Neurogenesis Using P19 Embryonal Carcinoma Cells
Summary
The P19 mouse embryonic carcinoma cell line (P19 cell line) is widely used for studying the molecular mechanism of neurogenesis with great simplification compared to in vivo analysis. Here, we present a protocol for retinoic acid-induced neurogenesis in the P19 cell line.
Abstract
The P19 cell line derived from a mouse embryo-derived teratocarcinoma has the ability to differentiate into the three germ layers. In the presence of retinoic acid (RA), the suspension cultured P19 cell line is induced to differentiate into neurons. This phenomenon is extensively investigated as a neurogenesis model in vitro. Therefore, the P19 cell line is very useful for molecular and cellular studies associated with neurogenesis. However, protocols for neuronal differentiation of P19 cell line described in the literature are very complex. The method developed in this study are simple and will play a part in elucidating the molecular mechanisms in neurodevelopmental abnormalities and neurodegenerative diseases.
Introduction
During embryonal development, a single cell layer is transformed into three separate germ layers1,2,3. To increase the research possibilities of phenomena occurring in vivo, generation of three-dimensional aggregates (embryonic bodies) have been developed as a convenient model. Cellular aggregates formed in this way can be exposed to various conditions causing cell differentiation, which reflect development of the embryo4,5. The P19 murine embryonic carcinoma cell line (P19 cell line) is commonly used as a cellular model for neurogenesis studies in vitro6,7,8. The P19 cell line exhibits typical pluripotent stem cell features and can differentiate into neurons in the presence of retinoic acid (RA) during cell aggregation followed by neurite outgrowth under adherent conditions. Moreover, the undifferentiated P19 cell line is also capable of forming muscle- and cardiomyocyte-like cells under the influence of dimethyl sulfoxide (DMSO)9,10,11,12.
Many methods13,14,15,16 have been reported for neuronal differentiation, but the methodology is sometimes complicated and not easy to grasp by only reading the descriptions. For example, protocols sometimes require a combination of Dulbecco's Modified Eagle Medium (DMEM) medium supplemented with a mixture of calf serum (CS) and fetal bovine serum (FBS)13. Moreover, media used for neuronal development are often composed of Neurobasal and B27 supplements13,14,15,16. As such, existing methods contain complexity in their preparation and our goal here is to simplify the protocols. In this study, we demonstrated that DMEM with FBS can be utilized for maintaining the P19 cell line (DMEM + 10% FBS) as well as for neuronal development (DMEM + 5% FBS + RA). This simplified method for neurogenesis using the P19 cell line allows us to study the molecular mechanism of how neurons are developed. Moreover, research on neurodegenerative diseases such as Alzheimer's disease is also conducted using P19 cell line17,18, and we believe that the method developed in this study will play a part in elucidating the molecular mechanisms in neurodevelopmental abnormalities and neurodegenerative diseases.
Protocol
1. Culture Maintenance
- Culture the P19 cell line in Maintenance Medium (Dulbecco's modified Eagle's medium with 4,500 mg/L of glucose supplemented with 10% FBS, 100 units/mL penicillin and 100 units/mL streptomycin). Incubate at 37 °C and 5% CO2.
2. Sub-culturing Cells
- When cells reach approximately 80% confluence, remove the spent medium from the cell culture flasks (surface area 25 cm2).
- Wash the cells with 2 mL of phosphate buffered saline (PBS) free of calcium and magnesium.
- Add 1 mL of 0.25% trypsin-EDTA (ethylenediaminetetraacetic acid) onto the cell monolayer.
- Put the flask in the CO2 incubator (37 °C and 5% CO2) for 2-3 min.
- Assess the cell attachment to the flask surface. Ensure that all of the cells are detached and floating in the medium.
- Add 9 mL of Maintenance Medium to stop the enzymatic activity of trypsin.
- Resuspend the cells in Maintenance Medium.
- Transfer cells to a 15 mL tube and centrifuge for 5 min at 200 x g and room temperature (RT).
- Discard the supernatant and add 10 mL of fresh Maintenance Medium into the 15 mL tube.
- Use the cell suspension to determine the cell number using a cell counter according to manufacturer's instructions.
- Seed cells at 2 x 104 cells/cm2 in a new 25 cm2 flask.
- Add the Maintenance Medium up to 10 mL.
