Genetic Incorporation of Biosynthesized L-dihydroxyphenylalanine (DOPA) and Its Application to Protein Conjugation
Summary
Here, we present a protocol for the genetic incorporation of L-dihydroxyphenylalanine biosynthesized from simple starting materials and its application to protein conjugation.
Abstract
L-dihydroxyphenylalanine (DOPA) is an amino acid found in the biosynthesis of catecholamines in animals and plants. Because of its particular biochemical properties, the amino acid has multiple uses in biochemical applications. This report describes a protocol for the genetic incorporation of biosynthesized DOPA and its application to protein conjugation. DOPA is biosynthesized by a tyrosine phenol-lyase (TPL) from catechol, pyruvate, and ammonia, and the amino acid is directly incorporated into proteins by the genetic incorporation method using an evolved aminoacyl-tRNA and aminoacyl-tRNA synthetase pair. This direct incorporation system efficiently incorporates DOPA with little incorporation of other natural amino acids and with better protein yield than the previous genetic incorporation system for DOPA. Protein conjugation with DOPA-containing proteins is efficient and site-specific and shows its usefulness for various applications. This protocol provides protein scientists with detailed procedures for the efficient biosynthesis of mutant proteins containing DOPA at desired sites and their conjugation for industrial and pharmaceutical applications.
Introduction
DOPA is an amino acid involved in the biosynthesis of catecholamines in animals and plants. This amino acid is synthesized from Tyr by tyrosine hydroxylase and molecular oxygen (O2)1. Because DOPA is a precursor of dopamine and can permeate the blood-brain barrier, it has been used in the treatment of Parkinson's disease2. DOPA is also found in mussel adhesion proteins (MAPs), which are responsible for the adhesive properties of mussels in wet conditions3,4,5,6,7. Tyr is initially encoded at the positions where DOPA is found in MAPs and is then converted into DOPA by tyrosinases8,9. Because of its interesting biochemical properties, DOPA has been used in a variety of applications. The dihydroxyl group of DOPA is chemically prone to oxidation, and the amino acid is easily converted into L-dopaquinone, a precursor of melanins. Owing to its high electrophilicity, L-dopaquinone and its derivatives have been used for crosslinking and conjugation with thiols and amines10,11,12,13. 1,2-Quinones can also function as a diene for cycloaddition reactions and have been used for bioconjugation by strain-promoted oxidation-controlled cyclooctyne-1,2-quinone (SPOCQ) cycloaddition14. In addition, the dihydroxyl group can chelate metal ions such as Fe3+ and Cu2+, and proteins containing DOPA have been utilized for drug delivery and metal ion sensing15,16.
DOPA has been genetically incorporated into proteins by using an orthogonal aminoacyl-tRNA (aa-tRNA) and aminoacyl-tRNA synthetase (aaRS) pair17 and used for protein conjugation and crosslinking10,11,12,13. In this report, experimental results and protocols for the genetic incorporation of DOPA biosynthesized from cheap starting materials and its applications to bioconjugation are described. DOPA is biosynthesized using a TPL and starting from catechol, pyruvate, and ammonia in Escherichia coli. The biosynthesized DOPA is directly incorporated into proteins by expressing an evolved aa-tRNA and aaRS pair for DOPA. In addition, the biosynthesized protein containing DOPA is site-specifically conjugated with a fluorescent probe and crosslinked to produce protein oligomers. This protocol will be useful for protein scientists, to biosynthesize mutant proteins containing DOPA and conjugate the proteins with biochemical probes or drugs for industrial and pharmaceutical applications.
Protocol
1. Plasmid Construction
- Construct an expression plasmid (pBAD-dual-TPL-GFP-WT) that expresses the TPL gene from Citrobacter freundii under the control of a constitutive promoter and the green fluorescent protein (GFP) gene with a His6-tag under the control of the araBAD promoter. For pBAD-dual-TPL-GFP-E90TAG, replace the codon for the site (E90) of DOPA with an amber codon (TAG), using a site-directed mutagenesis protocol. The details for the construction of this plasmid was described in our previous report18.
- Construct a tRNA/aaRS-expressing plasmid (pEVOL-DHPRS2)18,19 for the genetic incorporation of DOPA. pEVOL is a special plasmid vector expressing two copies of aaRS genes with independent promoters, and the detailed information of the plasmid is described in a previous report19.
- Use commercially available plasmid preparation kits to obtain high purity plasmid DNAs. Check the purity of the prepped DNAs by using agarose gel electrophoresis, if necessary.
2. Culture Preparation
- Electroporation
- Use the electro-competent E. coli DH10β strain for this experiment. Add 1 µL of each plasmid DNA (pEVOL-DHPRS2 and pBAD-dual-TPL-GFP-E90TAG) to 20 µL of competent cells. Mix them gently to make a homogeneous mixture, using a pipette.
