Expression and Purification of the Human Lipid-sensitive Cation Channel TRPC3 for Structural Determination by Single-particle Cryo-electron Microscopy
Summary
This protocol describes techniques used to determine ion channel structures by cryo-electron microscopy, including a baculovirus system used to efficiently express genes in mammalian cells with minimum effort and toxicity, protein extraction, purification, and quality checking, sample grid preparation and screening, as well as data collection and processing.
Abstract
Transient receptor potential channels (TRPCs) of the canonical TRP subfamily are nonselective cation channels that play an essential role in calcium homeostasis, particularly store-operated calcium entry, which is critical to maintaining proper function of synaptic vesicle release and intracellular signaling pathways. Accordingly, TRPC channels have been implicated in a variety of human diseases including cardiovascular disorders such as cardiac hypertrophy, neurodegenerative disorders such as Parkinson's disease, and neurologic disorders such as spinocerebellar ataxia. Therefore, TRPC channels represent a potential pharmacologic target in human diseases. However, the molecular mechanisms of gating in these channels are still unclear. The difficulty in obtaining large quantities of stable, homogeneous, and purified protein has been a limiting factor in structure determination studies, particularly for mammalian membrane proteins such as the TRPC ion channels. Here, we present a protocol for the large-scale expression of mammalian ion channel membrane proteins using a modified baculovirus gene transfer system and the purification of these proteins by affinity and size-exclusion chromatography. We further present a protocol to collect single-particle cryo-electron microscopy images from purified protein and to use these images to determine the protein structure. Structure determination is a powerful method for understanding the mechanisms of gating and function in ion channels.
Introduction
Calcium is involved in most cellular processes including signaling cascades, transcription control, neurotransmitter release, and hormone molecule synthesis1,2,3. The homeostatic maintenance of cytosolic free calcium is crucial to the health and function of cells. One of the major mechanisms of intracellular calcium homeostasis is store-operated calcium entry (SOCE), a process in which depletion of calcium stored in the endoplasmic reticulum (ER) triggers the opening of ion channels on the plasma membrane to facilitate the replenishment of ER calcium, which can then be used in further signaling4,5,6. Transient receptor potential channels (TRPCs), which are calcium-permeable channels belonging to the TRP superfamily, have been identified as a major participant in SOCE7,8,9 .
Among the seven members in the TRPC family, TRPC3, TRPC6, and TRPC7 form a homologue subgroup, and they are unique in the ability to be activated by the lipid secondary messenger diacylglycerol (DAG), a degradation product of the signaling lipid phosphatidylinositol 4,5-bisphosphate (PIP2)10,11. TRPC3 is highly expressed in smooth muscle and in the cerebral and cerebellar regions of the brain, where it plays essential roles in calcium signaling that impact neurotransmission and neurogenesis12,13. Dysfunction of TRPC3 has been linked to central nervous system disorders, cardiovascular disorders, and certain cancers such as ovarian adenocarcinoma14,15,16. Therefore, TRPC3 holds promise as a pharmaceutical target for treatment of these diseases. The development of specifically targeted drugs acting on TRPC3 has been limited by a lack of understanding of its molecular activation mechanisms, including lipid binding sites17,18. We have reported the first atomic-resolution structure of the human TRPC3 channel (hTRPC3) and its two lipid binding sites in a closed state, providing important insights into these mechanisms19.
The key factor for determining the structure of a membrane protein at high resolution is to obtain protein of high quality. The corresponding screening of expression and purification conditions necessary to obtain high quality protein can be a time-consuming and costly endeavor. Here we present a protocol describing in detail how we identify the optimal conditions for the expression and purification of hTRPC3, which behaved poorly in our initial screening. We present several key points on how to troubleshoot and optimize the protein behavior, which lay a solid foundation for our cryo-electron microscopy (cryo-EM) studies. We use a modified baculoviral generating vector (pEG), developed by Gouaux and colleagues, which is optimized for screening assays and efficient generation of baculovirus in mammalian cells20. This expression method is appropriate for rapid and cost-effective overexpression of proteins in the mammalian cell membrane. We combine the use of this vector with a fluorescence-detection size-exclusion chromatography-based (FSEC) prescreening method21. This method uses a green fluorescent protein (GFP) tag fused to the construct of interest and improves visualization of the target protein in small, whole-cell solubilized samples. This allows for screening of protein stability in the presence of different detergents and additives, with thermostabilizing mutations, and allows the use of a small number of cells from small-scale transient transfection. In this way, a multitude of conditions can be rapidly screened before moving to a large-scale protein purification. Following expression, screening, and purification, we present a protocol for obtaining and processing images from cryo-EM to generate a de novo structural determination of the protein. We believe that the approaches described here will serve as a generalizable protocol for structural studies of TRP channel receptors and other membrane proteins.
Protocol
1. Transformation of DH10α Competent Cells to Produce Bacmid DNA
- Synthesize the gene of interest and subclone it into a modified version of the pEG vector containing a twin strep-tag, a His8-tag, and GFP with a thrombin cleavage site at the N terminus (pFastBacI)20.
- Transform competent cells by adding 5 ng of plasmid containing a desired gene in pFastBacI to 50 μL of DH10α cells in a 1.5 mL tube and incubate for 10 min on ice. Heat shock the cells for 45 s at 42 °C. Add 200 μL of super optimal broth with catabolic repressor (SOC) medium to the tube and incubate for 4 – 8 h at 37 °C in an orbital shaker at 225 rpm.
- Plate 5 μL of cells on a bacmid LB agar plate (50 μg/mL kanamycin, 7 μg/mL gentamicin, 10 μg/mL tetracycline, 100 μg/mL Bluo-gal, and 40 μg/mL isopropyl β-D-1-thiogalactopyranoside [IPTG], agar).
- Incubate the plate for 48 h at 37 °C.
NOTE: The Bluo-gal indicator stains colonies that are still expressing lacZ (vector insertion unsuccessful), allowing for selection of white (successfully transformed) colonies. - Carefully select an isolated white colony, avoiding any white colonies that are in contact with blue colonies, and grow cells overnight in 6 mL of bacmid LB medium (50 μg/mL kanamycin, 7 μg/mL gentamicin, 10 μg/mL tetracycline) at 37 °C in an orbital shaker at 225 rpm.
2. Bacterial Preparation for Isolation of Bacmid DNA
- To isolate bacmid DNA, spin down Escherichia coli cells for 10 min at 2880 x g.
- Discard the supernatant and resuspend the pellet in 200 µL of cell resuspension solution from the miniprep kit (see Table of Materials) by pipetting. Be sure the pellet is fully and homogenously suspended. Then, transfer the cell suspension into 1.5 mL tubes.
- Add 200 µL of the cell lysis solution from the miniprep kit and mix by inverting the tube a few times. Incubate for up to 5 min at room temperature (RT) to lyse the cells. Add 200 µL of neutralization solution from the miniprep kit and mix by inverting tube a few times to stop the lysis reaction.
- Spin down for 10 min at 21,130 x g in a table-top centrifuge. Collect 600 μL of supernatant in a 2 mL tube. Add 600 μL phenol:chloroform:isoamyl alcohol solution (see Table of Materials) and mix thoroughly to extract the DNA from the remainder of the cell lysis products.