- Put the flask with cells in the CO2 incubator (37 °C and 5% CO2) for 2-3 days.
3. Trypsin Digestion
- Aspirate Maintenance Medium from the cell flask. Wash the cells once with 2 mL of calcium and magnesium-free PBS.
- Add 1 mL of 0.25% trypsin-EDTA.
- Put the flask with cells into the CO2 incubator at 37 °C for 2-3 min.
- Use 1 mL pipette to dissociate the cells by pipetting cells ten times.
- Neutralize trypsin by adding 9 mL of Differentiation Medium (Dulbecco's modified Eagle's medium with 4500 mg/L of glucose supplemented with 5% FBS, 100 units/mL penicillin and 100 units/mL streptomycin) without RA to the cells.
- Transfer cells to a 15 mL tube and centrifuge for 5 min at 200 x g and RT.
- Discard the supernatant and add 1 mL of Differentiation Medium without retinoic acid (RA). Resuspend the cell pellet.
- Use the cell suspension to determine the cell number using a cell counter according to manufacturer's instruction.
4. Aggregate Generation
- Add 5 µL of RA (1 mM stock dissolved in 99.8% ethanol, stored at -20 °C) to the 10 mL of Differentiation Medium and mix well (final concentration of 0.5 µM RA).
NOTE: RA is light sensitive. Low concentration of EtOH does not affect cell differentiation19,20,21. - Add 10 mL of Differentiation Medium (with RA) to the 100 mm non-treated culture dish (dedicated to suspension culture).
- Seed the 1 x 106 cells in the 100 mm dish (Dish surface area 56.5 cm2).
- Put the flask with cells into the incubator at 37 °C and 5% CO2 for 2 days in order to promote aggregates formation.
- After 2 days, exchange the Differentiation Medium. Aspirate medium containing aggregates using a 10 mL pipette and transfer to a 15 mL tube at RT.
- Allow the aggregates to settle by gravity for 1.5 min at RT.
- Discard the supernatant.
- Add a fresh 10 mL of Differentiation Medium with 0.5 µM RA using a 10 mL serological pipette.
CAUTION: Do not pipette the cell aggregates up and down. - Seed the aggregates into new 100 mm non-treated culture dish (dedicated to suspension culture).
- Place the plate in the incubator (at 37 °C and 5% CO2) for 2 days.
5. Aggregates Dissociation
- Aspirate the cell aggregates using a 10 mL pipette.
- Transfer the aggregates to a 15 mL tube. Allow the cell aggregates to settle by gravity for 1.5 min.
- Remove the supernatant.
- Wash the aggregates with DMEM alone (serum- and antibiotic- free).
- Allow the cell aggregates to settle by gravity sedimentation for 1.5 min at RT.
- Aspirate the supernatant and add 2 mL of trypsin-EDTA (0.25%).
- Place the cell aggregates into a water bath (37 °C) for 10 min. Agitate the aggregates gently every 2 min by tapping with a hand.
- Stop the trypsinization process by adding 4 mL of Maintenance Medium.
- Pipette aggregates up and down 20 times using 1 mL pipette.
- Centrifuge cells for 5 min at 200 x g and RT.
- Remove the supernatant and resuspend the cell pellet in 5 mL of Maintenance Medium.
- Determine the cell number with a cell counter.
6. Plating Cells
- Add 3 mL per well of Maintenance Medium to a 6-well plate.
- Seed cells in the 6-well culture plate at a density of 0.5 x 106/well.
- Incubate at 37 °C with 5% CO2 concentration.
- Seed the cells on cover glass in 6 well culture plate and perform immunostaining with anti-MAP2 antibody (20% confluence). Use 6-well plate to isolate RNA and perform RT-PCR for Map2, NeuN, Oct4, Nanog, and Gapdh (20% confluence).
