Screening Cotton Genotypes for Reniform Nematode Resistance
Summary
Here, a protocol is presented for the rapid non-destructive screening of cotton genotypes for reniform nematode resistance. The protocol involves visually examining the roots of nematode-infected cotton seedlings to determine infection response. The vegetative shoot from each plant is then propagated to recover plants for seed production.
Abstract
A rapid non-destructive reniform nematode (Rotylenchulus reniformis) screening protocol is needed for the development of resistant cotton (Gossypium hirsutum) varieties to improve nematode management. Most protocols involve extracting vermiform nematodes or eggs from the cotton root system or potting soil to determine population density or reproduction rate. These approaches are generally time-consuming with a small number of genotypes evaluated. An alternative approach is described here in which the root system is visually examined for nematode infection. The protocol involves inoculating cotton seedling 7 days after planting with vermiform nematodes and determining the number of females attached to the root system 28 days after inoculation. Data are expressed as the number of females per gram of fresh root weight to adjust for variation in root growth. The protocol provides an excellent method for evaluating host-plant resistance associated with the ability of the nematode to establish an infection site; however, resistance that hinders nematode reproduction is not assessed. As with other screening protocols, variation is commonly observed in nematode infection among individual genotypes within and between experiments. Data are presented to illustrate the range of variation observed using the protocol. To adjust for this variation, control genotypes are included in experiments. Nonetheless, the protocol provides a simple and rapid method to evaluate host-plant resistance. The protocol has been successfully used to identify resistant accessions from the G. arboreum germplasm collection and evaluate segregating populations of more than 300 individuals to determine the genetics of resistance. A vegetative propagation method for recovering plants for resistance breeding was also developed. After removal of the root system for nematode evaluation, the vegetative shoot is replanted to allow the development of a new root system. More than 95% of the shoots typically develop a new root system with plants reaching maturity.
Introduction
Rotylenchulus reniformis (Linford and Oliveira), commonly referred to as the reniform nematode, is one of the major parasitic nematode species present in soils of the southeastern United States1,2,3. The nematode is an obligate, sedentary semi-endoparasite requiring a host plant to complete its life cycle2,4. Vermiform preadult female nematodes penetrate the host root system to establish a feeding site in the stele2,3. As the nematode feeds and matures, the posterior portion remaining outside of the host root will swell upon egg production, forming a characteristic kidney shape (Figure 1). Rotylenchulus reniformis is capable of feeding on the root system of more than 300 plant species, including cotton4. Upland cotton (Gossypium hirsutum L.) is widely cultivated in the southeastern United States, but the lack of R. reniformis resistant varieties hinders nematode management2,3. Management strategies such as nematicide treatment and rotation with non-host crop species have been used to reduce soil R. reniformis population densities5,6, but seed cotton yield losses can commonly range from 1 to 5%2. Symptoms of R. reniformis infection can include plant stunting, suppressed root growth, nutritional deficiencies, fruit abortion, and delayed maturity2. However, symptoms may not be apparent due to the uniformity of symptoms across the field; therefore, approaches to assess R. reniformis infection are needed to identify and develop resistant upland cotton varieties. Evaluation of R. reniformis resistance in cotton is considered difficult7, because the infected root system may appear normal even though the plant may show symptoms of infection8.
An effective nematode screening protocol is required for the identification of R. reniformis resistant accessions from the cotton germplasm collection, and for the determination of the resistance genetics for these accessions. Such a protocol will aid in the transfer of resistance genes to upland cotton. Various bioassay methods have been used to assess R. reniformis infection in cotton8,9,10,11,12,13,14,15. In general, two major approaches have been used for the identification of R. reniformis resistant cotton genotypes. The most frequently used approach involves extracting eggs and/or vermiform nematodes from infected plants or soil8,11,12,14,15. The general methodology for this approach involves planting seeds for the individual cotton genotypes in separate pots, allowing the seedlings to develop for 7 to 14 days, inoculating the seedlings by adding a mixture of vermiform stages of R. reniformis to the soil, and allowing the nematodes to infect the root system for 30 to 60 days. Next, vermiform nematodes and/or eggs are extracted from the infected root system of each plant or from the potting soil. The number of extracted nematodes or eggs is then determined to estimate the population density and reproduction rate, which are compared to control genotypes in order to identify resistant genotypes.