CAUTION: Phenol:chloroform:isoamyl alcohol solution is toxic by inhalation, in contact with skin, and if swallowed. It can cause chemical burns and may be carcinogenic. Wear gloves and a buttoned lab coat. Work in a fume hood. Dispose of this hazardous waste appropriately. - Spin the tube for 10 min at 21130 x g in a table-top centrifuge. Two separate liquid phases will be visible. Carefully transfer 300 μL of the upper aqueous phase to a new tube. Add 600 μL of 100% ethanol to wash the DNA. Gently invert the tube to mix.
NOTE: Do not vortex, as this can shear bacmid DNA. - Cool tubes by placing them in a -20 °C freezer for 10 min. Spin down for 10 min at 21,130 x g in a table-top centrifuge. Discard the supernatant and preserve the DNA pellet.
- Add 1 mL of 70% ethanol to wash the pellet. Gently invert the tube to mix. Spin for 10 min at 21,130 x g in a table top centrifuge. Discard the supernatant and allow the pellet to air dry for approximately 5 min or until no liquid is visible in the tube and the DNA pellet becomes translucent.
- Resuspend the dry pellet in 50 µL of sterile, DNase-free, deionized water. Measure the DNA concentration.
NOTE: Do not freeze bacmid DNA. Store at 4 °C for up to several days.
3. Transfection of Sf9 Insect Cells with Bacmid to Produce P1 Baculovirus
- Seed 0.9 x 106 Sf9 cells/well in 2 mL of appropriate medium (see Table of Materials) in each well of a 6-well tissue culture plate. Incubate cells at 27 °C for 20 min to promote attachment to the plate.
CAUTION: Cell cultures are a potential biohazard. Work in an approved laminar flow hood using aseptic techniques and check institutional and governmental guidelines for recommended protective clothing and proper disposal of waste prior to performing experiments. - After attachment, add 8 µL of transfection reagent (see Table of Materials) to 100 µL of media for each well of the 6 well plate being transfected in a sterile tube. Add 6 μg of bacmid DNA to 100 µL of medium in a separate sterile tube. Incubate 5 min at RT. Combine the two solutions and incubate for 45 min at RT.
- Replace the medium in the 6 wells with 2 mL of fresh medium. Add the mixture from previous step to each well dropwise (200 µL per well). Gently rock the plate to ensure mixing of the transfection solution into the medium.
NOTE: Do not swirl or shake the plate because this will cause cells to detach. - Incubate cells for 5 d (120 h) in a 27 °C humidified incubator. Check GFP fluorescence before harvesting to verify that virus is being produced in a large percentage of cells; if the percentage is low, extend the incubation time as necessary (see Figure 1C).
- Collect the supernatant containing P1 virus (about 2 mL from each well). Filter the medium containing P1 virus into 2-mL tubes using a 3 mL syringe and small 0.2 µm filter. Add sterile fetal bovine serum (FBS) to a final concentration of 1%.
NOTE: This stock of P1 virus should be stored at 4 °C and be protected from light.
4. Infection of Sf9 Insect Cells with P1 Baculovirus to Produce P2 Baculovirus
- Prepare 200 mL (or desired volume) of Sf9 cells at a concentration of 0.8 – 0.9 x 106 cells/mL in appropriate medium (see Table of Materials) in a flat bottom Erlenmeyer culture flask of sufficient size.
NOTE: For suspension culture, the volume used should not exceed 40% of the total capacity of the flask. - Add 1:2500 ratio (v/v) of P1 virus stock from 3.5 to the Sf9 cell suspension culture. Incubate for the time of optimal virus expression (usually 48 – 120 h depending on the protein construct) at 27 °C in an orbital shaker at 115 rpm.
NOTE: The relative virus expression can be determined by viewing the GFP fluorescence of the virus in a sample of the culture. - Centrifuge the cell suspension for 40 min at 11,520 x g and collect the supernatant containing P2 virus. Filter the supernatant using disposable 0.2 µm filters. Add FBS to a final concentration of 0.5%.
NOTE: This stock of P2 virus should be stored at 4 °C and be protected from light. - Obtain a titer for the P2 virus using Sf9easy cells or a virus counter.
5. Infection of HEK293 Mammalian Cells with P2 Baculovirus for Large-scale Protein Expression
- Prepare a desirable volume of HEK293 mammalian cell suspension culture (4 – 6 L is recommended for preparation of frozen grids) at a concentration of 3.5 – 3.8 x 106 cells/mL in the expression medium (see Table of Materials) supplemented with 1% (v/v) sterile FBS in baffled-bottom Erlenmeyer culture flasks of sufficient size.
NOTE: For suspension culture, the volume used should not exceed 40% of the total volume of the flask. - Add 8% (v/v) of P2 virus stock solution from step 4.3 to the HEK293 cell suspension culture. Incubate at 37 °C in an orbital shaker at 135 rpm.
- Add 10 mM sodium butyrate at 12 – 18 h post-infection. Incubate for the time of optimal protein expression (usually 36 – 72 h) at 30 °C.
- Harvest cells by centrifuging for 20 min at 2,880 x g. Wash cells by resuspending in approximately 100 mL tris-buffered saline (TBS) per liter of cells harvested. Centrifuge again for 20 min at 2,880 x g and collect the cell pellet.
NOTE: The protocol may be paused here. Cell pellets can be snap-frozen in liquid nitrogen and stored at -80 °C until purification.
CAUTION: Liquid nitrogen may cause cryogenic burns or injury. It may cause frostbite. It may displace oxygen and cause rapid suffocation. Wear cold insulating gloves and face shield. - Collect small 1 mL harvests at varying time points and solubilize for 2 h at 4 °C with rocking or stirring in the presence of different detergents and/or additives. These small whole-cell solubilized samples can be clarified by ultracentrifugation at 235,000 × g for 10 min at 4 °C and run as a 30 μL sample on a size-exclusion chromatography (SEC) column (see Table of Materials) to determine the best time for expression and the best solubilization conditions.
NOTE: In the case of hTRPC3, this screening included different buffers with pH values from 4.0 – 9.5 and salt concentrations of 50 – 500 mM; different ionic compositions (such as MgCl2 or NaCl); different detergents with critical micelle concentration (CMC) values of 0.1 – 20 mM; reducing additives such as dithiothreitol, tris(2-carboxyethyl)phosphine, and β-mercaptoethanol; and the calcium-chelating additive ethylenediaminetetraacetic acid (EDTA).
6. Purification of hTRPC3 Protein from the Frozen Cell Pellet
- Thaw the pellet in buffer containing 20 mM Tris (pH 8.0), 500 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.8 μM aprotinin, 2 μg/mL leupeptin, 2 mM pepstatin A, and 1% digitonin, using 100 mL of buffer per liter of cells harvested. Once thawed, ensure homogeneity of the solution by pipetting or stirring. Allow to solubilize for 2 h at 4 °C in a beaker immersed in ice with a stir bar rotating.
- Remove cell debris by ultracentrifugation at 235,000 × g for 1 h at 4 °C. Verify protein quantity by running a 30 μL sample on an SEC column (see Table of Materials) by high performance liquid chromatography (HPLC) and visualize the target protein by the GFP signal output.
- Incubate the solubilized protein (supernatant) with cobalt affinity resin for 1 – 2 h at 4 °C. Verify protein binding to the resin by running a 30 μL sample on an SEC column.