Representative Results
The simplified scheme of protocol for neurogenesis induction in P19 cell line is presented in Figure 1. In order to define the character of the P19 cell line in an undifferentiated state and during neurogenesis, the RT-PCR (reverse transcription-polymerase chain reaction) method was used. The undifferentiated P19 cell line expressed the pluripotency genes such as organic cation/carnitine transporter4 (Oct4) and Nanog homeobox (Nanog). Neurogenesis induced by cells aggregation in suspension culture in the presence of RA led to a rapid decrease of Oct4 and Nanog expression. In contrary, expression of neuron markers: microtubule-associated protein 2 (Map2), NeuN (also known as RNA binding protein, fox-1 homolog 3 (Rbfox3)) increased after triggered neurogenesis (Figure 2)6,14,15,22. The primers used for each gene are indicated along with nucleotide sequencesand the size of the product in Table 1. A microscopic image of the undifferentiated P19 cell line presented a round-shaped morphology (Figure 3A). After induction of neurogenesis, the neuronal structure of the cells was clearly visible 4 days after plating (Figure 3B). Additionally, Figure 4 represents the fluorescence image of MAP2 expression in the differentiated P19 cell line (4 days after plating cells)14.
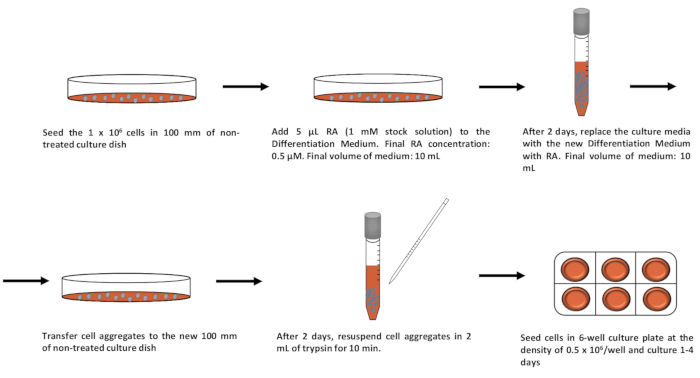
Figure 1: Protocol schematic for induction of neurogenesis in P19 embryonal carcinoma cells. Neurogenesis is induced by culturing the P19 cell line in a 100 mm non-treated culture dish with 5% of FBS and 0.5 µM RA. After 4 days, the cell aggregates are dissociated with trypsin and seeded on adherent cell culture plate for following next 4 days. Please click here to view a larger version of this figure.
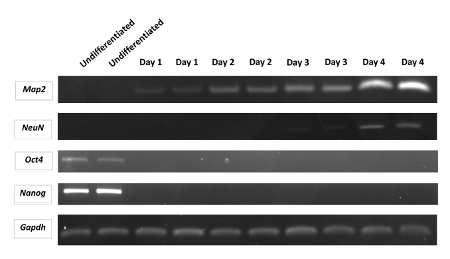
Figure 2: Changes of gene expression in P19 cell line. The band graph represents gene expression for undifferentiated P19 cell line (Oct4, Nanog) and during neurogenesis (Map2, NeuN). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as the reference gene. Samples are loaded in the agarose gel (1.5%) in double replications. Abbreviations: Undifferentiated represents the undifferentiated P19 cell line without RA treatment; Day 1-4 represents subsequent days after cell plating- following 4 days after RA treatment and cell aggregation stage. Please click here to view a larger version of this figure.
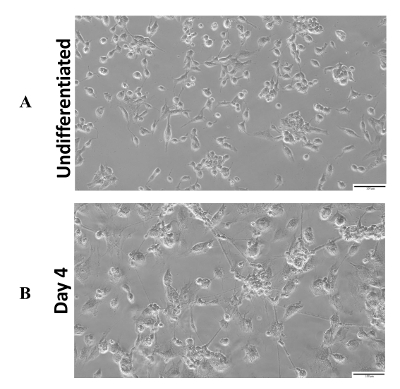
Figure 3: Representative images of analysis of P19 cell line. (A) Light microscopic images of undifferentiated P19 cell line. (B) Light microscopic images of P19 cell line after 4 days of neurogenesis- following 4 days after RA treatment and cell aggregation stage. Scale bar = 100 µm. Please click here to view a larger version of this figure.
