm the CO2-independent medium to 42 °C in a water bath for 30 min (up to 1 h). In a 50 mL tube, add 10 g of gelatin to 40 mL of warmed CO2-independent medium and vortex the solution.
- Dissolve the gelatin for 30–60 min at 42 °C. Vortex each 10 min to homogenize the solution.
- Once the solution is homogeneous, add 4 mL of the 20% gelatin solution to four 6 cm culture dishes. Avoid bubbles. Add a plastic paraffin film to protect the dishes, and allow the solution to solidify at 4 °C.
- Preparation of 8% gelatin solution
NOTE: The 8% gelatin solution may be prepared the day before use and stored at 4 °C.- Warm the CO2-independent medium to 42 °C for 30 min (up to 1 h). In a 50 mL tube, add 4 g of gelatin to 46 mL of warmed CO2-independent medium and vortex the solution.
- Dissolve the gelatin for 30–60 min at 42 °C. Add a plastic paraffin film to protect the tube and keep it in a water bath at 37 °C if it is to be used the same day, or store it at 4 °C if it is to be used later.
- Preparation of 20% gelatin solution and block
2. Thermolysin Treatment of Human Amniotic Membranes
- Human amniotic membrane wash
- Have hAMs supplied as small pieces (approximately 30 mm x 30 mm) fixed in a nylon scaffold, either already at 4 °C in PBS or frozen. If provided frozen, thaw the membranes at 37 °C in an incubator for 30 min.
- Wash the membranes:
- Place 1–4 membranes in a 250 mL bottle containing 80 mL of PBS. Use as many bottles as required.
- Shake each bottle in a plate shaker at a high speed for 5 min, then discard the PBS. Add 80 mL of PBS and repeat.
- Thermolysin treatment
- Add 40 mL of the working solution of thermolysin (1 U/mL) per bottle containing the membranes (1–4 membranes per bottles; up to 3 bottles at a time).
- Shake the bottles for 5 min at 450 rpm and then vortex them for 30 s. Repeat 1x.
- Discard the thermolysin solution and add 80 mL of PBS.
- Shake the bottles for 5 min at 450 rpm. Discard the PBS and add 80 mL of PBS. Repeat 3x.
3. Fixation of Human Amniotic Membranes on a Culture Insert
- Use long sterile forceps (that can reach the bottom of the bottle) to remove the hAMs one by one, and transfer each hAM to a 10 cm culture dish containing 10 mL of PBS.
- In a new 10 cm culture dish containing 10 mL of PBS, place one of the membranes with the nylon facing down. Detach two of the four clips that are used to fix the membrane to nylon.
- Insert the smaller ring of the culture insert between the nylon and the membrane.
- Make sure that the membrane covers the smaller ring completely. Clip the second part of the culture insert on top of the membrane. The basement membrane of the hAM is facing up inside the culture insert. Detach the last two clips.
- Cut, with sterile scissors, the excess of membrane outside the culture insert if necessary. Using forceps, transfer the membrane fixed in the culture insert to a 12-well plate, add PBS, and store the plate in an incubator at 37 °C and 5% CO2.
- Repeat these operations (from step 3.2 to 3.5) with the other membranes.
4. Thawing and Seeding of Retinal Pigment Epithelium Cells on Human Amniotic Membranes
- Thawing of retinal pigment epithelium cells
- The RPE cell bank is frozen at 1 million cells per cryogenic vial in liquid nitrogen. Thaw as many cryogenic vials as required (350,000 cells are seeded per membrane).
- Prepare a 15 mL tube containing 3 mL of RPE medium per cryogenic vial. Once the vial is thawed, transfer the cells to each tube. Repeat this operation for the other vials.
- Homogenize (pipette up and down a few times to resuspend the cells in the RPE medium) and take 10 µL of the cell solution and transfer it to a 1.5 mL tube. Add 10 µL of trypan blue. Place 15 µL of this solution in a counting chamber, then count the number of viable cells (not colored in blue) under a microscope with a 4X objective. Repeat this operation for the other vials.
- In the meantime, centrifuge the 15 mL tubes at 110 x g for 5 min. Discard the supernatant and resuspend the cells at 700,000 cells/mL.
- Seeding of retinal pigment epithelium cells into human amniotic membranes
- Remove the 12-well plate containing the membranes from the incubator.