- Transfer the mixture to the electroporation cuvettes. Place the cuvette in the electroporation chamber. Push the cuvette into the chamber to make firm cuvette-chamber contact.
- Shock the cuvette, using 25 µF and 2.5 kV for 0.1-cm cuvettes.
- Add 1 mL of prewarmed super optimal broth (SOC), containing 2% (w/v) tryptone, 0.5% (w/v) yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, and 20 mM glucose, to the cuvette, and transfer the cells to a culture tube. Rescue the cells by incubating them for 1 h at 37 °C with shaking.
- Spread 10 – 100 µL of the rescued cells on a lysogeny broth (LB) agar plate containing 35 µg/mL chloramphenicol and 100 µg/mL ampicillin. Incubate the cells at 37 °C for 16 h.
- Starter culture preparation
- Inoculate a single transformed colony in 5 mL of LB medium containing antibiotics. Incubate the colony for 12 h at 37 °C with shaking.
3. Expression and Purification of GFP-E90DOPA by a Biosynthetic System
- Expression of GFP-E90DOPA by a biosynthetic system
- Transfer the starter culture (2 mL) to 100 mL of PA-5052 medium20 (50 mM Na2HPO4, 50 mM KH2PO4, 25 mM [NH4]2SO4, 2 mM MgSO4, 0.1% [w/v] trace metals, 0.5% [w/v] glycerol, 0.05% [w/v] glucose, and 5% [w/v] amino acids [pH 7.25]) containing 35 µg/mL chloramphenicol, 100 µg/mL ampicillin, 100 mM pyruvate, 10 mM catechol, 25 mM ammonia, and 300 µM dithiothreitol (DTT) followed by an incubation at 30 °C with shaking.
- Add 2 mL of 0.2% (w/v) L-arabinose when the optical density (OD) at 550 nm reaches 0.8. Incubate the sample for 12 h at 30 °C with shaking.
- Spin down the cells at 11,000 x g for 5 min, discard the supernatant, and freeze the cell pellet at -80 °C.
- Cell lysis
- Thaw the cell pellet on ice and resuspend the cells in 10 mL of lysis buffer containing 50 mM NaH2PO4 (pH 8.0), 10 mM imidazole, and 300 mM NaCl.
- Sonicate the resuspended cells on ice for 10 min. Use 80% sonication amplitude with a pulse of 25 s on and 35 s off.
- Spin down the cell lysate at 18,000 x g at 4 °C for 15 min. Transfer the supernatant to a fresh tube for purification and discard the pellet.
- Ni-NTA affinity chromatography
- Add Ni-NTA (nitrilotriacetic acid) resin (300 µL of resin suspension for a 100-mL culture) to the supernatant and bind the proteins to the Ni-NTA resin at 4 °C by gently shaking the suspension for 1 h.
- Transfer the suspension to a polypropylene column and wash the resin 3x with 5 mL of wash buffer containing 50 mM NaH2PO4 (pH 8.0), 20 mM imidazole, and 300 mM NaCl.
- Elute the proteins 3x with 300 µL of elution buffer containing 50 mM NaH2PO4 (pH 8.0), 250 mM imidazole, and 300 mM NaCl.
- Determine the protein concentration by measuring the absorbance at 280 nm. The extinction coefficient (24,630 M-1cm-1) for GFP-E90DOPA at 280 nm was calculated by a protein extinction coefficient calculator (e.g., https://web.expasy.org/protparam/) and the independently measured extinction coefficiennt (2630 M-1cm-1) for DOPA. As an alternative method, use the Bradford protein assay21 for a determination of the protein concentration.
4. Oligomerization of Purified GFP-E90DOPA
- Add 1 µL of sodium periodate in H2O (6 mM) (1.0 equiv. to GFP-E90DOPA) to a solution of 20 µL of GFP-E90DOPA (300 µM) in a phosphate buffer containing 50 mM Na2HPO4 (pH 8.0) and 300 mM NaCl.
- Allow the oligomerization reaction to proceed at 25 °C for 48 h.
- Analyze the reaction mixture by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) (SDS-PAGE) as described in step 6.
5. Conjugation of GFP-E90DOPA with an Alkyne Probe by SPOCQ
- Add 1 µL of the Cy5.5-linked azadibenzocyclooctyne (Cy5.5-ADIBO)22 in H2O (4.0 mM) (10 equiv. to GFP-E90DOPA) to a solution of 20 µL of GFP-E90DOPA (20 µM) in a phosphate buffer containing 50 mM Na2HPO4 (pH 8.0) and 300 mM NaCl.
- Add 1 µL of sodium periodate in H2O (400 µM) (1.0 equiv. to GFP-E90DOPA) to the reaction mixture.
- Allow the SPOCQ reaction to proceed at 25 °C for 1 h. If a light-sensitive probe is used, cover the reaction vessel with aluminum foil.