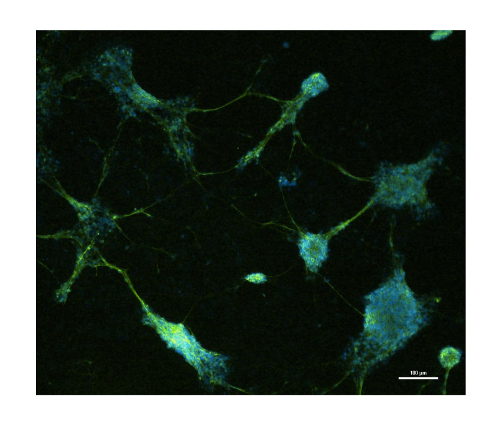
Figure 4: Representative immunofluorescence image of differentiated P19 cell line. Merged immunofluorescence image of P19 cell line stained with anti-MAP2 and DAPI at 4 days after plating. Scale bar = 100 µm. Please click here to view a larger version of this figure.
| Primer | Primer sequence | Product size (bp) |
| Gapdh | F: TGACCTCAACTACATGGTCTACA R: CTTCCCATTCTCGGCCTTG |
85 |
| Map2 | F: GCTGAGATCATCACACAGTC R: TCCTGCCAAGAGCTCATGCC |
211 |
| Oct4 | F: GGCGTTCTCTTTGGAAAGGTGTTC R: CTCGAACCACATCCTTCTCT |
313 |
| NeuN | F: GGCAAATGTTCGGGCAATTCG R: TCAATTTTCCGTCCCTCTACGAT |
160 |
| Nanog | F: AAAGGATGAAGTGCAAGCGGTGG R: CTGGCTTTGCCCTGACTTTAAGC |
520 |
Table 1: Primers used for RT-PCR.
Discussion
Here, we describe a simple protocol for neurogenesis using the P19 cell line. Although many reports have been published in this regard, a detailed methodology for neurogenesis induction using P19 cell line remains unclear. Moreover, we utilized a simple high glucose (4,500 mg/L) DMEM medium with 10% FBS for the entire experiment. This allowed us to perform the neurogenic experiment in a user-friendly manner and expand the usage of this method for the future.
The most critical points within this protocol are the RA concentration as well as the generation of cell aggregates in the suspension culture. The stimulation of neurogenesis in the P19 cell line can be carried out without the formation of aggregates, but the number of neuronal cells produced will be reduced by two-thirds in the cell culture22. Monzo et al. have shown neurogenesis induction in P19 cell line by culturing them in monolayer15. Although their method is quite convenient as we can eliminate suspension culture process, further studies are required to compare their method with other well-described methods.The RA concentration of 0.5 µM in the medium produced a high number of cell aggregates as well as neurons after plating as compared to 1 µM of RA. It is also important to note that we could not observe an efficient neurogenesis when most of the aggregates are attached to the bottom of the suspension culture dish during RA treatment. The optimal number of the P19 cell line to be used at the beginning of the procedure is 1 x 106 for every 10 mL of Differentiation Medium. During the induction of neurogenesis, the P19 cell line forms varying sized aggregates and even single cells are found in the culture. To overcome this problem, we collected the cell aggregates after 1.5 min of free fall in a 15 mL tube. We found that this approach allows the exclusion of contamination of single cells. It is also recommended to perform neuronal enrichment with the cell culture using anti-mitotic drugs (e.g., cytosine arabinoside) for long term culture to inhibit extensive proliferation of glial cells23.
Neurons derived from the P19 cell line express ionotropic glutamate receptors of both N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainite (KA) types24,25, as well as functional γ-aminobutyric acid (GABA) receptors25. Therefore, the P19 cell line is widely used in the studies on molecular mechanisms governing neuronal differentiation26,27,28. More importantly, the tumor development was not observed after cell transplantation29,30.