NOTE: If protein binding has occurred, the GFP tagged protein target will be retained on the column, not found in the flow-through. Therefore, no GFP signal will be present at the position corresponding to the target protein size when the flow-through is run on HPLC. - Wash the resin with 10 column volumes of buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 15 mM imidazole, and 0.1% digitonin). Check for protein loss by running a 30 μL sample on an SEC column.
NOTE: If protein loss from the column has occurred, the GFP tagged protein target will be found in the wash buffer that has passed over the column. Therefore, GFP signal will be present at the position corresponding to the target protein size when the wash buffer is run on HPLC. If protein loss has occurred, the imidazole concentration of the wash buffer may need to be lowered to prevent disrupting His tag binding to the affinity column. - Elute the resin-bound hTRPC3 with buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 250 mM imidazole, and 0.1% digitonin). Add thrombin at a 1:20 molar ratio (to cleave the GFP tag) and add 10 mM EDTA (an hTRPC3 stabilizing agent) to the eluted sample and incubate for 3 h at 4 °C. Check that protein has been eluted by running 90 μL of a sample diluted 1:100 on an SEC column and verifying the presence of a GFP signal at the position corresponding to the target protein size.
NOTE: At this point, the tryptophan signal from total protein in the elution can also be viewed. Only the target affinity-purified protein will remain in the elution and the GFP and tryptophan signals will be near identical in profile. If the target protein is not seen in large quantity in the elution but was not lost in the flow-through or wash, the protein has likely remained bound to the column and can be eluted using a higher concentration of imidazole in the buffer. - Concentrate the eluate to 500 μL or less in a 15 mL 100K centrifugal filter tube (see Table of Materials) by spinning at 2,880 x g at 4 °C in 5 min increments. Resuspend the protein by pipetting the solution up and down between spins to avoid overconcentrating.
NOTE: Centrifuge time may be shortened as the volume approaches the desired final volume. - Load the concentrate onto an SEC column in buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 1 mM EDTA, and 0.1% digitonin). Run .
- Combine peak fractions containing the intact TRPC3 tetramer, as visualized by UV absorbance signal, and concentrate again to a final concentration of at least 5 mg/mL.
7. Screening of Protein by Negative-stain Electron Microscopy
- Turn on the glow-discharge machine. Set the program for discharging a carbon-coated grid using argon and oxygen for 30 s. Run the program to make the carbon-coating on copper 400-mesh grids hydrophilic prior to addition of the protein solution.
- Set up five 40 μL drops of sterile water and two 40 μL drops of 1% uranyl formate solution (on lab film, wax paper, or a similar surface, see Table of Materials). Take the grid from step 7.1 and add 2.5 μL of protein sample 5 mg/mL (50 – 200 μM) onto the dark side and let it sit for 1 min.
- After 1 min, dry the grid using filter paper. Do not touch the filter paper directly to the grid surface; instead, bring the paper to the edge of the liquid droplet and allow capillary action to pull the liquid from the grid into the filter paper.
- Dip the grid into first drop of water. Dry with filter paper and repeat with the remaining drops of water and the first drop of uranyl formate. Allow the second drop of uranyl formate sit for 1 min and then dry with filter paper. Allow the grid to fully air dry (about 1 min) before storing.
NOTE: This staining protocol may not be ideal for all protein-detergent combinations. Different concentrations of uranyl formate stain and different lengths of time for stain exposure should be tested if the steps above do not provide a stain with good contrast. - Image the grids on an electron microscope (see Table of Materials) to check the protein particle quality. Ensure that the micrographs show numerous particles that are homogenous in general appearance and distribution, display good contrast, and match the predicted size of the target protein.
- Generate preliminary, low-resolution, two-dimensional (2D) classifications using 50 – 100 micrographs (see data processing – step 10) to check that the particles represent different views of a single consistent structure.
NOTE: Micrographs and preliminary 2D classes of sufficient quality, as described above, are a strong indicator that the protocol has been sufficiently optimized for protein purification. Preparation and screening of cryo-EM grids is warranted at this point.
8. EM Sample Preparation
- Glow-discharge a gold holey carbon grid (see Table of Materials) as described in step 7.1.
- Apply 2.5 μL of the concentrated hTRPC3 protein sample (5 mg/mL) onto the grid. Blot the grid for 1.5 s using a blot force of 1 and a wait time of 5 s at 100% humidity and 4 °C, then plunge the grid into liquid ethane cooled by liquid nitrogen using a vitrification machine.
NOTE: The humidity, temperature, blot-force, blot time, and wait time listed here were used for the authors’ hTRPC3 study19. They may need to be changed to produce optimal vitreous ice for other proteins and detergents. - Screen frozen grids for optimal ice conditions using a cryo-EM microscope (see Table of Materials) and manually view regions of thick ice (grid squares that appear smaller and darker), thin ice (grid squares that appear larger and brighter), and medium ice.
NOTE: Thicker ice often holds more particles, while thinner ice often yields better contrast and resolution. Use manual screening of images to determine which ice conditions results in a large number of monodispersed particles with good contrast and resolution. Once good conditions are verified, move to image collection on a 300 kV cryo-EM microscope.
9. EM Data Collection
- Using an automated acquisition program, record image stacks in super-resolution counting mode with a binned pixel size of 1.074 Å on an electron microscope operated at 300 kV with a nominal magnification of 130,000X direct electron detector.
- Dose-fractionate every image to 40 frames with a total exposure time of 8 s, with 0.2 s per frame and a dose rate of 6.76 e− Å−2 s−1 (nominal defocus values varied from 1.0 to 2.5 μm in the authors’ experiment).
10. EM Data Processing
- Implement motion correction of summed movie stacks22 and estimate defocus values23 using the data processing software (see Table of Materials)24.
- Pick particles from the micrographs. Use these picked particles to construct an initial reference-free 2D classification using the software24. Select ideal 2D class averages to use as templates for automated particle picking for the entire data set.
- Manually check the quality of the auto-picked particles and remove bad particles. Use multiple rounds of 2D classification to clean up picked particles.
- Generate an initial model25. Subject 2D picked particles to three-dimensional (3D) classification (about 5 classes) using C1 symmetry and an initial reconstruction low-pass filter of 60 Å as a reference model. Determine which classes have high-resolution features and combine particles within such a class.
- Further refine particles using the local refinement with C4 symmetry (in the case of hTRPC3) applied and a high-resolution limit for particle alignment set to 4.5 Å26.
11. Model Building
- Build a model (see Table of Materials for the software used). For hTRPC3, use the transmembrane domain (TMD) of the transient receptor potential melastatin 4 (TRPM4) structure protein data bank (PDB) 5wp6 as a guide27. Use bulky residues and secondary structure prediction to guide de novo building.
- Subject the initial model to real space refinement with secondary structure restraints28. Manually examine the refined model and remodify as needed (see Table of Materials for the software used).
- Apply Fourier shell correlation (FSC) curves to calculate the difference between the final model and the EM map for validation of the refined structure. Evaluate the geometries of the atomic models (see Table of Materials for the software used)29,30.
Representative Results
A schematic overview of the protocol for expression and purification of hTRPC3 is shown in Figure 1A. An image of the hTRPC3 bacmid plate with ideal white colonies, similar to the one selected for bacmid DNA purification, is shown in Figure 1B. We found that 48 h is ideal for clear Bluo-gal staining while maintaining the presence of isolated colonies. Peak production of P2 virus for hTRPC3, as visualized by GFP fluorescence, was seen after 4 d of infection in Sf9 insect cells (Figure 1C).
