Intra-Omental Islet Transplantation Using h-Omental Matrix Islet filliNG (hOMING)
Summary
Here, we present a protocol for in vivo validation of hydrogel-based cell therapy, illustrated by the example of islet transplantation. h-Omental Matrix Islet filliNG (hOMING) implantation allows implantation of a cell-hydrogel mixture between the omental layers, near to blood vessels, to maximize engraftment in a proper metabolic environment.
Abstract
Regenerative medicine based on cell therapy represents a new hope for curing disease. Current obstacles include proper in vivo validation of the efficiency of the therapy. For transfer to the recipient body, cells often need to be combined with biomaterials, especially hydrogels. However, validation of the efficacy of such a graft requires the right environment, the right hydrogel, and the right recipient site. The omentum might be such a site. Based on the example of islet transplantation, we developed the hOMING (h-Omental Matrix Islet filliNG) technique, which consists of the injection of the graft inside the tissue, in between the omental layers, to improve islet implantation and survival. To achieve this, islets have to be embedded in a hydrogel with a viscosity that enables its injection using an atraumatic needle. Syringes are loaded with a combination of hydrogel and islets. Several injections are performed inside the omental tissue at different entry points, and the deposition of the islet/hydrogel mixture is made along a line. We tested the feasibility of this innovative approach using dextran beads. The beads were well spread throughout the omental tissue, in close proximity to blood vessels. To test the efficacy of the graft, we transplanted islets into diabetic rats and perform a metabolic follow-up over two months. The transplanted islets exhibited a high rate of re-vascularization around and inside islets, and reversed diabetes. The hOMING technique could be applicable for other types of hydrogel or cell therapy, for cells with high metabolic activity.
Introduction
Cell therapy is a hot topic, as it aims to cure diseases based on the regenerative medicine. Biological material-assisted cell therapies have been increasingly studied in recent years, especially because cell implantation often requires a carrier for the transfer of the cells from the culture dish to the recipient. Biomaterial scaffolds are potentially valuable cell carriers that fulfill several roles1. A competent carrier should protect cells from mechanical stresses and provide favorable growth conditions, such as essential growth factors, metabolic waste excretion, exchange of nutrient substances, and oxygen2.
Among the different types of biomaterials used in cell therapy, hydrogels have many advantages. They are biocompatible, biodegradable, easy to handle, and facilitate oxygen diffusion3. Furthermore, current technology allows the use of hydrogels to help cells survive and engraft with, for example, supplementation with growth factors or extra-cellular matrix proteins4.
Hydrogel carriers containing stem cells can be injected as treatments, e.g., bone regeneration5 and nervous system disease6. The implantation of metabolically active cells is needed. While in vitro validation of the approach is possible, tools and techniques for in vivo validation remain to be refined.
Cell and hydrogel transplantation can easily be carried out by subcutaneous injection when biocompatibility tests are conducted. However, when grafted cells are meant to regulate systemic factors by their metabolic action, this subcutaneous localization is not optimal, essentially in terms of venous drainage7. Therefore, there are no current tools to quickly, safely, and efficiently evaluate the beneficial effects of a hydrogel. Based on the example of islet transplantation, which requires that hormones be released into the bloodstream from the graft in response to blood glucose levels, we developed a new method for cell/hydrogel implantation in vivo.
The first step was to identify an acceptor transplantation site, which can accept a hydrogel with cells. The omentum offers a large space for implantation, is highly plastic, and its dense vascularization combined with the intraperitoneal setting is interesting for studying cells having high metabolic activity8. We next needed to establish a surgical technique allowing the transfer of the cells and the hydrogel into the omentum. Inspired by the lipofilling used in plastic surgery9, we developed the h-Omental Matrix Islet filliNG (hOMING) approach. Islets embedded in hydrogel are injected inside the omental tissue. The technique also aimed to provide maximum engraftment by utilizing multiple depositions of the cell and hydrogel mixture into the omental tissue, where the large number of blood vessels also improves graft oxygenation.
In the present study, we describe a simple and innovative technique for islet implantation between omental sheets, inside the fat tissue closest to the blood vessels. This consists of micro-invasive surgery, which could be completed under laparoscopy, with the injection of islets contained in a hydrogel into the fat tissue. This technique is easily applicable to all hydrogel and cell combinations that need to be tested in a in a metabolically functional environment.