To this end, research on neurodegenerative diseases such as Alzheimer's disease17,18 is also conducted using P19 cell line, and we believe that the method developed in this study will thus play a part in elucidating the molecular mechanisms in neurodevelopmental abnormalities and neurodegenerative diseases.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The study was financially supported by National Science Centre, Poland (grant no. UMO-2017/25/N/NZ3/01886) and KNOW (Leading National Research Centre) Scientific Consortium "Healthy Animal – Safe Food", decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015
Materials
| 6x DNA Loading Dye | EURx | E0260-01 | |
| Agarose | Sigma- Aldrich | A9539 | |
| cDNA synthesis kit | EURx | E0801-02 | |
| DAPI (4′,6-Diamidine-2′-phenylindole dihydrochloride) | Sigma- Aldrich | 10236276001 | Working concentration: 1 μg/mL |
| DMEM high glucose (4.5 g/L) with L-glutamine | Lonza | BE12-604Q | |
| Ethanol 99.8% | Chempur | CHEM*613964202 | |
| Fetal Bovine Serum (FBS) | EURx | E5050-03 | |
| MAP2 antibody | Thermo Fisher Scientific | PA517646 | Dilution 1:100 |
| PCR reaction kit | EURx | E0411-03 | |
| Penicillin/Streptomycin 10K/10K | Lonza | DE17-602E | |
| Phosphate Buffered Saline (PBS), 1x concentrated without Ca2+, Mg2+ | Lonza | BE17- 517Q | |
| Retinoic acid | Sigma- Aldrich | R2625-50MG | dissolved in 99.8% ethanol; store in -20 °C up to 6 months |
| Secondary Antibody (Alexa Fluor 488) | Thermo Fisher Scientific | A11034 | Dilution 1:500 |
| Skim milk | Sigma- Aldrich | 1153630500 | |
| TBE Buffer | Thermo Fisher Scientific | B52 | |
| Triton-X 100 | Sigma- Aldrich | T8787-100ML | |
| Trypsin 0.25% – EDTA in HBSS, without Ca2+, Mg2+,with Phenol Red | biosera | LM-T1720/500 | |
| Cell Culture Plastics | |||
| 1 mL Serological Pipettes | Profilab | 515.01 | |
| 10 mL Serological Pipettes | Profilab | 515.10 | |
| 100 mm dish dedicated for suspension culture | Corning | C351029 | |
| 15 mL centrifuge tubes | Sigma- Aldrich | CLS430791-500EA | |
| 5 mL Serological Pipettes | Profilab | 515.05 | |
| 6-well plate | Corning | CLS3516 | |
| Cell culture flasks, surface area 25 cm2 | Sigma- Aldrich | CLS430639-200EA |
References
- Ramkumar, N., Anderson, K. V. SnapShot: mouse primitive streak. Cell. 146 (3), 488 (2011).
- Solnica-Krezel, L., Sepich, D. S. Gastrulation: making and shaping germ layers. Annual Review of Cell and Developmental Biology. 28, 687-717 (2012).
- Tam, P. P. L., Gad, J. M., Stern, C. D. Chapter 16: Gastrulation in the Mouse Embryo. Gastrulation: From Cells to Embryo. , 233-262 (2004).
- Sajini, A. A., Greder, L. V., Dutton, J. R., Slack, J. M. W. Loss of Oct4 expression during the development of murine embryoid bodies. Biologie du développement. 371 (2), 170-179 (2012).
- ten Berge, D., et al. Wnt Signaling Mediates Self-Organization and Axis Formation in Embryoid Bodies. Cell Stem Cell. 3 (5), 508-518 (2008).
- Bain, G., Ray, W. J., Yao, M., Gottlieb, D. I. From embryonal carcinoma cells to neurons: the P19 pathway. Bioessays. 16 (5), 343-348 (1994).
- Lin, Y. T., et al. YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Experimental Cell Research. 318 (15), 1877-1888 (2012).
- Neo, W. H., et al. MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. Journal of Biological Chemistry. 289 (30), 20788-20801 (2014).
- Jones-Villeneuve, E., McBurney, M. W., Rogers, K. A., Kalnins, V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. The Journal of Cell Biology. 94 (2), 253-262 (1982).
- McBurney, M. W., Rogers, B. J. Isolation of male embryonal carcinoma cells and their chromosome replication patterns. Biologie du développement. 89 (2), 503-508 (1982).
- Jones-Villeneuve, E., Rudnicki, M. A., Harris, J. F., McBurney, M. Retinoic acid-induced neural differentiation of embryonal carcinoma cells. Molecular and Cellular Biology. 3 (12), 2271-2279 (1983).
- Jasmin, D. C., Spray, A. C., Campos de Carvalho, R., Mendez-Otero, Chemical induction of cardiac differentiation in P19 embryonal carcinoma stem cells. Stem Cells and Development. 19 (3), 403-412 (2010).
- Solari, M., Paquin, J., Ducharme, P., Boily, M. P19 neuronal differentiation and retinoic acid metabolism as criteria to investigate atrazine, nitrite, and nitrate developmental toxicity. Toxicological Sciences. 113 (1), 116-126 (2010).