- Aspirate the PBS. Add 500 µL of the cell suspension prepared in step 4.1.4 in the center of the culture insert. Add 1 mL of RPE medium outside the culture insert (inside the well). Repeat for the other membranes.
- Place the 12-well plate containing the membranes in the incubator.
5. Maintenance of Retinal Pigment Epithelium Cell Cultures on Human Amniotic Membranes
- Renew the medium 2x per week, usually on Monday and Friday, until at least day 30 of the culture.
- Aspirate the medium inside and outside the culture insert for each well and renew it with fresh medium (1 mL outside the culture insert and 500 µL inside). Place the plate in the incubator at 37 °C and 5% CO2.
6. Preparation of the Retinal Pigment Epithelium Patch for Transplantation
NOTE: Starting at day 30 of the culture, the tissue is ready for transplantation.
- Warm the 8% gelatin solution in a water bath at 37 °C for 30 min. Fill the internal chamber of the vibratome with cold CO2-independent medium (at 4 °C).
- Place the 20% gelatin block into the vibratome.
- Take the 6 cm culture dish containing the block of 20% gelatin from the fridge. Using a scalpel, cut out a 5 cm x 5 cm block. Paste the top side of the block to the support with cyanoacrylate glue or its equivalent.
- Place a new blade in the vibratome.
- Adjust the level of the block until it is at the same level of the razor blade.
- Fill the bath around the support with fresh CO2-independent medium at 4 °C. Cut the block with the vibratome using a medium velocity until the block is cut uniformly, leading to a smooth surface. Set the position 0.
- Aspirate the medium around the block of gelatin until it is completely dry, and then take the 12-well culture plate containing the tissues from the incubator at 37 °C.
- Using forceps, carefully open the culture insert on top of the gelatin block. Cut, with the scissors, all parts of the membranes that do not contain cells (outside the ring where cells were not cultured). Aspirate the entire residual medium.
- Add 1 mL of the liquid 8% gelatin solution at 37 °C in order to cover the membranes. Carefully remove the excess. Wait 5–8 min to allow the gelatin to solidify.
- Add fresh CO2-independent medium (at 4 °C) to the bath until the membrane is covered.
- Cut, with the vibratome at a position of -100 µm, with a medium velocity, remaining aware of how the block behaves. At the end of the section, be careful to maintain the orientation of the tissue.
- With a scalpel, cut a corner to identify the orientation of the section.
- Collect the membrane embedded in gelatin with a spatula and place it in a 6-well plate filled with the conservation medium at 4 °C, until grafting it into the recipient eye.
- At the time of the transplantation, adjust the size of the implant to the size of the recipient eye under a surgical microscope (1–3 for rats, 10–15 mm2 for non-human primates).
Representative Results
hAMs contain an epithelial layer that should be removed before the seeding of RPE cells. An enzymatic treatment of the membrane is performed with the thermolysin under shaking. In order not to not lose the polarity of the membrane (the epithelium is on one side), it is fixed on a support which composition could be different depending on the provider (Figure 1A). Check the adhesion of the membrane to its support at this step and add clips if necessary. At the time of the fixation in the culture insert, work carefully to avoid making holes in the membrane and keep its polarity with the basement membrane facing up (Figure 1B). When cells are seeded on the membranes, they could escape from these holes and the final cell concentration will be reduced. The presence of holes could be visualized under a microscope or by adding PBS inside the culture insert and checking if PBS is leaking. Any membrane with holes should be discarded.
Following the fixation on the culture insert, the presence of residual cells in a phase-contrast microscope is evaluated (Figures 1C and 1D). Classically, a few dead cells could remain on the surface of the membrane, but those cells will be eliminated when the culture medium is changed (Figure 1C). The fibers of the basement membrane could be seen at a higher magnification (Figure 1D) if no cells remain. If that is not the case, the timing of the incubation with thermolysin might be adjusted.
In the days following the seeding of the RPE cells, check if the cells adhere. Depending on the microscope used, it could be difficult to clearly see the cells during the first few weeks, but if the cells do not adhere, cells will be seen floating around in the cell culture medium. Once the epithelium is formed and starts to mature, it becomes easier to see it under a microscope (Figures 2A, 2B, and 2C). At 3 weeks, the cells form a complete monolayer epithelium typical of RPE (cobblestone organization). After 4 weeks, the epithelium is enough mature for its preparation for implantation (Figure 2D). However, it could be kept in culture for more weeks for logistical reasons.
