Establishment of the Dual Humanized TK-NOG Mouse Model for HIV-associated Liver Pathogenesis
Summary
This protocol provides a reliable method to establish humanized mice with both human immune system and liver cells. Dual reconstituted immunodeficient mice achieved via intrasplenic injection of human hepatocytes and CD34+ hematopoietic stem cells are susceptible to human immunodeficiency virus-1 infection and recapitulate liver damage as observed in HIV-infected patients.
Abstract
Despite the increased life expectancy of patients infected with human immunodeficiency virus-1 (HIV-1), liver disease has emerged as a common cause of their morbidity. The liver immunopathology caused by HIV-1 remains elusive. Small xenograft animal models with human hepatocytes and human immune system can recapitulate the human biology of the disease's pathogenesis. Herein, a protocol is described to establish a dual humanized mouse model through human hepatocytes and CD34+ hematopoietic stem/progenitor cells (HSPCs) transplantation, to study liver immunopathology as observed in HIV-infected patients. To achieve dual reconstitution, male TK-NOG (NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(Alb-TK)7-2/ShiJic) mice are intraperitoneally injected with ganciclovir (GCV) doses to eliminate mouse transgenic liver cells, and with treosulfan for nonmyeloablative conditioning, both of which facilitate human hepatocyte (HEP) engraftment and human immune system (HIS) development. Human albumin (ALB) levels are evaluated for liver engraftment, and the presence of human immune cells in blood detected by flow cytometry confirms the establishment of human immune system. The model developed using the protocol described here resembles multiple components of liver damage from HIV-1 infection. Its establishment could prove to be essential for studies of hepatitis virus co-infection and for the evaluation of antiviral and antiretroviral drugs.
Introduction
Since the advent of antiretroviral therapy, there has been a substantial decrease in deaths related to HIV-1 monoinfection. However, liver disease has emerged as a common cause of morbidity in HIV-infected patients1,2. Coinfections of hepatitis viruses with HIV-1 infection are more common, accounting for 10% – 30% of HIV-infected persons in the United States3,4,5.
The host-specificity of HIV-1 and hepatitis viruses limits the utility of small animal models to study human-specific infectious diseases or to investigate multiple aspects of HIV-1-associated liver pathogenesis. Immunodeficient mice that permit the engraftment of human cells and/or tissues (termed humanized mouse models) are acceptable animal models for preclinical studies6,7,8. Since the introduction of humanized mice in the early 2000s, multiple preclinical studies of cholestatic human liver toxicity, human-specific pathogens, including HIV-1 and HIV-associated neurocognitive disorders, Epstein Barr virus, hepatitis, and other infectious diseases, have been investigated in these mice6,9,10,11. Multiple mouse models for CD34+ HSPCs and/or human hepatocyte transplantation have long been developed and have improved over time to study the disease pathogenesis of Hepatitis B virus (HBV)-associated liver disease12,13,14. Several models for HSPC and human hepatocyte (HEP) transplantation are based on strains, known as NOG (NOD.Cg-Prkdcscid Il2rgtm1Sug/JicTac)8,13, NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ)15, Balb/C-Rag2-/- γc-/- (Rag2tm1.1Flv Il2rgtm1.1Flv/J)12, and fah-/- NOD rag1-/-il2rγnull mouse16. However, each model has its own advantages and limitations; for example, AFC8 dual humanized mice for HEPs and human stem cells (HSCs) on a Balb/C-Rag2-/- γc-/- background enables the successful engraftment of immune cells and HSCs, but there is an absence of an antigen-specific T- and B-cell response in this model12. The major concerns in reconstituting double humanized mice include suboptimal engraftment, a lack of suitable models to support different tissues, mismatched conditions, immune rejection, or graft-versus-host disease (GVHD), and technical difficulties, such as risky manipulations with newborns and high mortality rates due to metabolic abnormalities13.
Although humanized mice have been used for HIV research for many years17,18,19, the use of humanized mice to study liver damage caused by HIV-1 has been limited20. We previously reported the establishment of a dual humanized TK-NOG mouse model and its application in HIV-associated liver disease8. This model shows the robust engraftment of liver and immune cells and recapitulates HIV infection pathogenesis. This discussion presents a detailed protocol, including the most critical steps in the transplantation of human hepatocytes. A description of the HSPCs required for a successful engraftment of HEPs and the establishment of a functional immune system in TK-NOG mice is also presented. The use of these mice to study HIV-associated liver immunopathogenesis is detailed. TK-NOG male mice carrying a liver-specific herpes simplex virus type 1 thymidine kinase (HSV-TK) transgene are used. Mouse liver cells expressing this transgene can easily be ablated after a brief exposure to a nontoxic dose of GCV. Transplanted human liver cells are stably maintained within the mouse liver without exogenous drugs21. The mice are also preconditioned with nonmyeloablative doses of treosulfan to create a niche in the mouse bone marrow for human cells8. Immunodeficient TK-NOG mice are intrasplenically injected with HEPs and multipotent HSPCs. The mice are then regularly monitored for blood and liver reconstitution by blood immunophenotyping and measurements of serum human-albumin levels, respectively. Mice with a successful reconstitution of more than 15% for both human immune cells and HEPs are intraperitoneally injected with HIV-1. The effect of HIV on the liver can be assessed as early as 4 – 5 weeks postinfection. It is critical to note that, because HIV-1 is used, all necessary precautions must be taken while handling the virus and injecting it into mice.
Protocol
This protocol has been approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Nebraska Medical Center.
NOTE: Obtain approval from the local IACUC before performing experiments on animals.
1. Processing of Umbilical Cord Blood and the Isolation of Human HSPCs
- Perform all steps of the protocol under sterile conditions in laminar flow cabinets.