An alternative approach, as described here, involves microscopically examining the cotton root system that has been infected with nematodes to determine the number of female nematodes parasitizing the roots10,16. Similar to other approaches, cotton genotypes are planted in separate pots and inoculated with vermiform nematodes approximately 7 days after planting. Within 30 days after inoculation, the root system is removed from individual plants and the soil is rinsed from the roots. Next, the nematodes attached to the root system are stained with red food coloring17, and roots are microscopically examined to determine the number of infection sites with resistant cotton genotypes (identified based on the number of nematodes per gram of root) compared to a susceptible control16. This second approach has the advantage of increased throughput by reducing the number of days required for evaluation and increasing the number of individual genotypes evaluated in a single experiment. Screening methodologies that evaluate population density or reproduction rate are often more time-consuming than those based on visual observations of infection signs7. However, one limitation of this approach is that host-plant resistance that hinders nematode reproduction as determined by egg production is not assessed13.
Screening protocols for R. reniformis resistance often destroy the root system during evaluation7 and involve the vegetative shoot being discarded. To overcome this limitation, a method of vegetative propagation has been developed to allow the recovery of plants for seed production18. After removal of the root system for nematode evaluation, the vegetative shoot is planted in potting soil to allow the root system to regrow. This method has broad applications for most R. reniformis screening protocols. A simple and rapid method of vegetative propagation is of critical importance for breeding R. reniformis resistant upland cotton varieties, where the recovery of the progeny is required to advance resistant genotypes to the next generation.
A protocol is presented for the large-scale screening of cotton genotypes for reniform nematode resistance. The goal is to develop a simple and rapid non-destructive screening method to evaluate cotton breeding populations for nematode resistance in order to aid in the breeding of resistant upland cotton varieties. Using this protocol, data are typically obtained within 35 days, with more than 300 genotypes evaluated in a single experiment. Data are presented for resistant and susceptible genotypes to illustrate the variation commonly observed using these methods.
Protocol
1. Maintaining a Source of R. reniformis Inoculum
- Fill terra cotta clay pots (15 cm in diameter, 13.5 cm in height) with a steam pasteurized mix of 1-part sandy loam and 2-parts sand. Plant a susceptible tomato (Solanum lycopersicon) variety in each pot and place the pots in a glasshouse.
NOTE: Other susceptible plant varieties such as cotton can be used instead of tomato. - Inoculate the tomato plants with vermiform reniform nematodes (see step 3.3). Maintain the plants in the glasshouse at a temperature of approximately 28 °C.
2. Planting Cotton Genotypes for R. reniformis Resistance Evaluation
- Prepare soil by combining 2-parts fine sand with 1-part sandy loam collected from the field.
- Steam pasteurize the soil mixture to ensure that the soil is free of nematodes and soil-borne plant pathogens.
- Add the soil mixture to conical plastic pots (4 cm in diameter, 21 cm in height). Prior to filling the pots, place a ball of cotton in the bottom of the pot to prevent soil loss. Partially fill the pots with soil approximately 2 cm from the top.
- Prepare a plastic stake for each pot to designate the genotype to be planted.
- Plant seeds of the cotton genotypes selected for evaluation.
- Plant a single seed in each pot for the evaluation of segregating populations, in which each seed represents a unique genotype.
- For cotton varieties or germplasm accessions, plant 2 to 3 seeds in a single pot to insure germination of at least one plant with the other seedlings removed from the pots prior to nematode inoculation.
Note: Alternatively, seeds can be germinated for 24 to 72 h prior to planting to minimize the number of pots with non-viable seeds.
- Plant seeds of selected resistant and susceptible control genotypes.
Note: Control genotypes are replicated 5 to 10 times to assess natural variations inherent to the screening methodology. - Fill pots with additional soil to cover the seed in each pot.
- Place the pots in the growth chamber. Maintain a constant temperature of 28 °C, an ambient air temperature, for the growth chamber. Provide artificial lighting with a mixture of fluorescent and incandescent lamps with a 16 h photoperiod.
- Place water emitters in each pot, and water the pots twice per day using an automatic watering system. Adjust the watering system to supply additional water as the plant grows.