P2 baulovirus was harvested from the media, supplemented with 1% FBS, and used to infect a suspension of HEK293 mammalian cells. Sodium butyrate was added to the infected cells 12 – 18 h after the virus to boost protein expression20. Cells were then incubated for an additional 36 h at 30 °C. The cells were harvested and subjected to solubility and stability screening by FSEC (Figure 2A)21. The cells were solubilized using seven different detergents with CMC values of 0.01 – 20 mM at a detergent concentration approximately 10 times the CMC value. After whole-cell solubilization, the cell lysis debris was removed by ultracentrifugation and the supernatant containing solubilized protein was loaded on an SEC column and run on HPLC in n-dodecyl-β-D-maltopyranoside/cholesteryl hemisuccinate (DDM/CHS) detergent-containing buffer to compare absolute solubility and peak volume position of hTRPC3 under different conditions relative to a TRPM4 control (Figure 2B). We chose to use DDM/CHS as the initial running buffer for the samples solubilized in different detergents because DDM/CHS is the most commonly used detergent when solving the structures of membrane proteins. All of the different detergent-solubilized TRPC3 samples showed peak positions at about 11.9 mL, which is likely too large, because the tetrameric form of hTRPC3 has a smaller molecular weight than the positive control human TRPM4 (Figure 2A and Figure 2B).
Nevertheless, because there was no structure of any TRPC channel receptor to compare our results to and because the large molecular weight might be caused by the architecture of TRPC3 or other factors, we carried out a small-scale purification of hTRPC3 using 25 mL of cells using DDM/CHS throughout the experiment as DDM/CHS gave the best solubility of hTRPC3. The SEC profile of hTRPC3 showed a monodisperse peak but was still in a peak position representing a higher molecular weight than TRPM4 (data not shown). We checked the protein in the peak fraction of SEC profile by negative-stain EM, because it is a rapid method of verifying the protein quality and requires a very small amount of protein. In the micrograph, particles were observed with two transmembrane domains present in the same particle. It appeared that two hTRPC3 particles dimerized in a head-to-head manner through the interaction between two cytosolic domains (Figure 3D). We then included the reducing reagent dithiothreitol (DTT) in the purification buffer for the second small-scale purification, hoping to disrupt the dimerization of hTRPC3. Indeed, a second peak with lower molecular weight appeared by SEC fractionation. However, the particles appeared too small to be an intact tetramer and showed no features of membrane proteins such as a transmembrane domain. It turned out that DDM/CHS was not a suitable detergent for purifying hTRPC3, despite giving the best solubility for hTRPC3.
Next, we tried a milder detergent, digitonin, to solubilize hTRPC3, and compared it with the protein solubilized in DDM/CHS. Here we used two different running buffers containing DDM/CHS and digitonin, respectively. This was important given that the detergent in the running buffer often contributes to protein stability and that the membrane protein may become instable when changing the detergents from solubilization to FSEC. The protein run in buffer containing digitonin showed a promising peak shift toward a lower molecular weight when solubilized in either DDM/CHS or digitonin. The protein solubilized by and run in digitonin yielded the highest peak in a reasonable position relative to the position of the positive control, human TRPM4 (Figure 3A). Then we moved forward by performing a small-scale purification using 25 mL of cells. Although multiple and broad peaks were observed by SEC (Figure 3B), the protein showed features of a single tetrameric hTRPC3 channel in 2D classification by negative-stain EM (Figure 3C and Figure 3D). We conclude that digitonin is an ideal detergent for purifying hTRPC3.
We scaled up the expression of hTRPC3 and performed a medium-scale purification using 400 mL of cells in digitonin. By doing so, we hoped to obtain sufficient protein for a few frozen grids without wasting medium and detergent before we figured out the final conditions for purifying hTRPC3 on a large scale. It happens often that membrane proteins show instability when highly concentrated for the preparation of frozen grids. The purified and concentrated protein not only shifted toward a higher molecular weight by FSEC, but also showed noisy background in cryo-EM grids imaged using an electron microscope equipped with an EMCCD camera. Even though we were able to observe single, intact tetrameric receptors, hTRPC3 purified in digitonin was not ideal for high-resolution cryo-EM studies.
To further improve the protein stability, we screened a great number of conditions described in Protocol step 5. We chose to screen additives based on the physiological character of hTRPC3; e.g., EDTA was selected because hTRPC3 is permeable to calcium and removing the calcium may stabilize the protein. Of all the samples tested by FSEC running in the digitonin-containing buffer,10 mM EDTA showed a remarkable effect on stabilizing hTRPC3 in an intact tetrameric peak position (Figure 4A). It also significantly increased the number of particles in the cryo-EM micrograph and decreased the noise in the background, as shown in Figure 5.
Having identified the ideal conditions for expression and purification, we performed a large-scale expression (2 L) of hTRPC3. The cells containing hTRPC3 were harvested and then solubilized. Ultracentrifuge-clarified lysate was subjected to metal-affinity column purification by batch-binding with a cobalt resin followed by purification in a gravity column. Retention of solubilized protein within the column and elution of affinity-purified protein from the column was verified by FPLC (Figure 4B). We found that for hTRPC3, an imidazole concentration of 15 mM in the wash buffer was sufficient to remove contaminating proteins with nonspecific resin binding, and 250 mM imidazole in the elution buffer was sufficient to elute the majority of solubilized hTRPC3 protein bound to the resin. All the samples were checked by FSEC, and the eluted protein showed a sharp, monodisperse peak at the correct peak position. As intrinsic flexibility between the GFP tag and the hTRPC3 protein may make the alignment of protein particles during image processing more challenging, and because a thrombin cleavage site is located between GFP and hTRPC3, we tested a small amount of purified protein at different cleavage times using thrombin protease. Given that the eluted protein incubated with thrombin at a 1:20 molar ratio showed reasonable stability and complete cleavage after 2 h at 4 °C, we cleaved off the GFP from all the purified protein using the same conditions and checked by HPLC to confirm that GFP has been completely removed (Figure 4C). The protein was further purified by S, and the cleavage of GFP can again be visualized by the peak position shift toward a smaller molecular weight (Figure 4D). A small unconcentrated sample from the main peak fraction was used to make grids for negative-stain EM. All fractions containing the tetrameric hTRPC3 were then combined and reconcentrated to a final concentration of 5 mg/mL, which was used to prepare grids for cryo-EM imaging. As we have observed that part of the protein still gradually shifted toward a larger molecular weight, we collected only the fractions at the correct molecular weight and froze the grids immediately after protein purification. We usually completed the sequence of experiments from purification of protein to preparation of frozen grids within a single day.
A 2.5 μL sample of purified hTRPC3 protein (concentration 5 mg/mL) was applied onto a glow-discharged holey carbon grid. A vitrification machine was used to prepare the grid, using a 1.5 s blotting time under 100% humidity followed by a plunge into liquid ethane cooled by liquid nitrogen. This step freezes the sample into vitreous ice for imaging. Images were captured at 130,000X nominal magnification by a cryo electron microscope operated at 300 kV. Image stacks were recorded in super-resolution counting mode with a binned pixel size of 1.074 Å and a nominal defocus value ranging from 1.0 to 2.5 μm using a direct electron detector and automated acquisition software. Each image was dose-fractionated to 40 frames with a total exposure time of 8 s (0.2 s per frame) and a dose rate of 6.76 e− Å−2 s−1. A representative micrograph from this data set is shown in Figure 5.