Protocol
All animal experiments were performed according to the National Institutes of Health guidelines, with the authorization number: AL/60/67/02/13.
1. Recipient Preparation
- Chemically induce diabetes in recipient rats.
- Inject 75 mg/kg of streptozotocin (STZ, in sterile 0.1 M citrate buffer, pH 4) intraperitoneally to rats10.
NOTE: For the transplantation studies, 6 week-old Lewis strain rats, weighing 150-190 g were used. - Check the diabetes status by daily blood glucose measurements during the first four days. Inject long-acting insulin 6 U/day subcutaneously when rats exhibit glycemia over 2 g/L to prevent diabetes complications and weight loss, until insulin pellet implantation.
- Include rats in the cohort when two measures of tail vein blood glucose are >4-5 g/L for 2 consecutive days, and C-peptide level is <200 pM. Measure glycemia using a glucometer and C-peptidemia by an enzyme-linked immunosorbent assay (ELISA).
- Implant insulin pellets under the skin (see 1.2).
NOTE: Chronic insulin therapy allows better glycemia regulation and avoids diabetic complications (which exacerbate oxidative stress at the transplantation site)11. Furthermore, the therapy conserves the diabetic state along with a normal growth curve (without usual weight loss observed in a diabetic animal). This can result in a bigger omental fat pad, which is ideal for carrying out transplantation.
- Inject 75 mg/kg of streptozotocin (STZ, in sterile 0.1 M citrate buffer, pH 4) intraperitoneally to rats10.
- Implantation of insulin pellets
- Anesthetize the rat using gas anesthesia (3% isoflurane in 500 mL/min O2) and place the rat in the prone position.
- Verify anesthesia status by checking the absence of reflex (paw pinching). Clean the neck using povidone iodine, and shave the area using a razor blade. Apply povidone iodine again and let it stand for 3 min.
- Place 1.5 insulin pellets (3 Units (U)/200 g rat) in a 1:5 diluted povidone iodine solution to sterilize the pellets. Pierce the neck skin using a 16 G trocar and insert the pellet using the furnished guide and stylet. Retrieve the guide and stylet and stitch a single point. Use povidone iodine to clean the stitch.
NOTE: No post-surgical pain management was necessary as the intervention was comparable to a single subcutaneous (SC) injection. - Let the rat recover from anesthesia and ensure that rats have access to food to avoid hypoglycemia.
- Measure the efficiency of pellet by measuring glycaemia decrease after implantation.
- Check the pellet efficiency by monitoring glycemia level every week for 1 month.
- Include rats in the cohort when a measure of tail vein blood C peptide level is maintained under 200 pM 1 month after insulin pellet implantation.
NOTE: Checking C-peptide levels is mandatory to evaluate animals at baseline before transplantation and to confirm their diabetic state. Low C-peptide regeneration always occurs during follow-up. The lowest C-peptidemia is indicative of the lowest regeneration.
2. hOMING: Intra-omental Matrix Islet Filling
- Islet-matrix mixture preparation.
- Prepare the viscous islet carrier in a laminar flow hood. Dissolve alginate powder in sterile PBS at a concentration of 1.5%. Sterilize the preparation by passage through a 0.22 µm filter. Prepare 400 µL per recipient.
NOTE: Any kind of hydrogel with a viscosity suitable for injection through a 21 G needle can be used. - Isolate islet from healthy Lewis rats (200-250 g) as previously described12.
- Count islet number in islet equivalents (IEQ) (one IEQ is considered equivalent to a pancreatic islet with a diameter of 150 µm)13.
- In a laminar flow hood, prepare aliquots of 7660-islet equivalent (IEQ) in a 1.5 mL tube.
- Wash islets aliquots with 500 µL of CMRL (Connaught Medical Research Laboratories) medium free of fetal bovine serum.
- Pellet islets by centrifugation (2 min at 500 x g and 4 °C). Discard the supernatant.
- Add 150 µL of alginate hydrogel carrier over the islets, mix carefully by pipetting up and down and place the mix on ice.
- Prepare an atraumatic 21 G needle and a 1 mL syringe without dead volume by loading 150 µL of empty alginate into the syringe.
- Fill the syringe with the mixture of islets and alginate (150 µL, total volume 300 µL). Keep the syringe on ice.