The preparation of the graft for implantation is described in Figure 3. Upon apposition of the membrane to the 20% gelatin block, aspirate all the excess cell culture medium. This step is important, as any remaining medium could preclude the adhesion of the 8% gelatin to the RPE and the membrane. Indeed, the medium will form a film of liquid between the layers of gelatin.
The gelatin used for the embedding of the implant could be of a varying strength, depending on its Bloom index (i.e., a quality control test, performed and provided by suppliers, that evaluates the strength of a gel at a standardized temperature and concentration). If the gelatin used is not rigid enough and disaggregates during the grafting, the used gelatin reference should be changed to another one with a higher strength. The concentration of gelatin could also be changed, based on experimentation, to adjust its strength and elasticity.
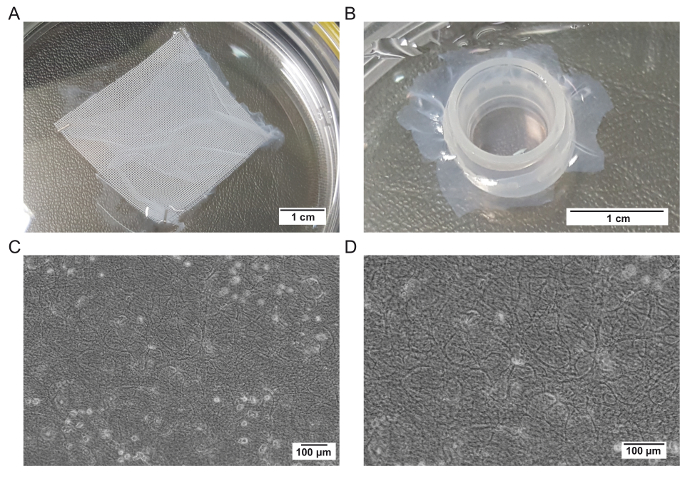
Figure 1: Representative images of the hAM before and after the thermolysin treatment and fixation on a culture insert. (A) Representative image of a membrane supplied by a tissue bank. (B) Representative image of a membrane fixed on a culture insert. (C) Representative image of a membrane treated with thermolysin at a low magnification. (D) Representative image of a membrane treated with thermolysin at a high magnification. Please click here to view a larger version of this figure.
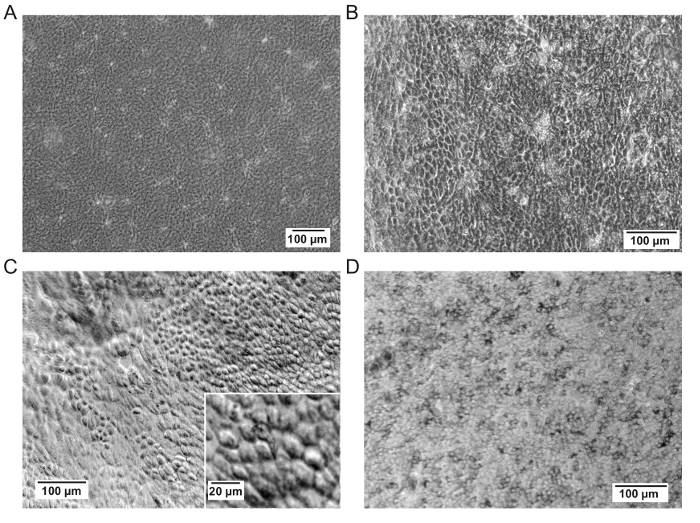
Figure 2: Representative images of the hAM upon seeding the RPE cells. (A) Representative image of the membrane before seeding.(B and C) Representative images of RPE cells on membranes at 3 weeks post-seeding. The membrane may not be completely planar as seen in panel C. Scale bar = 100 µm (C), 20 µm for the magnification. (D) Representative image of the RPE cells on a membrane at 30 days post-seeding, corresponding to the time of embedding them in gelatin. Please click here to view a larger version of this figure.