3. Nematode Inoculation of Cotton Plants and Preparation of Root Samples
- Extract vermiform reniform nematodes maintained on susceptible tomato plants (see step 1) using elutriation19 and centrifugal flotation20 methodologies the day before inoculation. Store the extracted nematodes at 4 °C.
NOTE: Baermann funnel extraction21 is an alternate method for nematode extraction. - Determine the number of nematodes extracted by counting the number of nematodes in a 100 µL subsample and prepare a suspension of 1,000 nematodes/mL in tap water for inoculations.
- Inoculate the cotton seedlings 7 d after planting with the nematode suspension. Create a small depression in the soil next to the plant, and pipette 1 mL of the reniform nematode suspension into the depression.
- Remove plants from the pots 28 d after inoculation for nematode evaluation.
NOTE: At this stage, plants are approximately 15 cm tall with 4 to 6 fully expanded leaves.- Remove most of the fully expanded leaves from the plants using scissors prior to removing the plants from the pots.
- To remove plants from the pots, squeeze the pot and slide the soil out into the hand.
- Gently remove the soil from the roots by agitating the root system in tap water in a 10 L container. Briefly rinse the root system in a container of clean tap water.
- Remove the root system from the plant approximately 1 cm below the soil line using scissors.
- Place the root system into a 120 mL plastic, non-sterile, disposable specimen container along with the plastic stake from the pot used for identification.
NOTE: Multiple samples are processed before proceeding to step 3.7. Proceed to step 4 for the vegetative propagation of the plant shoot. - Prepare a 12.5% (v/v) solution of red food coloring17 in tap water to stain the nematodes attached to the root system.
- Add approximately 30 mL of the red food coloring solution to the root sample in the specimen container to completely cover the root system.
- Place the specimen container in a microwave oven and heat the root sample until the staining solution starts to boil. Remove the sample from the microwave oven and allow the sample to cool at room temperature.
- Decant the red food coloring solution from the root sample and add approximately 100 mL of tap water to the specimen container to remove excess stain. Place the cover on the specimen container and store the sample in a refrigerator at 4 °C. Proceed to step 5 for evaluation of root infection.
NOTE: The protocol can be paused here before proceeding to step 5.
4. Vegetative Propagation to Recover Plants for Seed Production
- Place a ball of cotton in the bottom of a conical plastic pot (see step 2.3) and partially fill the pot with peat moss potting media. Then, place the vegetative shoot in the pot and firmly add potting media to fill the pot. Place a new labeled plastic stake in each pot to designate the cotton genotype.
- Place the tray of pots in a plastic container (73.6 cm length x 45.7 cm width x 15.2 cm height) with water and briefly water the plants to moisten the potting media. Place the pots in a growth chamber with a constant temperature of 28 °C using a 16 h photoperiod. Add additional water to the plastic container as needed to maintain soil moisture.
- Transplant the plants to larger pots for seed production after approximately 30 d. Partially fill a 6 L plastic pot with potting media (see step 4.1), remove the plant from the small pot, place the plant into the 6 L pot, and firmly add potting media to fill the pot.
- Place the plants in a glasshouse and add water to moisten the potting media. Maintain the temperature in the glasshouse at approximately 28 °C (artificial lighting is not required).
- Water plants by hand as needed for approximately 30 d.
- When approximately 75% of the plants require daily watering, water plants daily using an automatic watering system. Adjust the automatic watering system to water more frequently as needed for plant growth.
- Add approximately 10 g of a slow-release fertilizer to each pot prior to the start of floral initiation.
- Harvest plants at maturity and process the cotton seed samples to obtain seeds for further evaluation.
- To harvest cotton seeds, remove the cotton from the open bolls on the plant by hand and place it in a labeled paper bag. The seeds are attached to the cotton fibers, which are removed in the following steps.
- Remove lint fibers from the seed samples using a 10-saw laboratory gin.
- Remove fuzz fibers from the seed samples using concentrated sulfuric acid. Neutralize seed samples in a 15% (v/v) solution of sodium carbonate, rinse the samples with tap water, and dry the samples in a forced air drier.
- Place the seed samples in labeled envelopes for storage.
5. Evaluation of R. reniformis Root Infection
- Remove the root sample from the specimen container and count the number of female nematodes attached to the root system using a stereomicroscope (20X magnification).