6. Purification of the Labeled GFP
- Dilute the SPOCQ reaction mixture by adding a phosphate buffer, containing 50 mM Na2HPO4 (pH 8.0) and 300 mM NaCl, up to 500 µL.
- Transfer the diluted solution to a centrifugal filter spin column. Centrifuge the spin column at 14,000 x g at 4 °C for 15 min and discard the flow-through. Repeat this step 3x in order to remove any excess Cy5.5-ADIBO.
- Transfer the purified sample from the spin column to a 1.5-mL microcentrifuge tube.
- Alternatively, carry out a purification by dialysis against the same buffer.
- Store the purified GFP at 4 °C.
7. SDS-PAGE Analysis and Fluorescence Gel Scanning
- Prepare protein samples for SDS-PAGE analysis by adding 5 µL of protein sample buffer (see Table of Materials) and 2 µL of DTT (1.00 M) to 13 µL of purified, or crude, labeled GFP (13.6 µM or 0.38 mg/mL). For an analysis of oligomeric GFP, use a higher concentration of GFP (67.8 µM or 1.9 mg/mL). Denature the proteins by incubating them at 95 °C for 10 min.
- Place a 4% – 12% Bis-Tris SDS-PAGE gel cassette (purchased, premade gel cassettes) to the electrophoresis cell and add a running buffer (see Table of Materials). Load the protein samples and a molecular weight marker. Run the electrophoresis for 35 – 40 min at 200 V.
NOTE: Avoid any exposure of the protein samples to light by carrying out all processes in the dark, to minimize photo-bleaching of the fluorescent probe. - Use a fluorescence scanner for fluorescence imaging. Wrap a protein gel from electrophoresis and place it on a fluorescence scanner. Scan the gel by using an appropriate wavelength setting. For Cy5.5, select the Cy5 mode provided in the scanner software.
- Stain the gel with a commercial protein staining reagent.
8. MALDI-TOF MS Analysis by Trypsin Digestion
- Incubate 100 µL of purified GFP-E90DOPA (2.2 mg/mL or 80 µM) in a buffer containing 50 mM Tris-HCl (pH 8.0), 0.1% (w/v) SDS, and 25 mM DTT at 60 °C for 1 h.
- Add 1 µL of Iodoacetamide in H2O (IAA, 4.0 M) (500 equiv. to GFP-E90DOPA) to the purified GFP-E90DOPA solution (80 µM) in the same buffer. Incubate the mixture at 25 °C for 30 min with protection from light.
- Digest the GFP-E90DOPA sample by adding trypsin (1:100 protease-to-substrate-protein ratio [w/w]) and incubate the reaction mixture at 37 °C for 4 h.
- Purify the digested peptide sample by using a standard desalting method with C18 spin columns.
- Analyze the digested peptides by the reported protocol for matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using alpha-cyano-4-hydroxycinnamic acid (CHCA) as a matrix23.
Representative Results
The expression system for the direct incorporation of DOPA biosynthesized from a TPL is shown in Figure 1. The genes for the evolved aa-tRNA and aaRS pair are placed in a plasmid, and the GFP gene (GFP-E90TAG) containing an amber codon at position 90 is located in another plasmid to evaluate the incorporation of DOPA by GFP fluorescence. The TPL gene is placed in the same expression plasmid containing the GFP gene and constitutively expressed to maximize the yield of the DOPA biosynthesis. Using this expression system, optimal conditions for DOPA biosynthesis were screened. The growth media used for this experiment contained 25 mM ammonia and 100 mM pyruvate, with varying concentrations of catechol (0 – 10 mM). The catechol concentration was critical for the DOPA biosynthesis, while ammonia and pyruvate concentrations over 25 mM and 100 mM, respectively, did not affect the biosynthesis and incorporation of DOPA. The optimal concentration of catechol was 10 mM, and a concentration of more than 10 mM significantly decreased the bacterial cell growth due to its cytotoxicity. In this optimal condition, DOPA was biosynthesized by TPL from its starting materials, and the biosynthesized DOPA was directly incorporated into GFP. The expressed mutant GFP (GFP-E90DOPA) was purified and analyzed by SDS-PAGE (Figure 2A). The full-length GFP was purified, and the DOPA incorporation was confirmed by MALDI-TOF MS analysis (Figure 2B). The protein was digested with trypsin, the peptide fragment containing DOPA was analyzed, and the result showed the exclusive incorporation of DOPA with no detectable Tyr or other natural amino acid incorporation. The incorporation efficiency of DOPA biosynthesized by TPL was similar to the incorporation efficiency with 3 mM DOPA, which is the maximum concentration of DOPA because of its cytotoxicity19. Although 10 mM catechol showed moderate toxicity, the cell density after 16 h of culture time was three- to fourfold higher than in the presence of 3 mM DOPA. The purified protein yield by biosynthesis was threefold higher than that from the experiment using 3 mM DOPA (Figure 3).