- Take umbilical cord blood (CB) collected in heparinized tubes and make the volume up to 35 mL by adding phosphate-buffered saline (PBS). Layer the sample on top of the lymphocytes separation medium (LSM) as illustrated in Figure 1 and centrifuge the LSM with the layered CB at 400 x g for 35 min at 4 °C with no brakes.
NOTE: Dilute the blood carefully and gently to avoid mixing at the interface. - Remove the top LSM and plasma layer carefully and transfer the white buffy coat interface to a new tube using a transfer pipette.
- Resuspend the buffy coat in 30 – 40 mL of ice-cold buffer (PBS + 0.5% bovine serum albumin [BSA] + 2 mM ethylenediaminetetraacetic acid [EDTA]). Using a pipette, combine 20 µL of the cell suspension with 20 µL of 0.4% trypan blue and pipette 10 µL of the mixture into the outer opening of either of the two chambers of a counting slide and insert the slide in an automated cell counter to count the cells.
NOTE: Use ice-cold buffer in all steps, as it helps keep the cells viable. - Centrifuge the cells at 300 x g for 10 min at 4 °C and aspirate the supernatant carefully. Then, add 300 µL of ice-cold buffer.
- Add 100 µL of human Fc receptor blocking reagent and 100 µL of monoclonal mouse anti-human CD34 antibody-conjugated microbeads for up to 1 x 108 cells (see the Table of Materials). Incubate for 30 min at 4 °C, add 10 mL of ice-cold buffer to wash the cells, and centrifuge at 300 x g for 10 min at 4 °C.
NOTE: Scale this up according to the cell number if more than 1 x 108 cells are present. - Carefully remove the supernatant, resuspend the pellet in 500 µL of buffer, and proceed with the magnetic separation step to enrich HSPCs.
- Place a positive selection LS column (see the Table of Materials) in the magnetic-activated cell sorting field and pass it through with 3 mL of buffer.
- Load the sample to the LS column that can entrap microbeads bound to human CD34+ in samples and allow it to flow under the influence of gravity into the collection tube.
- Wash the column 3x with buffer and collect the elute in the same collection tube of the CD34– fraction of cells.
- Plunge the column with 5 mL of buffer to elute the CD34+ cells into a new collection tube. Repeat the procedure to achieve a purity of >90%.
- Count the eluted CD34+ cells using trypan blue dye in a hemocytometer. After counting, centrifuge the CD34+ cells at 300 x g for 5 min and discard the supernatant.
- Resuspend the CD34+ cells in 25 µL of PBS for an injection to be used immediately in transplantation or cryopreserve the cells at a concentration of 1-2 million/mL in freezing medium (Roswell Park Memorial Institute medium [RPMI 1640 medium] + 50% fetal bovine serum (FBS) + 10% dimethyl sulfoxide [DMSO]) for further use in transplantation.
NOTE: Always recount viable cells before using them in transplantation. - To check the purity of the CD34+ elute, take 50 µL of the suspension and incubate it with 10 µL of PE-conjugated anti-human CD34 antibody for 30 min at 4 °C. After the antibody incubation, wash the stained cells with PBS, resuspend them in 100 µL of PBS, and then, proceed to perform flow cytometry. Add an additional tube of cells with no antibody to design the gate in the flow cytometer.
- After acquisition, analyze the data by selecting the region of interest on a forward scatter (FSC) and side scatter (SSC) plot, followed by gating for single cells on FSC-area and FSC-height plots. Gate CD34-positive cells on single cells in the PE channel and SSC-area plot.
2. Preparation of Human Hepatocytes for Transplantation
- Remove the cryopreserved hepatocytes from the liquid nitrogen, quickly submerge the vial in the water bath, and thaw for approximately 90 – 120 s.
- Remove the vial cap and pour the thawed hepatocytes into the 50 mL conical tube of the warmed thawing medium.
- Suspend the cells by rocking the 50 mL tube by hand for a few seconds.
NOTE: Do not vortex the tube. - Pellet the cells at 100 x g for 8 min at room temperature.Wash the pelleted cells in PBS with 0.1% BSA and pool them with either fresh or thawed HSPCs (ratio 10:1) in PBS in a final volume of 80 µL/mouse.
3. Animal Handling, Screening, Genotyping, and Treatment for Human HSPC and Hepatocyte Transplantation
- Animal handling
- As a result of severe immunodeficiency, breed, house, and handle TK-NOG mice under aseptic conditions.
- Always wear a lab coat, gloves, shoe covers, and a face mask to prevent infection with potentially pathogenic microorganisms.
- Use sterile gloves and instruments for surgery and handle the animals aseptically throughout the surgery.
- Selecting TK-NOG mice for the experiment
- Maintain the TK-NOG strain colony by breeding female TK-NOG mice with male non-TK-NOG littermates and select transgenic offspring by genotyping.
NOTE: Perform genotyping (see step 3.3) to determine the presence or absence of the transgene in newborn male and female mice at the time of weaning. - Select males at 6 – 8 weeks of age for transplantation due to their high sensitivity to the GCV-mediated depletion of HSV-TK transgene-expressing hepatocytes21.
- Ear-tag the mice at the time of weaning or surgery to ease identification. Note down the weight and health status of the animals.
- Maintain the TK-NOG strain colony by breeding female TK-NOG mice with male non-TK-NOG littermates and select transgenic offspring by genotyping.
- Genotyping for the presence of the HSV-TK transgene using quantitative real-time polymerase chain reaction
- Perform genotyping at the time of weaning (usually at 3 – 4 weeks of age). For genotyping, cut a piece of the mouse ear in a laminar flow biological safety cabinet to maintain sterility and extract genomic DNA by using a genomic DNA isolation kit.