- Prepare the viscous islet carrier in a laminar flow hood. Dissolve alginate powder in sterile PBS at a concentration of 1.5%. Sterilize the preparation by passage through a 0.22 µm filter. Prepare 400 µL per recipient.
- Surgical procedure
- Sterilize surgical instruments using cold sterilization (2% Steranios for 20 min).
- Anesthetize the rat using isoflurane anesthesia and place the rat in the prone position.
- Shave the neck area using a razor blade and sterilize the area with povidone iodine. Let the iodine stand for 3 min.
- Make an incision using a scalpel and remove the 1.5 insulin pellets using forceps. Close the skin using one or two single stitch points. Do not remove the rat from anesthesia.
NOTE: After 1 month, the pellet can be friable as some fibrotic tissue can wrap as pellets; use scissors to properly dissect it. - Place the rat in the supine position. Shave and sterilize (with povidone iodine) the peritoneal area. Let the iodine stand for 3 min.
- Create a 1.5 cm laparotomy just under the sternum using a scalpel. Place wet-sterile gauze around the incised area.
- Identify the omentum that is the fat pad localized next to stomach. Use forceps to carefully catch the omentum, pull it gently out of the peritoneal cavity, and spread it on the gauze.
NOTE: The omental tissue extends from the spleen to the duodenum and attaches at its mid-point to the stomach. The normal omentum of diabetic rats receiving insulin therapy is approximately 2 cm² when spread on the gauze. - Hydrate the omental tissue well using 2 mL of pre-warmed 37 °C sterile saline. Use small curved forceps to manipulate the tissue and penetrate the omental edge with the needle between the omental layers. Insert the needle entirely.
- Start the injection of the islet preparation slowly and carefully move the needle backward to inject the islets in several places (as lines). Prior to withdrawing the needle, ensure that the hydrogel has stopped exiting the needle to avoid the loss of the dispersed islets.
- Repeat this manipulation as needed to inject the entire contents of the syringe using different entry points to distribute the islets throughout the omental tissue.
NOTE: In rats, four to five injections are generally needed. - Check that islets are not clustered in the syringe at the end of the injections.
NOTE: If some islets are still visible, it is possible to withdraw the needle from the syringe, fill the syringe directly with 100 µL of empty hydrogel, and reconnect the needle. A second round of injection can be done to flush out the remaining islets. - Use sterile saline again to hydrate the omental tissue and the wall of the laparotomy. Use forceps to carefully replace omentum in the abdominal cavity.
- Inject 2 mL of pre-warmed sterile saline into the abdominal cavity to rehydrate the rat.
- Close the muscle wall using a continuous thread suture. Then stitch the cutaneous layer with single stitch point (point-by-point).
- Inject meloxicam (1.5 mg/kg) subcutaneously as an analgesic for 5 days once a day.
- Place the rat in a cage on a heating pad until recovery from anesthesia. Repeat the procedure for all the recipient rats.
- Measure the blood glucose after transplantation every day. If glycemia is >2 g/L, inject 6 U of long-acting insulin subcutaneously once a day.
- Assess graft function by glycemia and c-peptidemia monitoring over 1 or 2 months.
NOTE: In cases of successful transplantation, glycemia should stabilize within 2-5 days after transplantation, and rats can be taken off insulin.
3. Omental Graft Explantation
NOTE: This procedure will permit the confirmation of good graft function. After retrieval of a functional graft, rats should return to a diabetic state. This step is performed after 1 or 2 months of metabolic follow-up.
- Anesthetize the rat with gas anesthesia and place it in the supine position.
- Shave the peritoneal area and sterilize it using povidone iodine for 3 min.
- Create a 1.5 cm laparotomy just under sternum using a scalpel. Place wet-sterile gauze all around the incised area.
- Identify the omentum, which is next to the stomach. Use forceps to carefully spread it on the gauze.
- Use scissors to excise the omentum. Start from the part that adheres onto the pancreas tail (next to the spleen). If bleeding occurs, use dry sterile gauze to stop it.
- Continue excision along the part attached to the stomach and retrieve the omentum.