The mutant GFP containing DOPA was used for protein conjugation. DOPA can be oxidized into L-dopaquinone by mild oxidants, and the electrophilic quinone reacts with strained alkynes and nucleophiles, such as thiols and amines, and can be used for bioconjugation (Figure 4). GFP-E90DOPA was tested for SPOCQ cycloaddition by reacting with sodium periodate, followed by a treatment with Cy5.5-ADIBO22. This SPOCQ reaction was analyzed by SDS-PAGE and fluorescence imaging (Figure 5A). The intense fluorescence band was observed with a treatment of sodium periodate and Cy5.5-ADIBO, while no fluorescence band was shown in control reactions with GFP-WT or without a sodium periodate treatment, confirming the efficiency and specificity of the conjugation reaction with the genetically incorporated DOPA. In addition, the DOPA-containing GFP was used for protein oligomerization. L-dopaquinone reacts with Lys or Cys in the same protein, resulting in oligomeric proteins. GFP-E90DOPA was treated with sodium periodate and the oligomerization was carried out for 48 h, and the reaction was analyzed by SDS-PAGE in comparison with a control sample without a sodium periodate treatment (Figure 5B). The analysis showed a clear oligomerization pattern with equally spaced protein bands for the periodate-treated sample, while the control sample showed the intact protein without any other bands.
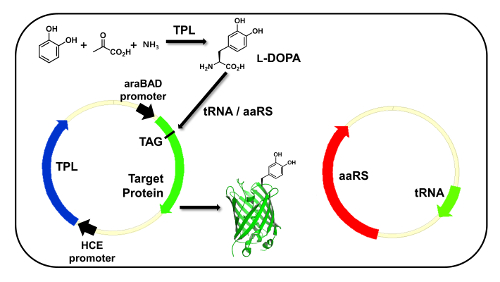
Figure 1: Direct incorporation of DOPA biosynthesized from catechol, pyruvate, and ammonia. DOPA is biosynthesized by heterologously expressed TPL from catechol, pyruvate, and ammonia in growth medium. The evolved tRNA/aaRS pair for DOPA is co-expressed to incorporate the biosynthesized DOPA into the target protein, GFP. The TPL gene was placed under a constitutive promoter in order to start the DOPA biosynthesis before inducing the GFP expression. Please click here to view a larger version of this figure.
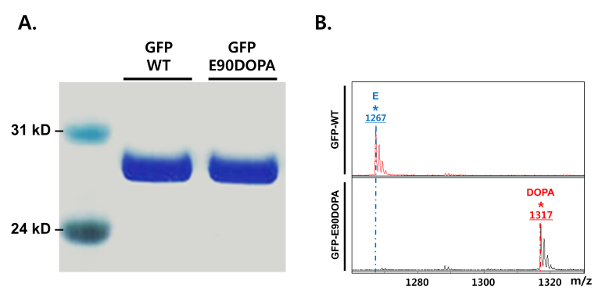
Figure 2: SDS-PAGE and MALDI-TOF MS analyses of GFP-E90DOPA expressed by the designed biosynthetic system. (A) GFP-E90DOPA was expressed by the designed biosynthetic system in the presence of 10 mM catechol, 100 mM pyruvate, and 25 mM ammonia, and then purified by Ni-NTA affinity chromatography. GFP-WT was also analyzed for comparison. The samples were separated by Bis-Tris 4% – 12% SDS-PAGE, and the gel was stained with Coomassie Brilliant Blue R-250. (B) MALDI-TOF MS analysis of the peptide fragment containing DOPA from the tryptic digestion of GFP-E90DOPA. The peptide fragment from GFP-WT contains the residues 86 – 96 (SAMPEGYVQER). The Glu in the fragment from GFP-WT is replaced with DOPA in the peptide fragment from GFP-E90DOPA. Please click here to view a larger version of this figure.
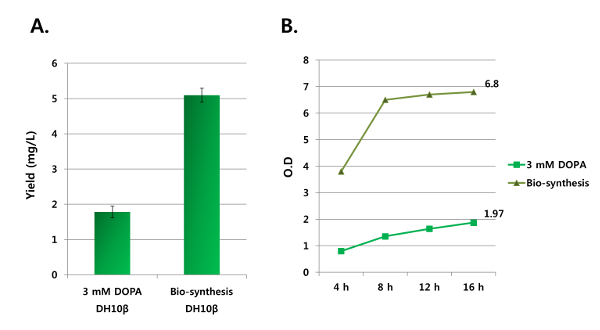
Figure 3: Comparison of a direct incorporation of biosynthesized DOPA with a general genetic incorporation of DOPA. These panels show GFP-E90DOPA expressed by the designed biosynthetic system and the genetic incorporation in the presence of 3 mM DOPA without additional pyruvate, ammonia, and catechol. (A) The protein yields were obtained from purified GFP-E90DOPA, and (B) the cell densities were measured at 16 h after the induction. Please click here to view a larger version of this figure.