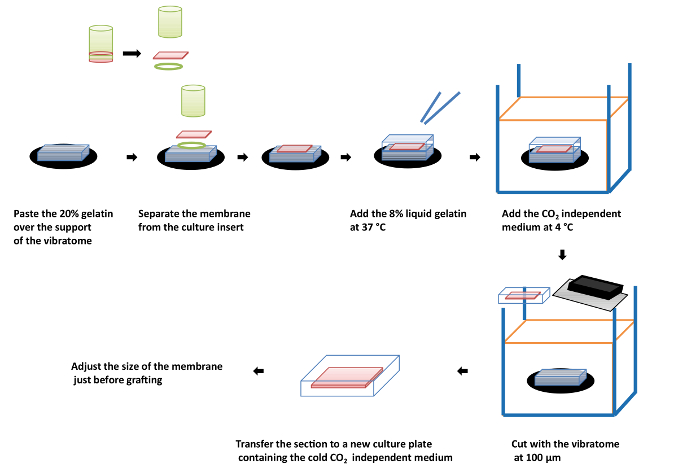
Figure 3: Scheme describing the sequential steps for the inclusion of the engineered retinal tissue containing the RPE cell layer on the hAM inside a gelatin film. Please click here to view a larger version of this figure.
Discussion
We described a method for the culture of RPE cells on a biological scaffold and its preparation for implantation in animal models. One of the critical steps of the protocol is the maintenance of the orientation of the hAM all along the procedure until its inclusion into gelatin. Indeed, the native epithelium of the membrane is removed and its basement membrane becomes exposed9. The RPE cells have to be seeded on top of this basement membrane. Upon preparation for gelatin embedding, it is crucial to work with all the products at the defined temperature. Indeed, gelatin has the property to be rigid at 4 °C and liquid at body temperature (37 °C)9. If the temperature is not respected, the gelatin could solidify or liquefy at a step where this effect is not desired.
Several biological scaffolds have been proposed, like Descemet's membranes25 or hAMs26. In particular, hAMs, from a cesarean section27, were demonstrated to be well-tolerated in the subretinal space, causing limited inflammation and reducing choroidal neovascularization26. The membrane successfully supports the culture of human RPE cells9,28. Moreover,these membranes have also a long history in clinics29, making them good candidates for a scaffold for RPE cell therapy. Other biological supports could be easily implemented with this protocol. Synthetic scaffolds based on polymer are already rigid and might not need a gelatin embedding prior to implantation13,30,31,32,33. Other systems for implantation have been recently developed for the subretinal delivery in the human eye of an hESC-derived RPE monolayer on a rigid polyester scaffold34 or on a synthetic parylene substrate designed to mimic Bruch's membrane35. Even though these strategies are promising, we believe that biological scaffolds might provide the best platform for RPE tissue engineering.
In this protocol, the seeded RPE cells were derived from human ES cells using a spontaneous differentiation method. However, different types of RPE cells could be used, either differentiated from human induced pluripotent stem cells or from primary RPE cells obtained from cadavers or even from an RPE cell line19,36,37,38. Moreover, if using pluripotent stem cells, several protocols were developed in the past years to obtain RPE cells based on a spontaneous differentiation or using small molecules to guide the differentiation18,22. RPE cells obtained from these different differentiation protocols could be also used with this method for tissue engineering.
One limit of this method is the stability of the embedded tissue at 4 °C. As it is included in gelatin just before the shipment to the surgery site, it has to be maintained at this temperature until the engraftment. In that context, the surgeries should be performed within 48 h.
This method could be easily transferred to the clinic. The RPE tissue embedded in gelatin is conserved at 4 °C in the CO2-independent medium. This medium could be substituted, if required, with others already approved by the US Food and Drug Administration (FDA) for the conservation of corneas obtained post-mortem and which are used for transplantations into humans. We successfully demonstrated the efficiency of this strategy for transplantation in a proof-of-concept study into rodent models9, and we are currently validating the surgical approach in non-human primates before implementing it for a clinical trial to treat patients with specific forms of RP affecting the RPE.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Jérôme Larghero and Valérie Vanneaux (Hôpital Saint Louis, Paris, France) for their input during the setting-up of the method described here.