NOTE: At this location, gastroepiploic arteries (Figure 1) can cause a large amount of bleeding if they are accidentally cut. Artery incisions are inevitable to retrieve the graft, but bleeding can be managed using forceps and gauze. If an accidental cut happens, compress firmly with dry gauze and maintain the compression for at least 1 min. The bleeding should stop. If not, use clips or use an electric bistoury to cauterize the vessels.
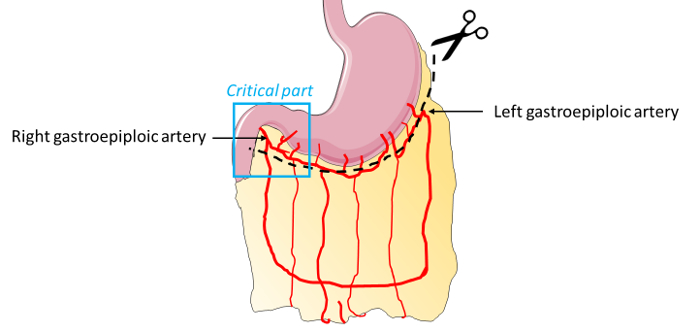
Figure 1: Omental artery distribution. For omentum graft explantation, the critical area composed of gastroepiploic arteries is represented in blue. During resection of this part of the omental tissue, attention must be to be paid to the right gastroepiploic artery section. Compression, ligature, or cauterization can be used to limit bleeding. Please click here to view a larger version of this figure.
- Check if any bleeding persists. If not, inject 2 mL of pre-warmed saline, close the rat, and process as previously described.
Explanted animals return to a diabetic state. Insulin injection (6 U/SC/day) is then mandatory to assure well-being of animals. - Euthanize the rat 10 to 12 days after explantation using an overdose of pentobarbital (182.2 mg/kg).
4. Histological Analysis: Hematoxylin and Eosin Staining
- Fix the retrieved omenta using 4% paraformaldehyde (PFA) and embed in paraffin.
- Cut sections 4 µm in thickness and apply hematoxylin and eosin stain for morphological evaluation of the transplant.
5. Statistical Analysis
- Determine statistical significance using statistical analysis software and repeated measures analysis of variance (ANOVA) with Tukey’s honest significance difference test as a post hoc test. Represent p values as: *p < 0.05; ** p < 0.01; *** p < 0.001.
Representative Results
The hOMING method permits the avoidance of intravascular implantation and the confinement of islets in an organ. A maximum time of 8-10 min is required for the entire islet implantation procedure, including anesthesia, which a timeline comparable to classical liver transplantation.
To study the way islets are distributed inside the omental tissue, dextran beads were transplanted using the hOMING method (Figure 2). One day after implantation, rats were sacrificed, and omental tissues were retrieved for histological analysis. Hematoxylin and eosin staining revealed a uniform distribution of the beads throughout the tissue (Figure 2, bottom right). Very often, beads were close to blood vessels and were well-implanted in the fat tissue. Immediately after implantation, an inflammatory reaction occurs around the beads, resulting in tissue rearrangement to nest the islets in the tissue.
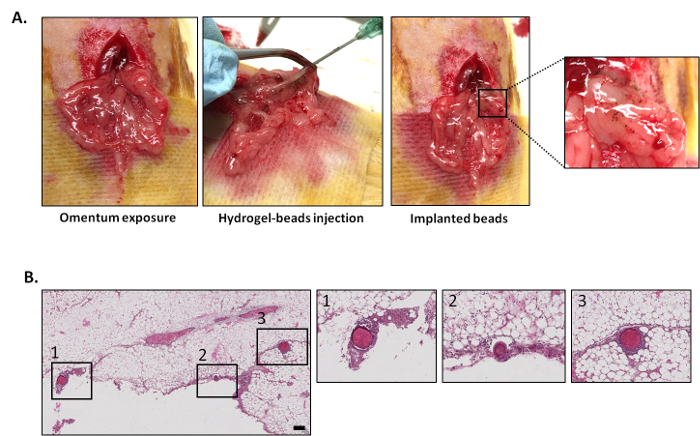
Figure 2: Description of hOMING technique and bead distribution through the omental tissue one day after implantation. (A) Illustration of the hOMING technique. After organ exposure (A, left), the islet-hydrogel mix (replaced here by blue-colored dextran beads for better visualization) was carefully injected in the tissue using an atraumatic needle (A, middle). Beads implanted in the tissue are visible (A, right). (B) Hematoxylin and eosin staining of omentum explanted 1 day after bead injection. Beads are found in the tissue with a uniform distribution. Scale bar = 100 mm. Please click here to view a larger version of this figure.