Motion correction and estimation of defocus values of the summed movie stacks was performed. Approximately 200 resulting micrographs were used as the source for manual particle picking and initial reference-free 2D classification31. Nine representative 2D class averages from this initial classification were selected and used as templates for automated particle picking for the entire data set. A manual check of autopicked particles was performed to remove obviously bad particles, then three rounds of 2D classification were applied to refine the selection of autopicked particles (Figure 5). These 2D-class selected particles were classified into five 3D classes using a low-pass-filter of 60 Å as an initial reference model. Of these five classes, only one displayed high-resolution features (Figure 5). Particles from this class were further subjected to local refinement with a high-resolution limit of 4.5 Å and C4 symmetry applied (Figure 5)32. The refined model was manually examined and remodified. The refined structure was validated by calculation of FSC curves to determine the difference between the final model and EM map and by evaluation of the geometries of the atomic models33.
Initial model construction was performed using the TMD domain of the TRPM4 structure (PDB 5wp6) as a guide34. De novo building of the model of hTRPC3 was mainly guided by bulky residues and secondary structure prediction, with the many α helices in the structure greatly facilitating register assignment. In the initial de novo-built model, the order, length, and position of secondary structural features and bulky residues are in close agreement with the prediction. During refinement, the resolution was held to a lower limit than the resolution estimated for the final reconstruction. Three-dimensional FSC was used to measure the normalized cross-correlation coefficient between two independently generated 3D maps (each using half of the data set) over corresponding shells in Fourier space (as a function of spatial frequency). We employed a soft mask of 4.3 Å from the reconstruction and an additional 4.3 Å cosine soft edge along with a low-pass filter of 10 Å, then used the gold standard FSC 0.143 cutoff threshold. This was used for final resolution reporting.
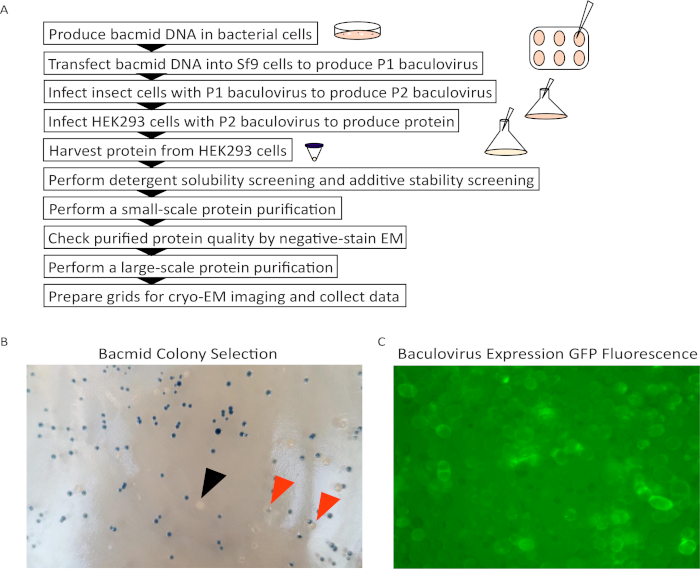
Figure 1: Expression and purification of hTRPC3 using the BacMam system. (A) Schematic procedure for hTRPC3 expression and purification. (B) Bacmid colony selection on blue-white indicator plate. Choose an isolated white colony (black arrow), not a white colony with an imbedded blue colony or a white colony in contact with a blue colony (red arrows). (C) GFP fluorescence of P2 baculovirus in Sf9 cells under 20X magnification. Fluorescence should be bright and present on the cell membrane and/or the interior of the majority of cells for a potent virus. Please click here to view a larger version of this figure.
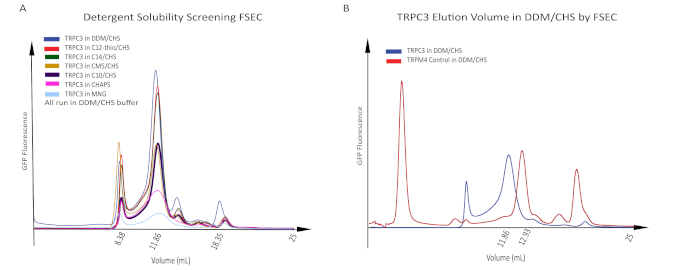
Figure 2: Stability screening of hTRPC3 by FSEC. (A) Detergent screening of hTRPC3. The hTRPC3 was whole-cell solubilized in a variety of detergents with a critical micelle concentration of 0.01 – 20 mM and was run in DDM/CHS-containing buffer. Although DDM/CHS shows the highest solubility of hTRPC3, the peak appears at the wrong position, too large to be the tetrameric hTRPC3 (blue trace). (B) TRPM4 control, with a tetrameric molecular weight of approximately 540 kDa, solubilized and run in DDM/CHS, had an elution volume of approximately 12.9 mL, while TRPC3, with a tetrameric molecular weight of approximately 400 kDa, solubilized and run in DDM/CHS, had an elution volume of approximately 11.9 mL. With a smaller molecular weight, hTRPC3 would be expected to have an elution volume slightly larger than TRPM4, not slightly smaller, suggesting that DDM/CHS may not be an ideal detergent for hTRPC3 solubilization and purification. Please click here to view a larger version of this figure.
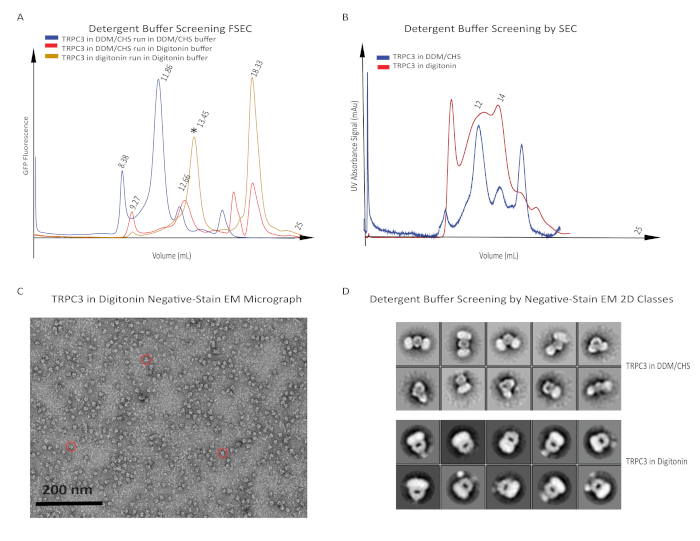
Figure 3: Detergent buffer screening of hTRPC3 by FSEC. (A) Solubility and stability test of hTRPC3 using digitonin. Digitonin was tested both for solubilization and in running buffer. Note the peak position of hTRPC3 protein solubilized in digitonin shifted toward a larger elution volume in comparison to the protein solubilized in DDM/CHS (yellow trace vs. blue and red traces). Only the combination using digitonin in both solubilization and running buffers shows the correct size of hTRPC3 by FSEC (yellow trace, asterisk). (B) Similar to the FSEC results of whole-cell solubilized hTRPC3,the peak position of hTRPC3 protein affinity purified in digitonin (red trace, 14 mL) shifted toward a larger elution volume in comparison to the protein affinity purified in DDM/CHS (blue trace, 12 mL) as measured by SEC-fractionation. (C) Negative-stain EM was used to verify the quality of purified protein prior to cryo-EM data collection. An ideal stain should present particles (red circles) negatively stained (appearing white) and surrounded by a detergent ring (appearing black). A representative, ideal micrograph in which these particles are abundant, monodispersed, and present in multiple orientations is shown. (D) Quality data from negative-stain EM should provide 2D classes with a clear and consistent general architecture and should encompass multiple orientations of the protein, with averages contained and centered within the mask (black circle). 2D classes from negative-stain EM micrographs of hTRPC3 solubilized in DDM/CHS present particles that appear to be a head-to-head dimerization of hTRPC3 monomers. In contrast, a rough (but clear and consistent) overall acorn-like tetrameric structure is apparent in 2D classes from negative-stain EM micrographs of hTRPC3 solubilized in digitonin. Please click here to view a larger version of this figure.