This work was supported by grants from the ANR [GPiPS: ANR-2010-RFCS005; SightREPAIR: ANR-16-CE17-008-02], the Fondation pour la Recherche Médicale [Bio-engineering program – DBS20140930777] and from LABEX REVIVE [ANR-10-LABX-73] to Olivier Goureau and Christelle Monville. It was supported by NeurATRIS, a translational research infrastructure (Investissements d'Avenir) for biotherapies in Neurosciences [ANR-11-INBS-0011] and INGESTEM, the national infrastructure (Investissements d'Avenir) engineering for pluripotent and differentiated stem cells [ANR-11-INBS-000] to Christelle Monville. Karim Ben M'Barek was supported by fellowships from DIM Stempole and LABEX REVIVE [ANR-10-LABX-73]. I-Stem is part of the Biotherapies Institute for Rare Diseases supported by the Association Française contre les Myopathies (AFM)-Téléthon.
Materials
| Sterile biosafety cabinet | TechGen International | Not applicable | |
| Liquid waste disposal system for aspiration | Vacuubrand | BVC 21 | |
| CO2-controlled +37 °C cell incubator | Thermo Electron Corporation | BVC 21 NT | |
| 200 µL pipette: P200 | Gilson | F144565 | |
| 1 mL pipette: P1000 | Gilson | F144566 | |
| Pipet aid | Drummond | 75001 | |
| +4 °C refrigerator | Liebherr | Not applicable | |
| Vibratome | Leica | VT1000S | |
| Fine scissors | WPI | 501758 | |
| Forceps (x2) | WPI | 555227F | |
| Water bath | Grant subaqua pro | SUB6 | |
| Precision balance | Sartorius | CP225D | |
| Centrifuge | Eppendorff | 5804 | |
| Microscope | Olympus | SC30 | |
| Horizontal Rocking Shaker | IKA-WERKE | IKA MTS 214D | |
| Vortex | VWR | LAB DANCER S40 | |
| Disposable Scalpel | WPI | 500351 | |
| plastic paraffin film | VWR | PM992 | |
| 0.200 µm single use syringe filter | SARTORIUS | 16532 | |
| Syringe without needle 50 mL | Dutscher | 50012 | |
| Bottles 250mL | Dutscher | 28024 | |
| 15 mL sterile Falcon tubes | Dutscher | 352097 | |
| 50 mL sterile Falcon tubes | Dutscher | 352098 | |
| culture insert | Scaffdex | C00001N | |
| 60 mm cell culture disches: B6 | Dutscher | 353004 | |
| 12 well cell culture plate | Corning | 3512 | |
| 6-well culture plates | Corning | 3506 | |
| Razor blades | Ted Pella, Inc | 121-9 | |
| Cyanoacrylate glue | Castorama | 3178040670105 | |
| PBS 1X (500 mL) | Sigma | D8537 | |
| Thermolysine | Roche | 5339880001 | |
| DMEM, high glucose, GlutaMAX | Invitrogen | 61965-026 | |
| KSR CTS (KnockOut SR XenoFree CTS) | Invitrogen | 12618-013 | |
| MEM-NEAA (100X) | Invitrogen | 11140-035 | |
| b-mercaptoethanol (50 mM) | Invitrogen | 31350-010 | |
| Penicillin/Streptomycin | Invitrogen | 15140122 | |
| CO2-independent medium | GIBCO | 18045-054 | |
| Gelatin | MERCK | 104078 | |
| human amniotic membrane | Tissue bank St Louis hospital (Paris, France) | Not applicable |
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiological Reviews. 85 (3), 845-881 (2005).
- Hartong, D. T., Berson, E. L., Dryja, T. P. Retinitis pigmentosa. Lancet. 368 (9549), 1795-1809 (2006).
- Daiger, S. P., Sullivan, L. S., Bowne, S. J. Genes and mutations causing retinitis pigmentosa. Clinical Genetics. 84 (2), 132-141 (2013).
- Gehrs, K. M., Anderson, D. H., Johnson, L. V., Hageman, G. S. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Annals of Medicine. 38 (7), 450-471 (2006).
- Swaroop, A., Chew, E. Y., Rickman, C. B., Abecasis, G. R. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annual Review of Genomics and Human Genetics. 10, 19-43 (2009).
- Khandhadia, S., Cherry, J., Lotery, A. J. Age-related macular degeneration. Advances in Experimental Medicine and Biology. 724, 15-36 (2012).
- Wong, W. L., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet. Global Health. 2 (2), e106-e116 (2014).