- Amplify genomic DNA extracted from ear piece in a 20 µL reaction mixture to screen for HSV-TK transgene under control of human albumin promoter by adding 1 µL of forward primer 5'-CCATGCACGTCTTTATCCTGG-3', 1 µL of reverse primer 5'-TAAGTTGCAGCAGGGCGTC-3', 0.5 µL of FAM probe 5′-FAM-AATCGCCCGCCGGCTGC-MGB-3', and 10 µL of master mix on a real-time polymerase chain reaction (PCR) instrument22.
- Set the real-time PCR settings as follows: 60 °C for 30 s (preread stage), 95 °C for 10 min (hold stage), 40 cycles of 95 °C for 15 s and 60 °C for 1 min (PCR stage), and 60 °C for 30 s (postread stage).
NOTE: A cycle of threshold (Ct) below 22 is considered positive for HSV-TK transgene.
- Treatment using ganciclovir and treosulfan
- Using 27 G needle, inject the TK-NOG mice with intraperitoneal GCV injections (6 mg/kg) 2x a day at day 7 and at day 5 in 100 µL of saline before surgery to deplete the mice's transgenic parenchymal cells (as shown in the experimental strategy in Figure 2)23.
- On days 3, 2, and 1 before the surgery, precondition mice with nonmyeloablative intraperitoneal doses of treosulfan (1.5 g/kg/day) in 100 µL of saline, using a 27 G needle.
- One day before the surgery, draw two to three drops (~100 µL) of blood from the submandibular vein by pricking it with a 5 mm lancet, and isolate the serum by centrifuging (1,500 x g for 10 min at 4 °C) for the alanine aminotransferase (ALT) assay to assess the degree of liver damage.
- Preparation for the surgery
- Use clippers to shave the mouse's fur surrounding the incision site (at the left of the peritoneal wall) before surgery.
- Adjust the oxygen flow to 1 L/min and the isoflurane flow to 3% – 5% in an induction chamber using a mouse anesthesia machine. Place one mouse at a time in the induction chamber for anesthesia.
- Attach the one end of a sterile extension tube (with a holding capacity of 550 µL of suspension; see the Table of Materials for specifications) to a 30 G needle and the other end to a 1 mL syringe.
- Fill the syringe with the suspension (80 µL/mouse) of pooled HEPs and HSPCs (see section 2), fit the syringe in the notch of a repetitive dispensing pipette, and adjust the dispenser to dispense 10 µL in each press.
- Once the mice are anesthetized (usually after 3 – 4 min), switch the isoflurane flow to the nose cone and reduce the isoflurane flow rate to 2% – 3%.
- Intrasplenic transplantation of human HSPCs and hepatocytes in mice
- Perform all surgery steps in a laminar flow cabinet under sterile conditions.
- Place a clean sterile drape over the working surface and scrub the left side of the body of each mouse with 10 % povidone-iodine followed by 70 % isopropyl alcohol, before making an incision.
- Make a small incision (~1 – 1.5 cm in length and 5 mm deep) in the skin, muscle, and peritoneum at the left of the peritoneal wall with Vanna's type scissors to enter the peritoneal cavity approximately 5 mm below the lower edge of the rib cage.
- Locate the spleen, pull it slightly with forceps to the operating area for easy access, and insert the 30 G needle into the lower pole of the spleen.
- Unlock the plunger of the dispensing pipette and dispense 10 µL of the volume at a time, with a limit of 60 – 80 µL per spleen. Retract the needle slowly and clip the spleen with ligating clips using a ligation applier.
- Push the spleen back into the body cavity with cotton-tipped applicators wetted with sterile PBS.
- Close the muscle layer of the abdominal wall using an absorbable suture interrupted suture pattern (not continuous). Accomplish skin closure with an interrupted suture pattern using non-absorbable sutures.
- Use warm water circulating pads to protect the mice from hypothermia after surgery.
- Remove non-absorbable sutures 10-14 days after surgery.
- Postoperative care
- When the transplanted animal wakens, inject analgesic buprenorphine (0.1 mg/kg) intraperitoneally, 2x a day for a consecutive 3 days.
- Observe the animals at least 1x a day until they return to normal physical conditions.
NOTE: Check each animal's body weight, since some mice may lose weight postsurgery. Mice typically regain their original weight in 1 to 2 weeks.
4. Engraftment Validation of the Human Liver by ELISA and the Human Immune System by Flow Cytometry
NOTE: Evaluate the reconstitution of the human liver and immune system monthly, starting 1 month posttransplantation by enzyme-linked immunosorbent assay (ELISA) and flow cytometry, respectively.
- Collect blood samples from the submandibular vein using lancets in EDTA tubes, and centrifuge at 1,500 x g for 10 min at 4 °C. Isolate serum to check human albumin levels to assess the engraftment efficiency of the mouse liver for transplanted human hepatocytes, using a human albumin ELISA quantitation set (see the Table of Materials) by following the manufacturer's instructions.
NOTE: Do not discard the pellet and use the pelleted cells for a flow cytometry analysis to evaluate the human immune system reconstitution. - Resuspend the cell pellet without serum in 35 µL of FACS buffer (PBS + 2% FBS) and stained with 5 µL of mouse-specific CD45 (concentration 0.5 mg/mL), 5 µL each of human-specific antibodies CD45 (0.1 mg/mL), CD3 (0.2 mg/mL), CD8 (0.1 mg/mL), and CD19 (0.5 mg/mL), and 20 µL of CD4 (0.25 mg/mL) and CD14 (0.25 mg/mL) each for 30 min at 4 °C, to check the development of a functional immune system from CD34+ HSPCs.
NOTE: Consider adding one additional tube of unstained cells to determine the gating of the stained cells. - After incubation, transfer the stained suspension (~100 µL) in a polystyrene round-bottom flow cytometry tube and use 2 mL of 1x lysis buffer (see the Table of Materials) by diluting 1 part of 10x lysis buffer with 9 parts of distilled water and incubating 10 – 15 min to lyse red blood cells.