NOTE: Only female reniform nematodes are capable of infecting plant roots. - Place the root system on paper towels for approximately 10 min to remove excess moisture. Weigh the root system to determine the fresh root weight.
- Enter the nematode count and fresh root weight data into a computer spreadsheet program and calculate the number of females per gram of root.
Representative Results
Rotylenchulus reniformis infection of the root system for two varieties is presented in Figure 1. Relatively fewer female reniform nematodes are able to establish a feeding site for the resistant cotton genotype compared to the susceptible genotype. Variation in root growth is common between accessions, as illustrated in Figure 2. This variation as measured by fresh root weight can also be observed between plants of the same genotype (Table 1). Gossypium arboreum genotypes frequently show lower root growth rates than upland cotton genotypes. To compensate for this variation, data are collected on fresh root weights, which are used to calculate the number of female reniform nematode per gram root tissue for each genotype. The numbers of female reniform nematodes per gram of root for resistant genotypes are generally less than 10, whereas susceptible genotypes typically have greater than 30 nematodes per gram of root.
One challenge for screening cotton genotypes for R. reniformis resistance is the potential variation that can occur within and between experiments. To evaluate and adjust for this variation, resistant and susceptible cotton genotypes are included as controls and replicated in each experiment. Table 1 presents data for two genotypes used as controls in two separate experiments using the protocol described above. The genotypes were replicated 10 times and inoculated 7 days after planting with 1,000 vermiform nematodes, then root systems were harvested 28 days after inoculation to count nematodes attached to the roots. Because the experiments were conducted at different times, the source of the nematodes used for inoculations was different; otherwise, all other parameters were similar. These data illustrate the variation that can be observed in reniform nematode evaluations. Female nematode counts and root weights were higher in experiment 1 for the two controls compared to experiment 2, resulting in higher numbers of females per gram of root for experiment 1. Because the female counts were considerably higher for experiment 1, the increases in root weights did not lower the numbers of females per gram of root to the levels observed for experiment 2. Considerable variation between replications of individual genotypes was also observed. However, the resistant G. arboreum genotype PI 615699 frequently showed substantially lower female counts and lower numbers of females per gram of root than the susceptible G. hirsutum genotype PI 529251. The genotypes can be easy classified as resistant or susceptible when these means are used for comparison. Genotypes are classified as resistant when the numbers of females per gram of root are approximately 10% of the susceptible control.
A subset of data from a segregating F2 population that was evaluated using the protocol is presented in Table 2. The population included 300 F2 plants, and data for 50 plants representing the range in variation are presented. For the population of 300 plants, the number of observed nematodes infecting the root systems ranged from 0 to 50, with a mean of 9.4. Root weights ranged from 0.01-1.22 g, with a mean of 0.38 g. Female nematodes per gram of root ranged from 0-400, with a mean of 33.6. The parents were replicated in the evaluation. The resistant parent (PI 417895) showed a mean of 5.8 females per gram of root, with a mean root weight of 0.8 g; in contrast, the susceptible parent (PI 529729) showed a mean of 40.8 females per gram of root, with a mean root weight of 0.35 g. Twenty plants showed no nematode infection and were classified as resistant, but this may represent escapes. Both these plants and plants with poor root growth are typically removed from data analysis. This range in variation for root growth and nematode infection of the root systems are commonly observed for nematode evaluations; thus, the ability to screen a large number of plants in a single experiment can minimize this variation and allow for accurately assessing the genetics of resistance. The population showed quantitative variation for nematode infection, and plants were classified as resistant based on the data from the susceptible parent, which suggested that resistance was conferred by two recessive genes for this population. Additionally, the vegetative propagation protocol described above was successfully used to recover plants from this population. The rating of the F3 progeny derived from individual F2 plants frequently corresponded to the rating of the F2 plant.

Figure 1: Rotylenchulus reniformis infected root samples. The root sample from a resistant cotton genotype (lower left) shows a single female nematode attached to the root, whereas the susceptible genotype (upper right) shows multiple females attached to the root. The black bar represents a 0.1 mm scale. Please click here to view a larger version of this figure.