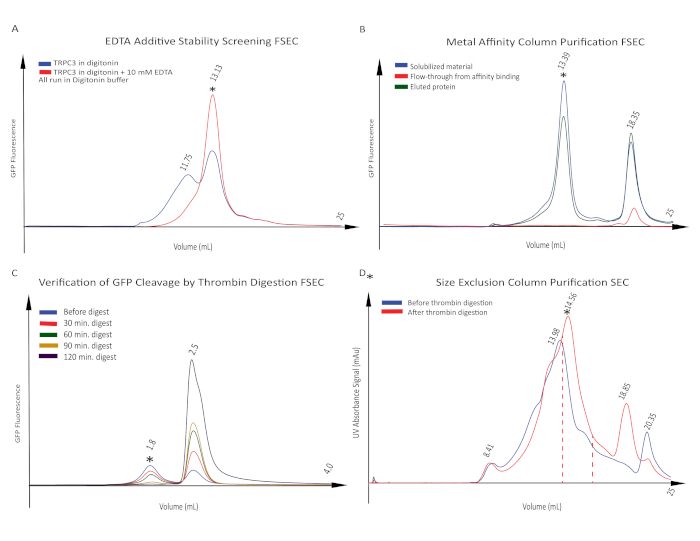
Figure 4: Purification of hTRPC3. (A) Stability test of hTRPC3 in the presence of EDTA. hTRPC3 was solubilized in digitonin in the presence (red trace) or absence (blue trace) of 10 mM EDTA. The former showed a single and narrower peak at the tetramer protein position (red trace, asterisk), indicating that EDTA stabilized hTRPC3 in its tetrameric conformation. (B) GFP fluorescence signal FSEC for hTRPC3 solubilized in digitonin and run in digitonin buffer. The tetramer peak is observed in the solubilized material before metal-affinity column purification (blue trace, asterisk). The absence of this peak in the flow-through fraction indicates a complete binding of hTRPC3 protein in the affinity column (red trace). After elution by imidazole, the fractions in the elution peak were checked by FSEC (green trace). All the fractions showing intact tetramer peak were collected for further purification. (C) Test of GFP cleavage of hTRPC3 using thrombin. Digestion time was tested using a small amount of protein at 4 °C, and the completeness of digestion was checked by FSEC. In each trace, the peak on the left corresponds to the hTRPC3 tetramer peak (asterisk) and the peak on the right is the free GFP peak. As the length of the digestion increased, the ratio of tetrameric protein to free GFP decreased, indicating that GFP was being cleaved from the protein. The 2 h digestion shows a complete cleavage of GFP. (D) SEC profile of hTRPC3 before or after thrombin digestion in digitonin containing running buffer with 10 mM EDTA added. As expected, the peak position is shifted toward the smaller elution volume after thrombin digestion (red trace). The main peak (asterisk) represents the tetrameric hTRPC3. The fractions between red lines were collected for cryo-EM experiments. Please click here to view a larger version of this figure.
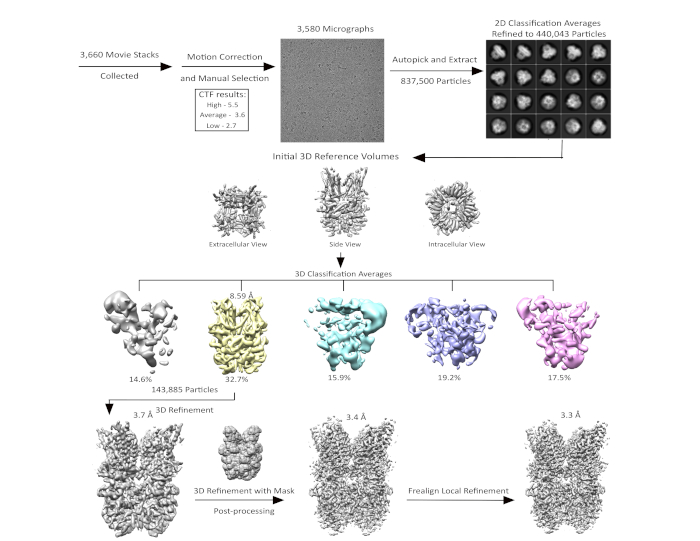
Figure 5: Schematic of cryo-EM data collection and processing for hTRPC3. The collection of 3,660 movie stacks of purified hTRPC3 was done using an electron microscope with a direct electron detector. Motion correction and manual selection were applied resulting in 3,580 micrographs. Particles were autopicked and subjected to 2D classification. 2D classes were further refined by 3D classification. Only one out of five 3D classes displayed high-resolution features. This class was selected for 3D refinement and Frealign local refinement, resulting in a structure for hTRPC3 at 3.3 Å resolution. Please click here to view a larger version of this figure.
Discussion
Structural determination of proteins by cryo-EM has revolutionized the field of structural biology in the past few years, thanks to the development of new cameras and algorithms that significantly speeds up the structure determination of proteins that do not readily crystalize, particularly membrane proteins. Despite all of the recent advances in the cryo-EM technique, the preparation of purified proteins sufficient in quality and quantity to facilitate high-quality imaging often remains time-consuming, costly, and challenging. The ability to rapidly express and screen multiple gene constructs under a variety of purification conditions, as described in the protocol above, improves the efficiency of this process by allowing the rapid and cost-effective production of high-quality purified protein for use in cryo-EM studies.