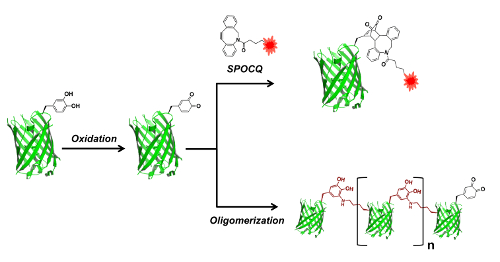
Figure 4: Reaction scheme of the bioconjugation of GFP-E90DOPA. GFP-E90DOPA is oxidized to convert DOPA into dopaquinone. The reaction of the dopaquinone-containing protein with a strained alkyne achieves site-specific protein labeling. The incubation of the dopaquinone-containing protein at a high concentration for a long time (48 h) causes protein self-conjugation to produce protein oligomers. Please click here to view a larger version of this figure.
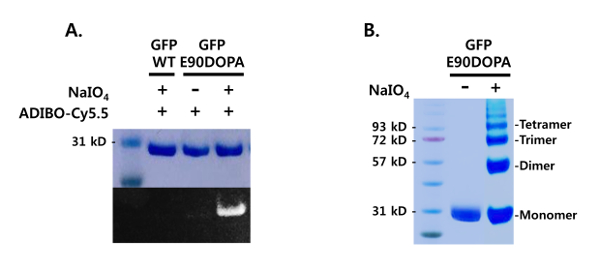
Figure 5: Bioconjugation of mutant proteins containing DOPA. (A) SPOCQ reactions of a DOPA-containing protein with a strained alkyne, ADIBO-Cy5.5. GFP-WT and GFP-E90DOPA were treated with sodium periodate and reacted with ADIBO-Cy5.5 for 60 min. The reactions were analyzed by SDS-PAGE, and Coomassie-stained (top) and fluorescence (bottom) images are shown. (B) Oligomerization of GFP-E90DOPA. The DOPA-containing GFP was treated with sodium periodate and incubated for 48 h. The reaction mixture was analyzed by SDS-PAGE. Please click here to view a larger version of this figure.
Discussion
In this protocol, the biosynthesis and direct incorporation of DOPA are described. The bacterial cell used in this method can synthesize an additional amino acid and use it as an unnatural building block for protein synthesis. The genetic incorporation of unnatural amino acids has been a key technology for the development of unnatural organism with an expanded genetic code. However, this method has been technically incomplete and is being modified to improve incorporation efficiency and minimize perturbation to endogenous translation systems. Recently, significant advances in the method have been achieved by reassigning codons24,25 for unnatural amino acids and engineering orthogonal translational components26,27,28,29. In addition to these improvements, the system described here offers another capability to an unnatural organism with an expanded genetic code: biosynthesis of an unnatural amino acid. This capability gives the unnatural system more independence as an organism with an expanded genetic repertoire. Furthermore, DOPA shows moderate toxicity on the bacterial strain used in this method, and at 3 mM DOPA, cell growth significantly reduced, which resulted in low protein yield. By using the direct incorporation system, toxicity was reduced and protein yield increased threefold.
The aaRS mutant used for the genetic incorporation of DOPA has moderate efficiency and fidelity and incorporates Tyr at a low concentration of DOPA.18 Therefore, the biosynthesis of DOPA in bacterial cells should be efficient enough to prevent Tyr incorporation. The DOPA biosynthesis by TPL from catechol, ammonia, and pyruvate is a reversible reaction, and high concentrations of the starting materials are required for an efficient biosynthesis. However, catechol cannot be used over 10 mM because it is toxic to the bacterial strain used in this protocol. The DTT concentration is also important in this protocol. DOPA shows its toxicity when it is converted into L-dopaquinone, and DTT is added in growth medium to reduce the oxidation rate. We observed a decrease in cell growth when the DTT concentration was over 300 µM. Therefore, for an optimal biosynthesis and incorporation of DOPA, the components (especially catechol and DTT) for culture medium should be used in appropriate concentrations as described in this protocol.
This protocol also describes applications of DOPA-containing proteins for site-specific protein conjugation. L-dopaquinone generated by the oxidation of DOPA efficiently reacts with a strained alkyne linked to a biochemical probe. This cycloaddition reaction is usually faster than nucleophilic addition reactions by amines or thiols. Nevertheless, the oxidation of DOPA should be carried out in the presence of both a DOPA-containing protein and a strained alkyne, to minimize the nucleophilic addition by nucleophiles in the protein. Therefore, the order of the addition of the reagents for this reaction is critical.
Many unnatural amino acids are available for protein conjugation, and some of them achieve a fast reaction rate and excellent conjugation efficiency30,31,32,33. These amino acids contain biorthogonal functional groups such as azides, alkynes, strained alkenes or alkynes, and tetrazines. Although they are useful for site-specific protein conjugation, many of them are expensive or commercially inaccessible, which limits their applications, especially in those requiring large-scale proteins. Therefore, the biosynthesis of DOPA from cheap starting materials and its direct incorporation will be useful for pharmaceutical and industrial applications requiring large-scale mutant proteins containing a biochemical probe or a drug (antibody-drug conjugate, ADC).