NOTE: Observe turbidity to evaluate the red blood cell lysis. Once the sample becomes clear, the lysis is complete. - After the lysis, add 3 mL of the FACS buffer in the tube and centrifuge at 300 x g at 4 °C for 5 min to get a pellet. Repeat the washing by adding 3 mL of FACS buffer to the pellet and centrifuge at 300 x g at 4 °C for 5 min.
- Fix the cells in freshly made 1% paraformaldehyde (PFA) and acquire stained cells on the flow cytometer, analyzed with flow software.
- For the analysis, select lymphocytes gating on an FSC/SSC plot, followed by single-cell gating on FSC-area/FSC-height.
- Further, gate for human-specific CD45 (hCD45) on the single-cell population and include mouse-specific CD45 (mCD45) for the exclusion of cells of murine origin. Strategize gating of the stained population based on the gating of unstained cells.
- Gate hCD45+ cells to determine the frequency of CD3+ T cells and CD19+ B cells. Gate T cells to determine CD4 and CD8 subsets. To evaluate monocytes, gate on hCD45 to determine CD14+ monocytes.
5. HIV Infection of TK-NOG Mice and Its Effect on the Human Liver and Immune System
- Handle the HIV-1 virus and all infected mice in a designated biosafety level 2 facility.
CAUTION: Autoclave and discard all HIV-infected wastes in double biohazard bags. For safety reasons, wear cut-resistant gloves while handing HIV-infected mice. - Wear personal protection equipment (PPE), including a disposable coverall gown, shoe covers, a face mask, and double gloves at all times while working with the virus.
- Select mice with a reconstitution of more than 15% of human CD45+ cells (tested in step 4.7) and with a presence of human albumin in the serum for HIV-1 infection (tested in step 4.1).
- Inject the mice with 1 x 103 to 1 x 104 tissue culture infectious doses 50 (TCID50) HIV-1ADA in a volume of 100 – 200 µL per mouse, intraperitoneally.
- Euthanize the HIV-infected mice 5 weeks postinfection by using isoflurane (with an isoflurane vapor concentration of >5%).
- After euthanizing the mice, collect blood in EDTA tubes by a cardiac puncture for the isolation of serum, to see the effect of HIV-1 on the liver by evaluating human-specific albumin levels by ELISA (see step 4.1) and blood cells to check for changes in the human immune cells using flow cytometry (see steps 4.2 – 4.8).
NOTE: Assess the peripheral viral load 5 weeks postinfection on a bioanalyzer to confirm if the mice are infected. - After drawing blood, excise the liver from the euthanized mice.
- For the liver excision, expose the abdominal cavity by making an incision of 1.5 – 2 cm long and 0.5 cm deep in the skin, muscles, and peritoneum, from the xyphoid. Make a cut perpendicular to the spine between the liver and the diaphragm. Lift the liver and sever any membranes attaching it to the stomach and intestines.
- Collect and fix the liver in 4% paraformaldehyde overnight and follow a standard immunohistochemical protocol to evaluate the effect of HIV on CK18+ hepatocytes by using human-specific CK18 antibody8.
Representative Results
The establishment of a dual humanized mouse model with human liver and immune cells can be easily monitored at each step with very simple ELISA and flow cytometry, respectively. Flow cytometry is regularly performed to evaluate the development of a functional immune system and to see the effect of HIV infection on immune cells. In dual humanized mice, the development of functional immune cells can range from 15% to 90% of the lymphocyte gate. Representative subsets of immune cells are shown in dot plots (Figure 3). For the evaluation of the engraftment of human hepatocytes, ELISA for human-specific albumin levels is performed monthly on mouse serum. Mice engrafted with both HSPCs and HEPs show human-specific albumin levels ranging from ~7 µg/mL to 377 µg/mL at one month, continuing to grow over the time of observation (6 months) (Figure 4). The effect of HIV infection on human immune cells in the blood of dual humanized mice is monitored by flow cytometry and on HEPs in the liver by human-specific albumin ELISA. By 5 weeks, HIV-1 causes a decrease in human albumin levels in the serum, as assessed by ELISA, and there is a depletion of human CK18+ hepatocytes in the liver sections of dual humanized mice, as evaluated by immunohistochemistry (Figure 5). A lower ratio of CD4:CD8 is typically observed, by flow cytometry, in the blood and liver of HIV-infected mice, compared to levels noted in the same mouse before infection (Figure 6). All reagents and materials important for the protocol are discussed in the Table of Materials.
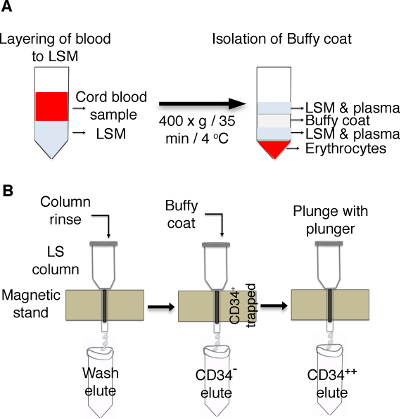
Figure 1: Schematic of the enrichment of CD34+ cells from cord blood. (A) Cord blood is layered on lymphocytes separation medium (LSM) and centrifuged to isolate buffy coat. (B) LS columns are placed on a magnetic stand and rinsed with BSA buffer, followed by adding buffy coat. Cells positive for CD34 are trapped in the columns, and CD34– cells are eluted in separate tubes. Trapped CD34+ cells in column resins are plunged with a plunger, and the cells are collected in a new tube. Please click here to view a larger version of this figure.