Figure 2: Variation observed in root growth for two cotton genotypes. The root systems for two reniform nematode resistant G.arboreum accessions are presented to illustrate the variation that can be observed for root growth. Please click here to view a larger version of this figure.
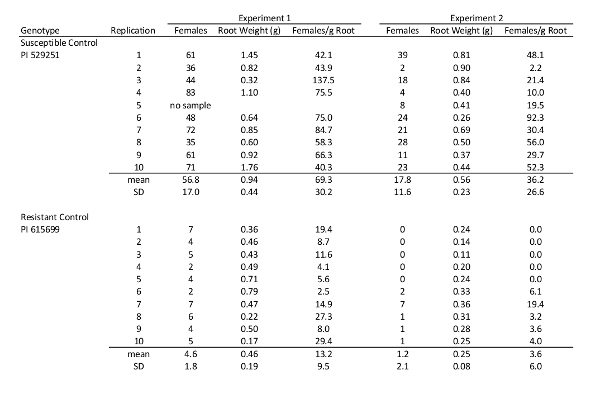
Table 1: Variation observed in reniform nematode infection response for two cotton genotypes included as controls. These data illustrate the variation that can occur within and between experiments for the susceptible G.hirsutum genotype PI 529251 and resistant G. arboreum genotype PI 615699; however, data means were significantly different, allowing the genotypes to be easily classified as resistant or susceptible.
| Genotype Designation | Females | Root Weight (g) | Females/g Root | Classification |
| 88 | 0 | 0.67 | 0.0 | Resistant |
| 156 | 0 | 0.10 | 0.0 | Resistant |
| 75 | 2 | 1.05 | 1.9 | Resistant |
| 298 | 2 | 0.58 | 3.4 | Resistant |
| 259 | 3 | 0.77 | 3.9 | Resistant |
| 208 | 1 | 0.21 | 4.8 | Resistant |
| 322 | 4 | 0.82 | 4.9 | Resistant |
| 189 | 2 | 0.35 | 5.7 | Resistant |
| 147 | 6 | 0.94 | 6.4 | Resistant |
| 267 | 2 | 0.18 | 11.1 | Moderately Resistant |
| 198 | 5 | 0.43 | 11.6 | Moderately Resistant |
| 251 | 2 | 0.17 | 11.8 | Moderately Resistant |
| 95 | 6 | 0.46 | 13.0 | Moderately Resistant |
| 248 | 3 | 0.23 | 13.0 | Moderately Resistant |
| 79 | 11 | 0.84 | 13.1 | Moderately Resistant |
| 340 | 4 | 0.29 | 13.8 | Moderately Resistant |
| 114 | 9 | 0.64 | 14.1 | Moderately Resistant |
| 168 | 6 | 0.40 | 15.0 | Moderately Resistant |
| 117 | 7 | 0.44 | 15.9 | Moderately Resistant |
| 77 | 10 | 0.57 | 17.5 | Moderately Resistant |
| 277 | 9 | 0.44 | 20.5 | Moderately Resistant |
| 47 | 8 | 0.34 | 23.5 | Moderately Susceptible |
| 96 | 20 | 0.85 | 23.5 | Moderately Susceptible |
| 139 | 15 | 0.60 | 25.0 | Moderately Susceptible |
| 253 | 2 | 0.08 | 25.0 | Moderately Susceptible |
| 247 | 15 | 0.53 | 28.3 | Moderately Susceptible |
| 308 | 8 | 0.28 | 28.6 | Moderately Susceptible |
| 152 | 9 | 0.31 | 29.0 | Moderately Susceptible |
| 123 | 8 | 0.26 | 30.8 | Moderately Susceptible |
| 296 | 18 | 0.58 | 31.0 | Moderately Susceptible |
| 138 | 10 | 0.31 | 32.3 | Moderately Susceptible |
| 151 | 5 | 0.15 | 33.3 | Moderately Susceptible |
| 102 | 31 | 0.77 | 40.3 | Moderately Susceptible |
| 67 | 5 | 0.12 | 41.7 | Susceptible |
| 51 | 18 | 0.43 | 41.9 | Susceptible |
| 311 | 21 | 0.48 | 43.8 | Susceptible |
| 334 | 4 | 0.09 | 44.4 | Susceptible |
| 266 | 33 | 0.74 | 44.6 | Susceptible |
| 260 | 7 | 0.14 | 50.0 | Susceptible |
| 49 | 16 | 0.32 | 50.0 | Susceptible |
| 149 | 20 | 0.39 | 51.3 | Susceptible |
| 104 | 22 | 0.34 | 64.7 | Susceptible |
| 238 | 39 | 0.57 | 68.4 | Susceptible |
| 144 | 24 | 0.33 | 72.7 | Susceptible |
| 225 | 24 | 0.30 | 80.0 | Susceptible |
| 87 | 38 | 0.43 | 88.4 | Susceptible |
| 126 | 50 | 0.51 | 98.0 | Susceptible |
| 272 | 3 | 0.03 | 100.0 | Susceptible |
| 154 | 24 | 0.12 | 200.0 | Susceptible |
| 286 | 3 | 0.01 | 300.0 | Susceptible |
Table 2: Reniform nematode infection response observed in a subset of 50 genotypes from a G. arboreum F2 population. These data illustrate the range of variation that can be observed for segregating populations. Root weights, nematode counts, and number of females per gram of root are presented for each genotype, with plants classified as resistant, moderately resistant, moderately susceptible, or susceptible.