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Global Frontier Research Program (NRF-2015M3A6A8065833), and the Basic Science Research Program (2018R1A6A1A03024940) through the National Research Foundation of Korea (NRF) funded by the Korea government.
Materials
| 1. Plasmid Construction | |||
| Plasmid pBAD-dual-TPL-GFP-E90TAG | optionally contain the amber stop codon(TAG) at a desired position. Ko, W. et al. Efficient and Site-Specific Antibody Labeling by Strain-promoted Azide-Alkyne Cycloaddition. BKCS. 36 (9), 2352-2354, doi: 10.1002/bkcs.10423, (2015) | ||
| Plasmid pEvol-DHPRS2 | 1. Young, T. S., Ahmad, I., Yin, J. A., and Schultz, P. G. An enhanced system for unnatural amino acid mutagenesis in E. coli. J. Mol. Biol. 395 (2), 361-374, doi: 10.1016/j.jmb.2009.10.030, (2010) 2. Kim, S., Sung, B. H., Kim, S. C., Lee, H. S. Genetic incorporation of l-dihydroxyphenylalanine (DOPA) biosynthesized by a tyrosine phenol-lyase. Chem. Commun. 54 (24), 3002-3005, doi: 10.1039/c8cc00281a (2018). | ||
| DH10β | Invitrogen | C6400-03 | Expression Host |
| Plasmid Mini-prep kit | Nucleogen | 5112 | 200/pack |
| Agarose | Intron biotechnology | 32034 | 500 g |
| Ethidium bromide | Alfa Aesar | L07482 | 1 g |
| LB Broth | BD Difco | 244620 | 500 g |
| 2. Culture preparation | |||
| 2.1) Electroporation | |||
| Micro pulser | BIO-RAD | 165-2100 | |
| Micro pulser cuvette | BIO-RAD | 165-2089 | 0.1 cm electrode gap, pkg. of 50 |
| Ampicillin Sodium | Wako | 018-10372 | 25 g |
| Chloramphenicol | Alfa Aesar | B20841 | 25 g |
| Agar | SAMCHUN | 214230 | 500 g |
| SOC medium | Sigma | S1797 | 100 mL |
| 3. Expression and Purification of GFP-E90DOPA by biosynthetic system | |||
| 3.1 Expression of GFP-E90DOPA by biosynthetic system | |||
| L(+)-Arabinose, 99% | Acros | 104981000 | 100 g |
| Pyrocatechol, 99% | SAMCHUN | P1387 | 25 g |
| Ammonium sulfate, 99% | SAMCHUN | A0943 | 500 g |
| pyruvic acid, 98% | Alfa Aesar | A13875 | 100 g |
| Sodium phosphate dibasic, anhydrous, 99% | SAMCHUN | S0891 | 1 kg |
| Potassium phophate, monobasic, 99% | SAMCHUN | P1127 | 1 kg |
| Magnesium sulfate, anhydrous, 99% | SAMCHUN | M0146 | 1 kg |
| D(+)-Glucose, anhydrous, 99% | SAMCHUN | D0092 | 500 g |
| Glycerol, 99% | SAMCHUN | G0269 | 1 kg |
| Trace metal mix a5 with co | Sigma | 92949 | 25 mL |
| L-Proline, 99% | SAMCHUN | P1257 | 25 g |
| L-Phenylalanine, 98.5% | SAMCHUN | P1982 | 25 g |
| L-Tryptophane | JUNSEI | 49550-0310 | 25 g |
| L-Arginine, 98% | SAMCHUN | A1149 | 25 g |
| L-Glutamine, 98% | JUNSEI | 27340-0310 | 25 g |
| L-Asparagine monohydrate, 99% | SAMCHUN | A1198 | 25 g |
| L-Methionine | JUNSEI | 73190-0410 | 25 g |
| L-Histidine hydrochloride monohydrate, 99% | SAMCHUN | H0604 | 25 g |
| L-Threonine, 99% | SAMCHUN | T2938 | 25 g |
| L-Leucine | JUNSEI | 87070-0310 | 25 g |
| Glycine, 99% | SAMCHUN | G0286 | 25 g |
| L-Glutamic acid, 99% | SAMCHUN | G0233 | 25 g |
| L-Alanine, 99% | SAMCHUN | A1543 | 25 g |
| L-Isoleucine, 99% | SAMCHUN | I1049 | 25 g |
| L-Valine, 99% | SAMCHUN | V0088 | 25 g |
| L-Serine | SAMCHUN | S2447 | 25 g |
| L-Aspartic acid | SAMCHUN | A1205 | 25 g |
| L-Lysine monohydrochloride, 99% | SAMCHUN | L0592 | 25 g |
| 3.2 Cell lysis | |||
| Imidazole, 99% | SAMCHUN | I0578 | 1kg |
| Sodium phosphate monobasic, 98% | SAMCHUN | S0919 | 1 kg |
| Sodium Chloride, 99% | SAMCHUN | S2907 | 1 kg |