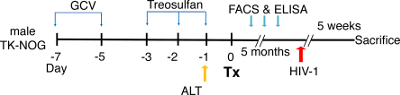
Figure 2: Schematic view of the experimental design for the dual reconstitution of humanized liver and immune system mice, followed by HIV-1 infection. TK-NOG mice are injected with ganciclovir (GCV) at a dose of 6 mg/kg, 2x a day, on day -7 and day -5, followed by a treosulfan injection on days -3, -2 and -1. To screen the mice for the transplantation (Tx), an alanine aminotransferase (ALT) assay is performed one day before the surgery, and mice with ALT levels of >200 and <600 U/L are selected. After transplantation, the mice are checked for a reconstitution of the human immune system by flow cytometry (FACS) and for liver reconstitution by assessing their albumin level using ELISA. The mice are infected with HIV-1 5 weeks before they are sacrificed. Please click here to view a larger version of this figure.
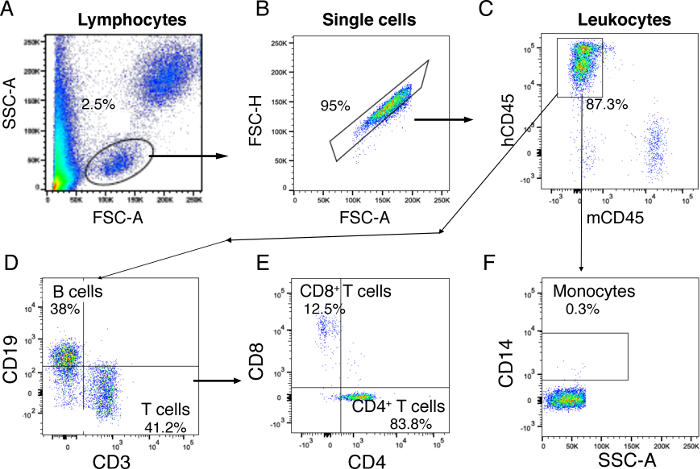
Figure 3: Flow cytometry analysis gating strategy for the human cell distribution of blood. (A) First, lymphocytes are gated on whole blood based on FSC-A and SSC-A. (B) Single cells are gated on lymphocytes. (C) Human CD45+ leukocytes are gated on single cells using mouse CD45 and human CD45. (D) CD3+ T cells and CD19+ B cells are identified on gated CD45+ human leukocytes. (E) CD4+ T helper cells and CD8+ cytotoxic T cells are identified in gated CD3+ T cells. (F) CD14+ monocytes are gated from human CD45+ leukocytes. The results represented here are from one mouse transplanted with dual human hepatocytes and HSPCs. Please click here to view a larger version of this figure.
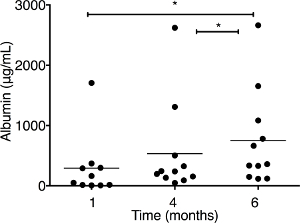
Figure 4: Albumin concentration is measured by ELISA in the serum of dual humanized mice. The mice are transplanted with both human hepatocytes (HEPs) and CD34+ hematopoietic stem/progenitor cells (HSPCs) (n = 11). Serum is collected at different times at 1, 4, and 6 months posttransplantation, and dilutions are made to adjust the unknown sample concentrations in the range of standards. Each symbol represents an individual mouse value. The results represent the median, as well as individual values. * P < 0.05, by one-way ANOVA. This figure has been modified from Dagur et al.8. Please click here to view a larger version of this figure.
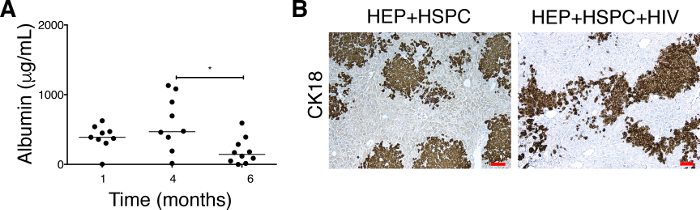
Figure 5: Effect on HIV-1 on albumin levels in serum and the depletion of CK18+ human hepatocytes in the liver of dual humanized mice. (A) Albumin concentrations are monitored in uninfected mice (n = 9) transplanted with both human HEPs and HSPCs at 1 and 4 months. The mice are infected (n = 10) with HIV at 4 – 5 months posttransplantation and sacrificed 5 weeks postinfection. Each symbol represents an individual mouse value. The results represent the median, as well as individual values. * P < 0.05, by one-way ANOVA. This figure has been modified from Dagur et al.8. (B) Five-micron liver sections from uninfected (HEPs + HSPCs, left panel) and HIV-infected TK-NOG mice (HEPs + HSPCs + HIV, right panel) are fixed, paraffin embedded, and stained for anti-human cytokeratin-18 (CK18) antibody. HIV-1 causing a depletion of CK18+ hepatocytes is evidenced by a less occupied area by the CK18+ human cells. The results represented here are from one uninfected and one HIV-infected mouse transplanted with dual human hepatocytes and HSPCs. Scale bars = 100 µm. Please click here to view a larger version of this figure.
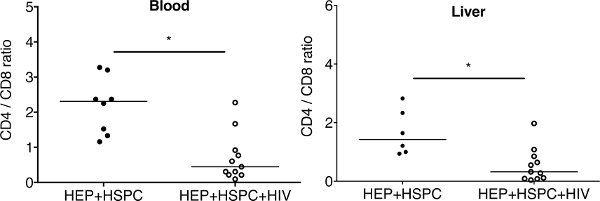
Figure 6: Ratio of CD4+ cells to CD8+ T cells in peripheral blood and in the liver of dual reconstituted uninfected and HIV-1-infected mice. For dual reconstituted uninfected mice: closed circle; HEPs + HSPCs; blood n = 7; liver n = 6. For HIV-1 infected mice: open circles; HEPs + HSPCs + HIV; blood n = 10; liver n = 11. The results represent the median, as well as individual values. * P < 0.05, by one-way ANOVA test between HIV-infected and uninfected mice. This figure has been modified from Dagur et al.8. Please click here to view a larger version of this figure.