Discussion
An effective screening protocol is required for 1) the identification of R. reniformis resistant cotton genotypes in order to evaluate the genetics of resistance and 2) the breeding of resistant varieties. Most protocols assess R. reniformis population densities or reproduction rates by extracting vermiform nematodes or eggs from the cotton root system or potting soil8,11,12,14,15. These approaches are often more time-consuming, and results tend to be more varied within and between experiments. Additionally, cotton genotypes may be more frequently misclassified in non-replicated glasshouse experiments using these protocols11. Nevertheless, similar results can be achieved for the various protocols or parameters evaluated9,13.
An alternative screening protocol is presented in which the screening of cotton genotypes is conducted by assessing the number of female reniform nematodes parasitizing the root system. Resistant and susceptible cotton genotypes may initially show a similar number of female nematodes penetrating the root systems within 16 hours after inoculation, but within 36 hours, resistant genotypes start to show significantly fewer attached female nematodes and nematode development is hindered9. Thus, this screening protocol provides a more direct measure of nematode infection of the cotton root system compared to protocols that rely on the extraction of nematodes or eggs from the root system or potting soil. The method of cotton seedling inoculation with vermiform nematodes is similar among the approaches. Inoculations are typically conducted 7 to 14 days after planting, but the timing of the inoculation is less critical, as seeds can also be directly planted into reniform nematode-infested soil. Inoculations are conducted using 1,000 vermiform nematodes, but the protocol can be modified to increase or decrease the number used for inoculation, or two inoculations can be conducted at 7 and 14 days after planting to insure sufficient female nematodes are present for root infection. In soybean, the number of nematodes used for inoculation had no significant effect on egg mass ratings 21 days after planting; although, higher ratings were typically observed at the higher nematode population density22. The protocol can be optimized to determine the minimum nematode density for inoculation.
Nematode infection of the root system is assessed 28 days after inoculation for this protocol, which is generally earlier than in other protocols. This is a critical step in the protocol, as assessments are conducted prior to egg hatch. A significant delay in harvesting root samples can result in a second round of infection. However, this earlier evaluation has the advantage of increasing throughput. For the protocol presented, cotton genotypes are planted in a mixture of sand and soil, which is critical for the simple and quick removal of the root system from the pot. The use of an automatic watering system is essential when using small pots with a sand and soil mixture in order to prevent the pots from drying out. Red food coloring is used to stain the nematodes attached to the root system, which is a simple and safe method17. Once the root systems are stained, they can be stored in tap water at 4 °C before counting the number of nematodes attached to the root system; thus, a greater number of cotton genotypes can be evaluated in a single experiment, because no additional processing of the samples is required prior to the assessment of nematode counts. Also, it is advantageous to store the root samples in tap water for several days, which allows roots to de-stain, making the counting easier.