| Ultrasonic Processor – 150 microliters to 150 milliliters | SONIC & MATERIALS | VCX130 | |
| 3.3 Ni-NTA Affinity Chromatography | |||
| Ni-NTA resin | QIAGEN | 30210 | 25 mL |
| Polypropylene column | QIAGEN | 34924 | 50/pack, 1 mL capacity |
| 4. Oligomerization of Purified GFP-E90DOPA | |||
| Sodium periodate, 99.8& | Acros | 419610050 | 5 g |
| 5. Conjugation of GFP-E90DOPA with an Alkyne Probe by Strain-Promoted Oxidation-Controlled Cyclooctyne–1,2-Quinone Cycloaddition (SPOCQ) | |||
| Cy5.5-ADIBO | FutureChem | FC-6119 | 1mg |
| 6. Purification of Labeled GFP | |||
| Amicon Ultra 0.5 mL Centrifugal Filters | MILLIPORE | UFC500396 | 96/pack, 500ul capacity |
| 7. SDS-PAGE Analysis and Fluorescence Gel Scanning | |||
| 1,4-Dithio-DL-threitol, DTT, 99.5 % | Sigma | 10708984001 | 10 g |
| NuPAGE LDS Sample Buffer, 4X | Thermofisher | NP0007 | 10 mL |
| MES running buffer | Thermofisher | NP0002 | 500 mL |
| Nupage Novex 4-12% SDS PAGE gels | Thermofisher | NO0321 | 12 well |
| Coomassie Brilliant Blue R-250 | Wako | 031-17922 | 25 g |
| G:BOX Chemi Fluorescent & Chemiluminescent Imaging System | Syngene | G BOX Chemi XT4 | |
| 8. MALDI-TOF MS analysis by Trypsin Digestion | |||
| 8.1 Preparation of the digested peptide sample by trypsin digestion | |||
| Tris(hydroxymethyl)aminomethane, 99% | SAMCHUN | T1351 | 500 g |
| Hydrochloric acid, 35~37% | SAMCHUN | H0256 | 500 mL |
| Dodecyl sulfate sodium salt, 85% | SAMCHUN | D1070 | 250 g |
| Iodoacetamide | Sigma | I6125 | 5 g |
| Trypsin Protease, MS Grade | Thermofisher | 90057 | 5 x 20 µg/pack |
| C-18 spin columns | Thermofisher | 89870 | 25/pack, 200 µL capacity |
| 8.2 Analysis of the digested peptide by MALDI-TOF | |||
| Acetonitirile, 99.5% | SAMCHUN | A0125 | 500 mL |
| α-Cyano-4-hydroxycinnamic acid | Sigma | C2020 | 10 g |
| Trifluoroacetic acid, 99% | SAMCHUN | T1666 | 100 g |
| MTP 384 target plate ground steel BC targets | Bruker | 8280784 | |
| Bruker Autoflex Speed MALDI-TOF mass spectrometer | Bruker |
References
- Nagatsu, T., Levitt, M., Udenfriend, S. Tyrosine hydroxylase: The initial step in norepinephrine biosynthesis. Journal of Biological Chemistry. 239, 2910-2917 (1964).
- Pinder, R. M. Possible dopamine derivatives capable of crossing the blood-brain barrier in relation to parkinsonism. Nature. 228 (5269), 358 (1970).
- Waite, J. H., Tanzer, M. L. Polyphenolic substance of mytilus edulis: novel adhesive containing L-DOPA and hydroxyproline. Science. 212 (4498), 1038-1040 (1981).
- Lee, H., Scherer, N. F., Messersmith, P. B. Single-molecule mechanics of mussel adhesion. Proceedings of the National Academy of Sciences of the United States of America. 103 (35), 12999-13003 (2006).
- Papov, V. V., Diamond, T. V., Biemann, K., Waite, J. H. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis. Journal of Biological Chemistry. 270 (34), 20183-20192 (1995).
- Waite, J. H., Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochimie. 40 (9), 2887-2893 (2001).
- Nicklisch, S. C., Waite, J. H. Mini-review: The role of redox in DOPA-mediated marine adhesion. Biofouling. 28 (8), 865-877 (2012).
- Silverman, H. G., Roberto, F. Understanding marine mussel adhesion. Marine Biotechnology. 9 (6), 661-681 (2007).
- Lee, B. P., Messersmith, P. B., Israelachvili, J. N., Waite, J. H. Mussel-inspired adhesives and coatings. Annual Review of Materials Research. 41, 99-132 (2011).