- Ben M’Barek, K., Regent, F., Monville, C. Use of human pluripotent stem cells to study and treat retinopathies. World Journal of Stem Cells. 7 (3), 596-604 (2015).
- Ben M’Barek, K., et al. Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Science Translational Medicine. 9 (421), (2017).
- Schwartz, S. D., et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 379 (9817), 713-720 (2012).
- Schwartz, S. D., et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet. 385 (9967), 509-516 (2015).
- Hsiung, J., Zhu, D., Hinton, D. R. Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Translational Medicine. 4 (1), 10-20 (2015).
- Diniz, B., et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Investigative Ophthalmology & Visual Science. 54 (7), 5087-5096 (2013).
- Kamao, H., et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2 (2), 205-218 (2014).
- Mandai, M., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. The New England Journal of Medicine. 376 (11), 1038-1046 (2017).
- Thomas, B. B., et al. Survival and functionality of hESC-derived retinal pigment epithelium cells cultured as a monolayer on polymer substrates transplanted in RCS rats. Investigative Ophthalmology & Visual Science. 57 (6), 2877-2887 (2016).
- Borooah, S., et al. Using human induced pluripotent stem cells to treat retinal disease. Progress in Retinal and Eye Research. 37, 163-181 (2013).
- Leach, L. L., Clegg, D. O. Concise review: Making stem cells retinal: Methods for deriving retinal pigment epithelium and implications for patients with ocular disease. Stem Cells. 33 (8), 2363-2373 (2015).
- Reichman, S., et al. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proceedings of the National Academy of Sciences of the United States of America. 111 (23), 8518-8523 (2014).
- Lustremant, C., et al. Human induced pluripotent stem cells as a tool to model a form of Leber congenital amaurosis. Cellular Reprogramming. 15 (3), 233-246 (2013).
- Reichman, S., et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS Cells in xeno-free and feeder-free conditions. Stem Cells. 35 (5), 1176-1188 (2017).
- Maruotti, J., et al. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 112 (35), 10950-10955 (2015).
- Stanzel, B. V., et al. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Reports. 2 (1), 64-77 (2014).
- Ilmarinen, T., et al. Ultrathin polyimide membrane as cell carrier for subretinal transplantation of human embryonic stem cell derived retinal pigment epithelium. PloS One. 10 (11), e0143669 (2015).
- Thumann, G., Schraermeyer, U., Bartz-Schmidt, K. U., Heimann, K. Descemet’s membrane as membranous support in RPE/IPE transplantation. Current Eye Research. 16 (12), 1236-1238 (1997).
- Kiilgaard, J. F., Scherfig, E., Prause, J. U., la Cour, M. Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cells International. 2012, 716968 (2012).
- Capeans, C., et al. Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmologica Scandinavica. 81 (3), 271-277 (2003).
- Ohno-Matsui, K., et al. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Molecular Vision. 11, 1-10 (2005).
- Paolin, A., et al. Amniotic membranes in ophthalmology: long term data on transplantation outcomes. Cell and Tissue Banking. 17 (1), 51-58 (2016).
- Hu, Y., et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Research. 48 (4), 186-191 (2012).
- Pennington, B. O., Clegg, D. O. Pluripotent stem cell-based therapies in combination with substrate for the treatment of age-related macular degeneration. Journal of Ocular Pharmacology and Therapeutics: The Official Journal of the Association. 32 (5), 261-271 (2016).
- Song, M. J., Bharti, K. Looking into the future: Using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling. Brain Research. 1638 (Pt A), 2-14 (2016).
- Ramsden, C. M., et al. Stem cells in retinal regeneration: Past, present and future). Development. 140 (12), 2576-2585 (2013).
- da Cruz, L., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nature Biotechnology. 36 (4), 328-337 (2018).
- Kashani, A. H., et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Science Translational Medicine. 10 (435), (2018).
- Binder, S., Stanzel, B. V., Krebs, I., Glittenberg, C. Transplantation of the RPE in AMD. Progress in Retinal and Eye Research. 26 (5), 516-554 (2007).
- Dunn, K. C., Aotaki-Keen, A. E., Putkey, F. R., Hjelmeland, L. M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Experimental Eye Research. 62 (2), 155-169 (1996).
- Salero, E., et al. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 10 (1), 88-95 (2012).