Discussion
The liver is compromised and damaged in HIV-infected patients24. Experimental small animal models for studying human liver diseases in the presence of HIV-1 is extremely limited, despite the availability of a few cotransplanted animal models with CD34+ HSPCs and hepatocytes7,12,25. In in vitro experiments, hepatocytes are shown to have low-level HIV-1 infection26. Humanized mice that carry both types of human cells are a desirable model. The liver of mice reconstituted with only human immune system has been shown to be affected by HIV infection under the experimental depletion of human regulatory T cells20,27. However, the difference in immune and functional properties of mouse and human hepatocytes may underline the differences in their responses to HIV-1 and immune cells. In this review, a protocol is described to reconstitute both human immune system and liver and to address HIV-1-associated liver immunopathology, as observed in HIV-1-infected patients. TK-NOG male mice were selected due to their liver-selective high mRNA expression of HSV-TK transgene and the susceptibility of GCV toxicity to mouse transgenic liver21. Moreover, they can be maintained for long periods after transplantation without the use of exogenous drugs and do not develop spontaneous systemic diseases28. To establish human immune system and liver reconstitution, ablation of the mouse immune system and damage to mouse-specific liver cells are required and achieved using nonmyeloablative doses of treosulfan and GCV, as shown previously in TK-NOG male mice13,23. Mice are injected with GCV and treosulfan at the age of 6 – 8 weeks, as the expression of transgene and GCV-induced hepatic injury as assessed by ALT levels are optimal, then, for providing niche-to-transplanted human cells21. Mice showing ALT levels of >200 U/L, but <600 U/L, are usually selected for transplantation. Mice showing ALT levels of >600 U/L are at a greater risk of death as human hepatocytes are not able to rescue the damaged mouse liver function.
Currently, dual humanization is shown by the transplantation of human CD34+ HSPCs and fetal liver cells; however, the manipulation of newborn animals creates technical problems13,14. HSPCs can be derived or isolated from multiple sources, such as fetal liver cells (FLCs), embryonic stem cells (ESCs), and CB. However, ethical issues constrain the use of ESCs and FLCs. CB has no such restriction and is a most useful alternative to obtain HSPCs, as well as being a precious source of primitive hematopoietic stem and progenitor cells that can reconstitute the functional immune system. Cord blood should not be older than one day when used to isolate HSPCs, as the yield of HSPCs is highly affected by age. The purity of the isolated HSPCs needs to be checked before cryopreserving the cells. The cross-contamination of CD3+ T cells is avoided, as it may lead to systemic mouse graft-versus-host disease and acute allorejection of HEPs while transplanting with mismatched cells.
Commercially available hepatocytes were used as a source for liver reconstitution8,13. Adult hepatocytes are preferred for establishing liver reconstitution due to their increased efficiency in engraftment and sustainability for a long period of time29.
The presence of human immune system in a mouse model increased ALB levels, as shown previously30,31. However, the efficiency of hepatocytes and immune system reconstitution may vary with different sources of donor cells and depend on the recipient mouse. So, each mouse needs to be assessed for engraftment, and the most critical part is to utilize the antibodies or reagent that are human-specific and do not cross-react with mouse cells. The human-specific reagents and antibodies used in the study presented here are detailed in the Table of Materials. If antibodies other than provided in the Table of Materials are used for the study, be sure to check for the human specificity.
The optimal condition would be the transplantation of syngeneic cells; however, that is technically difficult to achieve. Wherever possible, HSPCs and hepatocytes should be pooled from donors with partially matched human leukocyte class-1 antigens (like HLA-A2).
To screen mice for HIV studies, blood is drawn at multiple time points to determine the optimal immune and liver reconstitution; flow cytometry and ELISA are preferred as they can be performed with only a little amount of blood. Blood cells and serum from the same sample could be used for flow cytometry and ELISA, respectively. It is important to make proper dilutions of serum at each time point (1,000 – 40,000 range) to evaluate ALB levels so that the unknown concentrations can be brought within the range of standard concentrations (kit range: 6.25 – 400 ng/mL).
Proinflammatory cytokines in response to HIV-1 infection in the presence of human immune system can also be useful in addressing the interaction of hepatocytes and immune cells. The model is useful for showing the immunopathogenesis of HIV-1-induced liver disease, given that it recapitulates liver damage in the same manner as in humans, evidenced by a low ratio of CD4:CD8, a decrease in ALB levels, human hepatocyte death, and liver immune activation. The model also has some limitations, such as a low level of cytotoxic T cells activity and impaired immunoglobulin class switching. Due to the presence of both human immune system and liver, the model presented here is promising for studi coinfections of HIV-1 and hepatitis viruses, chronic hepatitis infection (to clarify the mechanisms of the anti-hepatitis immune response), and as a cirrhosis model.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institute of Health grant R24OD018546 (to L.Y.P. and S.G.). The authors would like to thank Weizhe Li, Ph.D., for the help in surgical procedures, Amanda Branch Woods, B.S., Yan Cheng for immunohistology, UNMC flow cytometry research facility members Director Phillip Hexley, Ph.D., Victoria B. Smith, B.S., and Samantha Wall, B.S., UNMC advanced microscopy core facility members Janice A. Taylor, B.S., and James R. Talaska, B.S., for the technical support. The authors acknowledge Drs. Mamoru Ito and Hiroshi Suemizu from CIEA for providing TK-NOG mice and Dr. Joachim Baumgart for providing treosulfan. The authors thank Dr. Adrian Koesters, UNMC, for her editorial contribution to the manuscript.