While the method described here provides a way to purify large quantities of mammalian membrane proteins at less cost than by direct transfection and more quickly than by generation of a stably transfected cell line, it has many steps, each of which must be optimized to provide a high-quality protein yield. Within the biochemical techniques used to produce and purify hTRPC3, there are a number of critical steps and checkpoints. The first critical checkpoint is the production of bacmid DNA. As described in the results, ideal colony selection is the first step in generation of a quality virus for protein expression. Additionally, if the concentration of bacmid DNA purified from the selected colony is less than 1 μg/μL, the resulting virus will not be sufficient for robust protein expression. The second critical checkpoint is the production of P1 and P2 baculovirus. Virus titer can be estimated by GFP fluorescence, as shown in Figure 1C or can be measured as described by Coleman et al.35. In addition to viral titer, a time course of infection time should be undertaken for each new construct to determine the optimal time of infection for maximal protein expression. Critical checkpoint three is the solubility and stability screening shown in Figure 2. Detergents with a high CMC, such as DDM, may provide high solubility, but they are not ideal for cryo-EM imaging because they can result in an abundance of contaminating micelles. A milder detergent, such as digitonin, can provide sufficient solubility while preserving the protein stability. The screening of additives, such as EDTA in the case of hTRPC3, is important for discovering a purification condition that results in intact and homogenous protein, probably by removing the calcium from hTRPC3, which is permeable to calcium. Critical checkpoint four is the verification of specific and efficient protein capture during the affinity column purification and the observation of an ideal protein peak during SEC-fractionation (Figure 4). Avoidance of the inclusion of contaminating protein particles while retaining all of the target protein is crucial to obtaining a high enough yield of protein for cryo-EM imaging. Alteration of the batch-binding time and ratio of resin to solubilized protein can help improve protein retention by the resin column. The overall quality of the SEC-fractionation peak is in our experience the best indicator, prior to imaging, of the quality of purified protein particles. A low or a broad peak during SEC-fractionation indicates possible problems of too few protein particles per image or the presence of contaminating protein aggregation/degradation products, respectively. Additionally, changes to the affinity tag within the construct itself has proven necessary in some cases to ensure sufficient affinity-tag binding. The final checkpoint prior to collection of cryo-EM data sets is the verification of protein particle quality by negative-stain EM. While not strictly necessary, we find that this provides an excellent opportunity to spot and correct protein quality issues before investing in the time- and cost-intensive process of collecting data by cryo-EM.
One alternative method of producing protein for cryo-EM structural studies is the direct purification of protein from the native tissue source. While this method often encounters issues with protein yield, it is an excellent alternative for proteins that are highly abundant in cells or that exist as part of a large multi-protein complex, which can be difficult to produce using a recombinant overexpression system36. However, for single proteins expressed in moderate or low amounts under physiologic conditions, the expression and purification system presented here is an efficient, relatively low-cost and high-yield method for producing purified mammalian membrane protein for cryo-EM structural studies. One method that could strongly complement that presented here is the reconstitution of purified protein into lipid nanodiscs prior to cryo-EM data collection37. Under this method, the proteins are imbedded into a small disk of lipid bilayer, probably providing a native-like microenvironment in comparison to detergent micelles, although there is no evidence so far that the structures dissolved in detergent and nanodisc are different38,39,40. Another approach is to extract the protein directly using amphipathic polymers like styrene maleic anhydride (SMA), which has been successfully used for determination of membrane protein structures41,42.
These approaches described in this manuscript have proven to be readily adaptable in our lab to a variety of other mammalian membrane proteins beyond ion channels. Therefore, we believe this protocol will be a valuable tool in the structural and functional analyses that underlie high-specificity targeted drug design. This will be particularly useful in neurodegenerative disease, cardiovascular disease, and cancer, in which ion channels are difficult but high-potential drug targets.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank G. Zhao and X. Meng for the support with data collection at the David Van Andel Advanced Cryo-Electron Microscopy Suite. We appreciate the VARI High-Performance Computing team for computational support. We give our gratitude to N. Clemente, D. Dues, J. Floramo, Y. Huang, Y. Kim, C. Mueller, B. Roth, and Z. Ruan for comments that greatly improved this manuscript. We thank D. Nadziejka for editorial support for this manuscript. This work was supported by internal VARI funding.
Materials
| pEG BacMam vector (pFastBacI) | addgene | 31488 | |
| DH10α cells | Life Technologies | 10361-012 | |
| S.O.C. media | Corning | 46003CR | for transformation of DH10α cells for Bacmid |
| Bacmam culture plates | Teknova | L5919 | for culture of transformed DH10α cells |
| Incubation shaker for bacterial cells | Infors HT | Multitron standard | |
| Incubated orbital shaker for insect cells | Thermo-Fisher | SHKE8000 | |
| Reach-in CO2 incubator for mammalian cells | Thermo-Fisher | 3951 | |
| Table-top orbital shaker | Thermo-Fisher | SHKE416HP | used in Reach-in CO2 incubator for mammalian cells |
| Incubator | VWR | 1535 | for bacterial plates |
| QIAprep Spin Miniprep Kit | Qiagen | 27106 | for plasmid extraction and purification |
| Phenol:Chloroform:Isoamyl alcohol | Invitrogen | 15593031 | for DNA extraction |
| Sf9 cells | Life Technologies | 12659017 | insect cells for producing virus |
| Sf-900 media | Gibco | 12658-027 | insect cell media |
| FBS | Atlanta Biologicals | S11550 | |
| Cellfectin II | Gibco | 10362100 | for transfecting insect cells |
| lipofectamine 2000 | Invitrogen | 11668-027 | for transfecting mamalian cells |
| 0.2 mm syringe filter | VWR | 28145-501 | for filtering P1 virus |
| 0.2 mm filter flasks 500ml resevoir | Corning | 430758 | for filtering P2 virus |
| erlenmeyer culture flask (flat bottom 2L) | Gene Mate | F-5909-2000 | for culturing insect cells |
| erlenmeyer culture flask (baffled 2L) | Gene Mate | F-5909-2000B | for culturing mammalian cells |
| nanodrop 2000 spectrophotometer | Thermo-Fisher | ND-2000 | for determining DNA and protein concentrations |
| HEK293 | ATCC | CRL-3022 | mammalian cells for producing protein |
| Freestyle 293 expression Medium | Gibco | 1238-018 | mammalian cell media for protein expression |
| Butyric Acid Sodium Salt | Acros | 263195000 | to amplify protein expression |
| PMSF | Acros | 215740500 | protease inhibitor |
| Aprotinin from bovine lung | Sigma-Aldrich | A1153-100MG | protease inhibitor |
| Leupeptin hydrochloride | Sigma-Aldrich | 24125-16-4 | protease inhibitor |
| pepstatin A | Fisher Scientific | BP2671-250 | protease inhibitor |
| digitonin | EMD Millipore | 300410 | detergent – to solubilize protein from membrane |
| imidazole | Sigma | 792527 | to elute protein from resin column |
| TALON resin | Clonetech | 635504 | for affinity purification by His-tag |
| superose6 incease columns | GE | 29091596; 29091597 | for HPLC and FPLC |
| Prominence Modular HPLC System | Shimadzu | See Below | |
| Controller Module | " | CBM20A | |
| Solvent Delivery System | " | LC30AD | |
| Fluorescence Detector | " | RF20AXS | |
| Autosampler with Cooling | " | SIL20ACHT | |
| Pure FPLC System with Fractionator | Akta | ||
| thrombin (alpha) | Haematologic Technologies Incorporated | HCT-0020 Human alpha | for cleaving GFP tag |
| Amicon Ultra 15 mL 100K centrifugal filter tube | Millipore | UFC910008 | for concentrating protein |
| EDTA | Fisher | E478500 | for stabilizing protein |
| 400 mesh carbon-coated copper grids | Ted Pella Inc. | 01754-F | grids for negative stain |
| Quantifoil holey carbon grid (gold, 1.2/1.3 μm size/hole space, 300 mesh) | Electron Microscopy Sciences | Q3100AR1.3 | grids for Cryo-EM |
| Vitrobot Mark III | FEI | for preparing sample grids by liquid ethane freezing | |
| liquid nitrogen | Dura-Cyl | UN1977 | |
| ethane gas | Airgas | UN1035 | |
| Solarus Plasma System | Gatan | Model 950 | for cleaning grids before sample freezing |
| Tecnai Spirit electron microscope | FEI | for negative stain EM imaging | |
| Talos Arctica electron microsocope | FEI | for screening and low resolution imaging of Cryo-EM grids | |
| Titan Krios electron microscope | FEI | for high-resolution Cryo-EM imaging | |
| Software | |||
| Gautomatch software | http://www.mrc-lmb.cam.ac.uk/kzhang/Gautomatch/ | to pick particles from micrographs | |
| Relion 2.1 software | https://github.com/3dem/relion | to construct 2D and 3D classification | |
| CryoSPARC software | https://cryosparc.com/ | to generate an initial structure model | |
| Frealign software | http://grigoriefflab.janelia.org/frealign | to refine particles | |
| Coot software | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ | to build a model | |
| MolProbity software | http://molprobity.biochem.duke.edu/ | to evaluate the geometries of the atomic model | |
| SerialEM software | http://bio3d.colorado.edu/SerialEM/ | for automated serial image stack acquisition | |
| MortionCor2 software | http://msg.ucsf.edu/em/software/motioncor2.html | for motion correction of summed movie stacks | |
| GCTF software | https://www.mrc-lmb.cam.ac.uk/kzhang/Gctf/ | for measuring defocus values in movie stacks | |
| Phenix.real_space_refine software | https://www.phenix-online.org/documentation/reference/real_space_refine.html | for real space refinement of the initial 3D model |
References
- Berridge, M. J., Bootman, M. D., Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 4 (7), 517-529 (2003).