- Umeda, A., Thibodeux, G. N., Zhu, J., Lee, Y., Zhang, Z. J. Site-specific protein cross-linking with genetically incorporated 3,4-dihydroxy-L-phenylalanine. ChemBioChem. 10 (8), 1302-1304 (2009).
- Umeda, A., Thibodeux, G. N., Moncivais, K., Jiang, F., Zhang, Z. J. A versatile approach to transform low-affinity peptides into protein probes with cotranslationally expressed chemical cross-linker. Analytical Biochemistry. 405 (1), 82-88 (2010).
- Xu, J., Tack, D., Hughes, R. A., Ellington, A. D., Gray, J. J. Structure-based non-canonical amino acid design to covalently crosslink an antibody-antigen complex. Journal of Structural Biology. 185 (2), 215-222 (2014).
- Burdine, L., Gillette, T. G., Lin, H. J., Kodadek, T. Periodate-triggered cross-linking of DOPA-containing peptide-protein complexes. Journal of the American Chemical Society. 126 (37), 11442-11443 (2004).
- Borrmann, E., et al. Strain-promoted oxidation-controlled cyclooctyne-1,2-quinone cycloaddition (SPOCQ) for fast and activatable protein conjugation. Bioconjugate Chemistry. 26 (2), 257-261 (2015).
- Ayyadurai, N., et al. Development of a selective, sensitive, and reversible biosensor by the genetic incorporation of a metal-binding site into green fluorescent protein. Angewandte Chemie International Edition. 50 (29), 6534-6537 (2011).
- Kim, B. J., Cheong, H., Hwang, B. H., Cha, H. J. Mussel-inspired protein nanoparticles containing iron(III)-DOPA complexes for pH-responsive drug delivery. Angewandte Chemie International Edition. 54 (25), 7318-7322 (2015).
- Alfonta, L., Zhang, Z., Uryu, S., Loo, J. A., Schultz, P. G. Site-specific incorporation of a redox-active amino acid into proteins. Journal of the American Chemical Society. 125 (48), 14662-14663 (2003).
- Kim, S., Sung, B. H., Kim, S. C., Lee, H. S. Genetic incorporation of L-dihydroxyphenylalanine (DOPA) biosynthesized by a tyrosine phenol-lyase. Chemical Communications. 54 (24), 3002-3005 (2018).
- Young, T. S., Ahmad, I., Yin, J. A., Schultz, P. G. An enhanced system for unnatural amino acid mutagenesis in E. coli. Journal of Molecular Biology. 395 (2), 361-374 (2010).
- Studier, F. W. Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification. 41 (1), 207-234 (2005).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72 (1-2), 248-254 (1976).
- Jang, S., Sachin, K., Lee, H. J., Kim, D. W., Lee, H. S. Development of a simple method for protein conjugation by copper-free click reaction and its application to antibody-free Western blot analysis. Bioconjugate Chemistry. 23 (11), 2256-2261 (2012).
- Gobom, J., et al. Alpha-cyano-4-hydroxycinnamic acid affinity sample preparation. A protocol for MALDI-MS peptide analysis in proteomics. Analytical Chemistry. 73 (3), 434-438 (2001).
- Mukai, T., et al. Highly reproductive Escherichia coli cells with no specific assignment to the UAG codon. Scientific Reports. 5, 9699 (2015).
- Lajoie, M. J., et al. Genomically recoded organisms expand biological functions. Science. 342 (6156), 357-360 (2013).
- Bryson, D. I., et al. Continuous directed evolution of aminoacyl-tRNA synthetases. Nature Chemical Biology. 13 (12), 1253-1260 (2017).
- Guo, J., Melancon, C. E., Lee, H. S., Groff, D., Schultz, P. G. Evolution of amber suppressor tRNAs for efficient bacterial production of proteins containing nonnatural amino acids. Angewandte Chemie International Edition. 48 (48), 9148-9151 (2009).
- Lee, S., et al. A facile strategy for selective phosphoserine incorporation in histones. Angewandte Chemie International Edition. 52 (22), 5771-5775 (2013).
- Neumann, H., Wang, K., Davis, L., Garcia-Alai, M., Chin, J. W. Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome. Nature. 464 (7287), 441-444 (2010).
- Lang, K., Chin, J. W. Bioorthogonal reactions for labeling proteins. ACS Chemical Biology. 9 (1), 16-20 (2014).
- Lang, K., Chin, J. W. Cellular Incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chemical Reviews. 114 (9), 4764-4806 (2014).
- Plass, T., Milles, S., Koehler, C., Schultz, C., Lemke, E. A. Genetically encoded copper-free click chemistry. Angewandte Chemie International Edition. 50 (17), 3878-3881 (2011).
- Seitchik, J. L., et al. Genetically encoded tetrazine amino acid directs rapid site-specific in vivo bioorthogonal ligation with trans-cyclooctenes. Journal of the American Chemical Society. 134 (6), 2898-2901 (2012).