Materials
| 27G1/2" needles | BD biosciences | 305109 | |
| 30G1/2" needles | BD biosciences | 305106 | |
| 5 mL polystyrene round-bottom tube 12 x 75 mm style | Corning | 352054 | |
| BD 1 mL Tuberculin Syringe Without Needle | BD biosciences | 309659 | |
| BD FACS array bioanalyzer | BD Biosciences | For purity check of eluted CD34+ cells | |
| BD FACS array software | BD Biosciences | Software to analysis acquired CD34+ cell on FACS array | |
| BD FACS lysing solution | BD Biosciences | 349202 | To lyse red blood cells |
| BD LSR II | BD Biosciences | Instrument for acquisiton of flow cytometry samples | |
| BD Vacutainer Plastic Blood Collection Tube | BD biosciences | BD 367874 | To collect Cord blood |
| Bovine Serum Albumin | Sigma-aldrich | A9576 | |
| Buprenorphine | Controlled substance and pain-killer | ||
| CD14-PE | BD Biosciences | 555398 | Specific to human |
| CD19-BV605 | BD Biosciences | 562653 | Specific to human |
| CD34 MicroBead Kit, human | Miltenyi Biotec | 130-046-702 | For isoation of CD34+ HSPC |
| CD34-PE, human | Miltenyi Biotec | 130-081-002 | Antibody used for purity check of eluted CD34+ cells |
| CD3-AF700 | BD Biosciences | 557943 | Specific to human |
| CD45-PerCPCy5.5 | BD Biosciences | 564105 | Specific to human |
| CD4-APC | BD Biosciences | 555349 | Specific to human |
| CD8-BV421 | BD Biosciences | 562428 | Specific to human |
| Cell counting slides | Bio-rad | 1450015 | |
| ChargeSwitch gDNA Mini Tissue Kit | Thermofisher scientific | CS11204 | for extraction of genomic DNA from ear piece |
| Cobas Amplicor system v1.5 | Roche Molecular Diagnostics | bioanalyzer to measure viral load | |
| Cotton-tipped applicators | McKesson | 24-106-2S | |
| Cytokeratin-18 (CK18) | DAKO | M7010 | Specific to human |
| DMSO (Dimethyl sulfoxide) | Sigma-aldrich | D2650-5X5ML | |
| Extension set Microbore Slide Clamp(s) Fixed Male Luer Lock. L: 60 in L: 152 cm PV: 0.55 mL Fluid Path Sterile | BD biosciences | 30914 | Attached to dispensing pippet and to load with HSPC and HEP suspesion |
| FACS Diva version 6 | BD Biosciences | flow cytometer software required for acqusition of sample | |
| Fetal Bovine Serum (FBS) | Gibco | 10438026 | |
| FLOWJO analysis software v10.2 |
FLOWJO, LLC | flow cytometry analysis software | |
| Ganciclovir | APP Pharmaceuticals, Inc. | 315110 | Prescripition drug |
| Greiner MiniCollect EDTA Tubes | Greiner bio-one | 450475 | |
| Hepatocytes thawing medium | Triangle Research Labs | MCHT50 | |
| Horizon Open Ligating Clip Appliers | Teleflex | 537061 | To hold the ligating clips |
| Hospira Sterile Water for Injection | ACE surgical supply co. Inc. | 001-1187 | For dilution of Buprenorphine (pain-killer) |
| Human Albumin ELISA Quantitation Set | Bethyl laboratories | E80-129 | For assesing human albumin levels in mouse serum |
| Human hepatocyte | Triangle Research Labs | HUCP1 | Cryopreserved human hepatocytes, induction qualified |
| Iris Scissors, Straight | Ted Pella, Inc. | 13295 | |
| Lancet | MEDIpoint | Goldenrod 5 mm | |
| LS columns | Miltenyi Biotec | 130-042-401 | Used to entrap CD34+ microbeads (positive selection) |
| Lymphocyte Separation Medium (LSM) | MP Biomedicals | 50494 | For isoation of lymphocytes from peripheral blood |
| MACS MultiStand | Miltenyi Biotec | 130-042-303 | holds Qudro MACS seperator and LS columns |
| McPherson-Vannas Micro Dissecting Spring Scissors | Roboz Surgical Instrument Co. | RS-5605 | Used to make an incision on skin to expose spleen |
| Micro Dissecting Forceps | Roboz Surgical Instrument Co. | RS-5157 | to hold and pull out spleen from peritoneal cavity |
| mouse CD45-FITC | BD Biosciences | 553080 | mouse-specific |
| PBS (Phosphate Buffered Saline) | Hyclone | SH30256.02 | |
| Qudro MACS separator | Miltenyi Biotec | 130-090-976 | holds four LS columns |
| RPMI 1640 medium | Gibco | 11875093 | |
| StepOne Plus Real Time PCR | Applied Biosystems | Instrument used to genotype | |
| Stepper Series Repetitive Dispensing Pipette 1ml | DYMAX CORP | T15469 | Used to dispense HSPC and HEP supension in controlled manner |
| Suturevet PGA synthetic absorbale suture | Henry Schein Animal Health | 41178 | Suturing of skin and peritoneum |
| TaqMan Gene Expression Master Mix | Thermofisher scientific | 4369016 | |
| TC20 automated cell counter | Bio-rad | 1450102 | |
| TK-NOG mice | Provided by the Central Institute for Experimental Animals (CIEA, Japan; Drs. Mamoru Ito and Hiroshi Suemizu) | ||
| Treosulfan | Medac GmbH | Provided by Dr. Joachim Baumgart (medac GmbH) | |
| Trypan Blue | Bio-rad | 1450022 | |
| Vannas-type Micro Scissors, Straight, 80mm L | Ted Pella, Inc. | 1346 | Used to make an incision on skin to expose spleen |
| Weck hemoclip traditional titanium ligating clips | Esutures | 523700 | To ligate the spleen post-injection |
References
- Smith, C., et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 24 (10), 1537-1548 (2010).