- Babuska, V., et al. Characterization of P19 cells during retinoic acid induced differentiation. Prague Medical Report. 111 (4), 289-299 (2010).
- Monzo, H. J., et al. A method for generating high-yield enriched neuronal cultures from P19 embryonal carcinoma cells. Journal of Neuroscience Methods. 204 (1), 87-103 (2012).
- Popova, D., Karlsson, J., Jacobsson, S. O. P. Comparison of neurons derived from mouse P19, rat PC12 and human SH-SY5Y cells in the assessment of chemical- and toxin-induced neurotoxicity. BMC Pharmacology and Toxicology. 18 (1), 42 (2017).
- Woodgate, A., MacGibbon, G., Walton, M., Dragunow, M. The toxicity of 6-hydroxydopamine on PC12 and P19 cells. Molecular Brain Research. 69 (1), 84-92 (1999).
- Tsukane, M., Yamauchi, T. Ca2+/calmodulin-dependent protein kinase II mediates apoptosis of P19 cells expressing human tau during neural differentiation with retinoic acid treatment. Journal of Enzyme Inhibition and Medicinal Chemistry. 24 (2), 365-371 (2009).
- Adler, S., Pellizzer, C., Paparella, M., Hartung, T., Bremer, S. The effects of solvents on embryonic stem cell differentiation. Toxicology in Vitro. 20 (3), 265-271 (2006).
- Jones-Villeneuve, E. M., McBurney, M. W., Rogers, K. A., Kalnins, V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. The Journal of Cell Biology. 94 (2), 253-262 (1982).
- Roy, B., Taneja, R., Chambon, P. Synergistic activation of retinoic acid (RA)-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha (RAR alpha)-, RAR beta-, or RAR gamma-selective ligand in combination with a retinoid X receptor-specific ligand. Molecular and Cellular Biology. 15 (12), 6481-6487 (1995).
- Hamada-Kanazawa, M., et al. Sox6 overexpression causes cellular aggregation and the neuronal differentiation of P19 embryonic carcinoma cells in the absence of retinoic acid. FEBS Letters. 560 (1-3), 192-198 (2004).
- Tangsaengvit, N., Kitphati, W., Tadtong, S., Bunyapraphatsara, N., Nukoolkarn, V. Neurite Outgrowth and Neuroprotective Effects of Quercetin from Caesalpinia mimosoides Lamk on Cultured P19-Derived Neurons. Evidence-Based Complementary and Alternative. , 838051 (2013).
- Magnuson, D. S., Morassutti, D. J., McBurney, M. W., Marshall, K. C. Neurons derived from P19 embryonal carcinoma cells develop responses to excitatory and inhibitory neurotransmitters. Developmental Brain Research. 90 (1-2), 141-150 (1995).
- MacPherson, P., Jones, S., Pawson, P., Marshall, K., McBurney, M. P19 cells differentiate into glutamatergic and glutamate-responsive neurons in vitro. Neurosciences. 80 (2), 487-499 (1997).
- Hong, S., et al. Methyltransferase-inhibition interferes with neuronal differentiation of P19 embryonal carcinoma cells. Biochemical and Biophysical Research Communications. 377 (3), 935-940 (2008).
- Wenzel, M., et al. Identification of a classic nuclear localization signal at the N terminus that regulates the subcellular localization of Rbfox2 isoforms during differentiation of NMuMG and P19 cells. FEBS Letters. 590 (24), 4453-4460 (2016).
- Harada, Y., et al. Overexpression of Cathepsin E Interferes with Neuronal Differentiation of P19 Embryonal Teratocarcinoma Cells by Degradation of N-cadherin. Cellular and Molecular Neurobiology. 37 (3), 437-443 (2017).
- Morassutti, D. J., Staines, W. A., Magnuson, D. S., Marshall, K. C., McBurney, M. W. Murine embryonal carcinoma-derived neurons survive and mature following transplantation into adult rat striatum. Neurosciences. 58 (4), 753-763 (1994).
- Magnuson, D. S., Morassutti, D. J., Staines, W. A., McBurney, M. W., Marshall, K. C. In vivo electrophysiological maturation of neurons derived from a multipotent precursor (embryonal carcinoma) cell line. Developmental Brain Research. 84 (1), 130-141 (1995).