The protocol described allows for the screening of larger populations in order to reduce environmental variation that will occur between experiments. Using a 900 m2 plant growth chamber equipped with an automatic watering system, populations of 480 individual plants can be evaluated. The protocol has been successfully used to evaluate segregating populations of 300 or more individuals to characterize the genetics of resistance10,18. These populations showed that quantitative variation for nematode infection and resistance in G. arboreum may be more often associated with multiple recessive genes; thus, larger populations are required for genetic studies. Additionally, quantitative variation is observed in segregating populations, regardless of the protocol employed, to evaluate nematode infection response.
Host-plant resistance in cotton can hinder the ability of the nematode to infect the root system and establish a feeding site, but it may also affect the reproductive ability of the nematode. The screening protocol described evaluates the number of nematodes that are able to establish a feeding site on the cotton root system. Nematode reproduction as measured by egg production was not assessed in this protocol, which is an important limitation. Nonetheless, the protocol can be modified to collect this type of data. Alternately, other methodology can be used to collect these data after resistant genotypes have been identified, which reduces the need to screen a large number of individuals.
Data from nematode evaluations of individual genotypes can be variable within and between experiments, which is a common problem with all screening protocols used to assess R. reniformis resistance for cotton genotypes. The use of an experimental design with multiple replications for the screening of germplasm accessions will aid in assessing this variation for the identification of resistant genotypes. Additionally, including the same resistant and susceptible control genotypes between experiments is helpful in assessing this variation and comparing results from multiple experiments. These controls are also used to monitor the success of nematode inoculation. Additionally, data means from these controls are used to classify genotypes as resistant or susceptible10,16. Cotton genotypes are typically classified as resistant if they show less than 10% of the infection observed on the susceptible control16,23. Root growth is another factor contributing to the variation observed in the data using the protocol, because plants having a tap root with fewer lateral roots offer less sites for infection, which may result in fewer nematodes per gram of root.
The difficulty in developing new upland cotton varieties using other cotton species as a source of resistance genes requires a vegetative propagation protocol in order to advance the breeding lines to the next generation for further selection or additional breeding. A simple vegetative propagation protocol as described was developed to recover plants after nematode evaluation. The protocol has been successfully used to recover plants from large populations18. Typically, the root system is recovered within 30 days after the vegetative shoot is planted. Survival rates are frequently greater than 95%. Plants showing poor vigor can be lost when propagating a large number of plants. In general, less than 1% of the plants failed to show root or shoot growth. The protocol can be easily modified and used with other nematode screening protocols.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was funded by the United States Department of Agriculture, Agricultural Research Service. Mention of trade names and commercial products in this article are solely for the purpose of providing specific information and do not imply recommendations or endorsements by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. The authors have no conflict of interest to declare. Technical assistance was provided by Kristi Jordan.
Materials
| Ray Leach Cone-tainer | Stuewe and Sons Inc. | SC10U | |
| Cone-tainer tray | Stuewe and Sons Inc. | RL98 | |
| Sand | various | ||
| Cotton balls | various | ||
| Pylon 4 inch plant labels (4 in L x 5/8 in W) | Pylon Platics | L-4-W | Any brand or vendor is acceptible. |
| 4 oz. specimen containers | Fisher Scientific | 16-320-731 | Any brand or vendor is acceptible. |
| Red food coloring | McCormick & Co., Inc. | ||
| 1 mL Pipet tips | various | ||
| 10 L container | various | Inexpensive buckets work well. | |
| 6 L pots | Nursery Supplies Inc. | Poly-Tainer-Can No2A | Any brand or vendor is acceptible. Different size pots can be used |
| Potting media | Sun Gro Horticulture | Metro-Mix 360 | Any brand or vendor is acceptible. |
| Fertilizer | Everris NA Inc. | Osmocote Plus | Any brand or vendor is acceptible. |
| Plastic container (73.6 cm L x 45.7 cm W x 15.2 cm D) | Rubbermaid | 3O29 | Any brand or vendor is acceptible. |
References
- Heald, C. M., Robinson, A. F. Survey of current distribution of Rotylenchulus reniformis. in the United States. Journal of Nematology. 22 (4), 695-699 (1990).
- Koenning, S. R., Wrather, J. A., Kirkpatrick, T. L., Walker, N. R., Starr, J. L., Mueller, J. D. Plant-parasitic nematodes attacking cotton in the United States: old and emerging production challenges. Plant Disease. 88 (2), 100-113 (2004).