- Puoti, M., et al. Mortality for liver disease in patients with HIV infection: a cohort study. Journal of Acquired Immune Deficiency Syndromes. 24 (3), 211-217 (2000).
- Rodriguez-Mendez, M. L., Gonzalez-Quintela, A., Aguilera, A., Barrio, E. Prevalence, patterns, and course of past hepatitis B virus infection in intravenous drug users with HIV-1 infection. The American Journal of Gastroenterology. 95 (5), 1316-1322 (2000).
- Scharschmidt, B. F., et al. Hepatitis B in patients with HIV infection: relationship to AIDS and patient survival. Annals of Internal Medicine. 117 (10), 837-838 (1992).
- Lacombe, K., Rockstroh, J. HIV and viral hepatitis coinfections: advances and challenges. Gut. 61, 47-58 (2012).
- Brehm, M. A., Jouvet, N., Greiner, D. L., Shultz, L. D. Humanized mice for the study of infectious diseases. Current Opinion in Immunology. 25 (4), 428-435 (2013).
- Billerbeck, E., et al. Humanized mice efficiently engrafted with fetal hepatoblasts and syngeneic immune cells develop human monocytes and NK cells. The Journal of Hepatology. 65 (2), 334-343 (2016).
- Dagur, R. S., et al. Human hepatocyte depletion in the presence of HIV-1 infection in dual reconstituted humanized mice. Biology Open. 7 (2), (2018).
- Gaska, J. M., Ploss, A. Study of viral pathogenesis in humanized mice. Current Opinion in Virology. 11, 14-20 (2015).
- Gorantla, S., Poluektova, L., Gendelman, H. E. Rodent models for HIV-associated neurocognitive disorders. Trends in Neurosciences. 35 (3), 197-208 (2012).
- Xu, D., et al. Chimeric TK-NOG mice: a predictive model for cholestatic human liver toxicity. The Journal of Pharmacology and Experimental Therapeutics. 352 (2), 274-280 (2015).
- Washburn, M. L., et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 140 (4), 1334-1344 (2011).
- Gutti, T. L., et al. Human hepatocytes and hematolymphoid dual reconstitution in treosulfan-conditioned uPA-NOG mice. The American Journal of Pathology. 184 (1), 101-109 (2014).
- Strick-Marchand, H., et al. A novel mouse model for stable engraftment of a human immune system and human hepatocytes. PLoS One. 10 (3), 0119820 (2015).
- Keng, C. T., et al. Characterisation of liver pathogenesis, human immune responses and drug testing in a humanised mouse model of HCV infection. Gut. 65 (10), 1744-1753 (2016).
- Li, F., Nio, K., Yasui, F., Murphy, C. M., Su, L. Studying HBV Infection and Therapy in Immune-Deficient NOD-Rag1-/-IL2RgammaC-null (NRG) Fumarylacetoacetate Hydrolase (Fah) Knockout Mice Transplanted with Human Hepatocytes. Methods in Molecular Biology. 1540, 267-276 (2017).
- Poluektova, L. Y., Garcia, J. V., Koyanagi, Y., Manz, M. G., Tager, A. M. . Humanized Mice for HIV Research. , (2014).
- Cheng, L., Ma, J., Li, G., Su, L. Humanized Mice Engrafted With Human HSC Only or HSC and Thymus Support Comparable HIV-1 Replication, Immunopathology, and Responses to ART and Immune Therapy. Frontiers in Immunology. 9, 817 (2018).
- Zhang, L., Su, L. HIV-1 immunopathogenesis in humanized mouse models. Cellular & Molecular Immunology. 9 (3), 237-244 (2012).
- Nunoya, J., Washburn, M. L., Kovalev, G. I., Su, L. Regulatory T cells prevent liver fibrosis during HIV type 1 infection in a humanized mouse model. The Journal of Infectious Diseases. 209 (7), 1039-1044 (2014).
- Hasegawa, M., et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochemical and Biophysical Research Communications. 405 (3), 405-410 (2011).
- Higuchi, Y., et al. The human hepatic cell line HepaRG as a possible cell source for the generation of humanized liver TK-NOG mice. Xenobiotica. 44 (2), 146-153 (2014).
- Kosaka, K., et al. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochemical and Biophysical Research Communications. 441 (1), 230-235 (2013).
- Crane, M., Iser, D., Lewin, S. R. Human immunodeficiency virus infection and the liver. World Journal of Hepatology. 4 (3), 91-98 (2012).
- Bility, M. T., Li, F., Cheng, L., Su, L. Liver immune-pathogenesis and therapy of human liver tropic virus infection in humanized mouse models. Journal of Gastroenterology and Hepatology. 28, 120-124 (2013).
- Kong, L., et al. Low-level HIV infection of hepatocytes. Virology Journal. 9, 1-7 (2012).
- Dash, P. K., et al. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 26 (17), 2135-2144 (2012).
- Sun, S., Li, J. Humanized chimeric mouse models of hepatitis B virus infection. International Journal of Infectious Diseases. 59, 131-136 (2017).
- Shafritz, D. A., Oertel, M. Model systems and experimental conditions that lead to effective repopulation of the liver by transplanted cells. The International Journal of Biochemistry & Cell Biology. 43 (2), 198-213 (2011).
- Almeida-Porada, G., Porada, C. D., Chamberlain, J., Torabi, A., Zanjani, E. D. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 104 (8), 2582-2590 (2004).
- Streetz, K. L., et al. Hepatic parenchymal replacement in mice by transplanted allogeneic hepatocytes is facilitated by bone marrow transplantation and mediated by CD4 cells. Hepatology. 47 (2), 706-718 (2008).