- Kumar, R., Thompson, J. R. The regulation of parathyroid hormone secretion and synthesis. Journal of the American Society of Nephrology. 22 (2), 216-224 (2011).
- Sudhof, T. C. Calcium control of neurotransmitter release. Cold Spring Harbor Perspectives in Biology. 4 (1), a011353 (2012).
- Ong, H. L., de Souza, L. B., Ambudkar, I. S. Role of TRPC Channels in Store-Operated Calcium Entry. Advances in Experimental Medicine and Biology. 898, 87-109 (2016).
- Smyth, J. T., et al. Activation and regulation of store-operated calcium entry. Journal of Cellular and Molecular Medicine. 14 (10), 2337-2349 (2010).
- Prakriya, M., Lewis, R. S. Store-Operated Calcium Channels. Physiological Reviews. 95 (4), 1383-1436 (2015).
- Liu, X., Singh, B. B., Ambudkar, I. S. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. Journal of Biological Chemistry. 278 (13), 11337-11343 (2003).
- Zhu, X., Jiang, M., Birnbaumer, L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. Journal of Biological Chemistry. 273 (1), 133-142 (1998).
- Zhu, X., et al. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 85 (5), 661-671 (1996).
- Itsuki, K., et al. Voltage-sensing phosphatase reveals temporal regulation of TRPC3/C6/C7 channels by membrane phosphoinositides. Channels (Austin). 6 (3), 206-209 (2012).
- Tang, J., et al. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. Journal of Biological Chemistry. 276 (24), 21303-21310 (2001).
- Gonzalez-Cobos, J. C., Trebak, M. TRPC channels in smooth muscle cells. Frontiers in Bioscience (Landmark Edition). 15, 1023-1039 (2010).
- Li, H. S., Xu, X. Z., Montell, C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF). Neuron. 24 (1), 261-273 (1999).
- Becker, E. B., et al. Candidate screening of the TRPC3 gene in cerebellar ataxia. Cerebellum. 10 (2), 296-299 (2011).
- Kitajima, N., et al. TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling. Scientific Reports. 6, 37001 (2016).
- Yang, S. L., Cao, Q., Zhou, K. C., Feng, Y. J., Wang, Y. Z. Transient receptor potential channel C3 contributes to the progression of human ovarian cancer. Oncogene. 28 (10), 1320-1328 (2009).
- Oda, K., et al. Transient receptor potential cation 3 channel regulates melanoma proliferation and migration. Journal of Physiological Sciences. 67 (4), 497-505 (2017).
- Xia, M., Liu, D., Yao, C. TRPC3: A New Target for Therapeutic Strategies in Chronic Pain-DAG-mediated Activation of Non-selective Cation Currents and Chronic Pain (Mol Pain 2014;10:43). Journal of Neurogastroenterology and Motility. 21 (3), 445-447 (2015).
- Fan, C., Choi, W., Sun, W., Du, J., Lu, W. Structure of the human lipid-gated cation channel TRPC3. Elife. 7, e36852 (2018).
- Goehring, A., et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nature Protocols. 9 (11), 2574-2585 (2014).
- Hattori, M., Hibbs, R. E., Gouaux, E. A fluorescence-detection size-exclusion chromatography-based thermostability assay to identify membrane protein expression and crystallization conditions. Structure (London, England: 1993). 20 (8), 1293-1299 (2012).
- Zheng, S. Q., et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nature Methods. 14 (4), 331-332 (2017).
- Zhang, K. Gctf: Real-time CTF determination and correction. Journal of Structural Biology. 193 (1), 1-12 (2016).
- Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology. 180 (3), 519-530 (2012).
- Punjani, A., Rubinstein, J. L., Fleet, D. J., Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nature Methods. 14 (3), 290-296 (2017).
- Grigorieff, N. Frealign: An Exploratory Tool for Single-Particle Cryo-EM. Methods in Enzymology. 579, 191-226 (2016).
- Emsley, P., Lohkamp, B., Scott, W. G., Cowtan, K. Features and development of Coot. Acta Crystallographica Section D: Biological Crystallography. 66 (Pt 4), 486-501 (2010).
- Afonine, P. V., et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica Section D: Biological Crystallography. 68 (Pt 4), 352-367 (2012).
- Chen, V. B., et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D: Biological Crystallography. 66 (Pt 1), 12-21 (2010).
- Scheres, S. H. W., Chen, S. Prevention of overfitting in cryo-EM structure determination. Nature Methods. 9 (9), 853-854 (2012).
- Scheres, S. H. W. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology. 180 (3), 519-530 (2012).
- Grigorieff, N. Frealign: An Exploratory Tool for Single-Particle Cryo-EM. Methods in Enzymology. 579, 191-226 (2016).
- Chen, V. B., et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D-Biological Crystallography. 66, 12-21 (2010).
- Emsley, P., Lohkamp, B., Scott, W. G., Cowtan, K. Features and development of Coot. Acta Crystallographica Section D-Biological Crystallography. 66, 486-501 (2010).
- Green, E. M., Au, Thermostabilization, Expression, Purification, and Crystallization of the Human Serotonin Transporter Bound to S-citalopram. Journal of Visualized Experiments. (117), e54792 (2016).
- Mesa, P., Deniaud, A., Montoya, G., Schaffitzel, C. Directly from the source: endogenous preparations of molecular machines. Current Opinion in Structural Biology. 23 (3), 319-325 (2013).
- Bayburt, T. H., Sligar, S. G. Membrane Protein Assembly into Nanodiscs. FEBS letters. 584 (9), 1721-1727 (2010).
- Winkler, P. A., Huang, Y., Sun, W., Du, J., Lü, W. Electron cryo-microscopy structure of a human TRPM4 channel. Nature. 552, 200-204 (2017).
- Autzen, H. E., et al. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science. 359 (6372), 228-232 (2017).
- Guo, J., et al. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature. 552 (7684), 205-209 (2017).
- Parmar, M., et al. Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1860 (2), 378-383 (2018).
- Gulati, S., et al. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochemical Journal. 461 (2), 269-278 (2014).