- Robinson, A. F. Reniform in U.S. cotton: when, where, why, and some remedies. Annual Review of Phytopathology. 45, 263-288 (2007).
- Robinson, A. F., Inserra, R. N., Caswell-Chen, E. P., Vovlas, N., Troccoli, A. Rotylenchulus species: identification, distribution, host ranges, and crop plant resistance. Nematropica. 27 (2), 127-180 (1997).
- Davis, R. F., Koenning, S. R., Kemerait, R. C., Cummings, T. D., Hurley, W. D. Rotylenchulus reniformis management in cotton with crop rotation. Journal of Nematology. 35 (1), 58-64 (2003).
- Starr, J. L., Koenning, S. R., Kirkpatrick, T. L., Robinson, A. F., Roberts, P. A., Nichols, R. L. The future of nematode management in cotton. Journal of Nematology. 39 (4), 283-294 (2007).
- Weaver, D. B., Lawrence, K. S., van Santen, E. Reniform nematode resistance in upland cotton germplasm. Crop Science. 47 (1), 19-24 (2007).
- Robinson, A. F., Cook, C. G., Percival, A. E. Resistance to Rotylenchulus reniformis and Meloidogyne incognita race 3 in the major cotton cultivars planted since 1950. Crop Science. 39 (3), 850-858 (1999).
- Carter, W. W. Resistance and resistant reaction of Gossypium arboreum to the reniform nematode, Rotylenchulus reniformis. Journal of Nematology. 13 (3), 368-374 (1981).
- Erpelding, J. E., Stetina, S. R. Genetics of reniform nematode resistance in Gossypium arboreum germplasm line PI 529728. World Journal of Agricultural Research. 1 (4), 48-53 (2013).
- Robinson, A. F., Bridges, A. C., Percival, A. E. New sources of resistance to the reniform (Rotylenchulus reniformis) and root-knot (Meloidogyne incognita) nematode in upland (Gossypium hirsutum L.) and sea island (G. barbadense L.) cotton. Journal of Cotton Science. 8 (3), 191-197 (2004).
- Robinson, A. F., Percival, A. E. Resistance to Meloidogyne incognita race 3 and Rotylenchulus reniformis in wild accessions of Gossypium hirsutum and G. barbadense from Mexico. Journal of Nematology. 29 (4), 746-755 (1997).
- Stetina, S. R., Young, L. D. Comparisons of female and egg assays to identify Rotylenchulus reniformis resistance in cotton. Journal of Nematology. 38 (3), 326-332 (2006).
- Usery, S. R., Lawrence, K. S., Lawrence, G. W., Burmester, C. H. Evaluation of cotton cultivars for resistance and tolerance to Rotylenchulus reniformis. Nematropica. 35 (2), 121-133 (2005).
- Yik, C. -. P., Birchfield, W. Resistant germplasm in Gossypium species and related plants to Rotylenchulus reniformis. Journal of Nematology. 16 (2), 146-153 (1984).
- Stetina, S. R., Erpelding, J. E. Gossypium arboreum accessions resistant to Rotylenchulus reniformis. Journal of Nematology. 48 (4), 223-230 (2016).
- Thies, J. A., Merrill, S. B., Corley, E. L. Red food coloring stain: new, safer procedures for staining nematodes in roots and egg masses on root surfaces. Journal of Nematology. 34 (2), 179-181 (2002).
- Erpelding, J. E., Stetina, S. R. Genetic characterization of reniform nematode resistance for Gossypium arboreum accession PI 417895. Plant Breeding. 137 (1), 81-88 (2018).
- Byrd, D. W., et al. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematology. 8 (3), 206-212 (1976).
- Jenkins, W. R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 48 (9), 692 (1964).
- Robinson, A. F., Heald, C. M. Carbon dioxide and temperature gradients in Baermann funnel extraction of Rotylenchulus reniformis. Journal of Nematology. 23 (1), 28-38 (1991).
- Williams, C., Gilman, D. F., Fontenot, D. S., Birchfield, W. A rapid technique for screening soybeans for reniform nematode resistance. Plant Disease Reporter. 63 (10), 827-829 (1979).
- Schmitt, D. P., Shannon, G. Differentiating soybean responses to Heterodera glycines races. Crop Science. 32 (1), 275-277 (1992).