To validate this technique, we performed isogenic studies using Lewis rats (n=8). Diabetic rats receiving islet transplants (7660 islet equivalent, IEQ) per kg rat body weight using hOMING were monitored for glycemia and C-peptidemia for two months. Glycemia was controlled by implantation of insulin pellet (as attesting to the first drop in glycemia observed in the Figure 3A). Graft function was reflected by glycemia of approximately 2 g/L and C-peptidemia >500 pM. Before transplantation, rats were diabetic (glycaemia >5 g/L and C-peptidemia <200 pM). After transplantation and insulin pellet retrieval, glycemia maintenance and normalization was observed just 3 days post-hOMING transplantation and was maintained until graft retrieval (p < 0.05 compared to pre-transplant levels). After omental explantation, glycemia rose again to the pre-transplant level, attesting to the functionality of islets that were transplanted by hOMING (Figure 3A). The C-peptidemia pattern was exactly the opposite, with low to undetectable levels before the graft, followed by an increase and maintenance at this increased level throughout the course of the study (p <0.05), and, after omental explantation, a decrease to pre-transplant levels (Figure 3B). Analysis of the explanted omentum by histology revealed highly re-vascularized islets, most likely as a result of their proximity to blood vessels (Figure 3C).
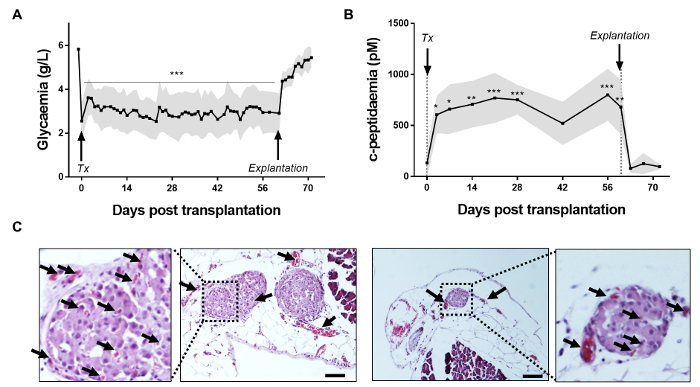
Figure 3: Two-month metabolic follow-up of rats receiving hOMING and graft assessment. (A) Glycemia measurement and (B) C-peptide assessment after hOMING using alginate as an islet carrier (Tx: Transplantation and insulin pellet retrieval; Explantation: Explantation of the omentum). Grafts are functional, as is shown by the maintenance of normoglycemia after insulin pellet retrieval and increase in C-peptidemia after islet implantation. Gray shaded areas represent the minimal and maximal recorded values at each time point. (C) Hematoxylin and eosin staining of an omental section after islet transplantation using the hOMING method. Islets are well-integrated into the tissue two months after implantation without any surrounding fibrotic tissue. Vessels have grown around and inside islets, as shown by the arrows, and thus completely restore islet function. Morphology of the islets also seems well-preserved. Scale bars = 50 µm. (n = 8) (*p < 0.05; ** p < 0.01; *** p < 0.001 determined using repeated measures analysis of variance (ANOVA) with Tukey's honest significance difference test as a post hoc test). Please click here to view a larger version of this figure.
Discussion
Some critical steps can be highlighted in this protocol. First, the person performing the surgery and manipulating the tissues must be delicate with the fat tissue, as it is fragile. Crushing or damaging the omentum needs to be avoided. Omentum, as a defensive tissue, is enriched in macrophages and other leukocytes. These immune cells can be activated by excessive manipulation and could negatively affect the graft. Secondly, graft (islet-hydrogel mixture) loading is also critical. The person performing the procedure must avoid dead volumes and must load all of the hydrogel-islet mixture into the injection device. Immediately after this step, another critical point is the injection itself. Injection must be performed slowly, with care, and delicately. The syringe must be flushed by the empty hydrogel loaded initially to retrieve any remaining islets. Thirdly, suturing must be done carefully and in two steps. The muscular plan should be sutured first, taking care not to suture the omentum with the muscle, and the skin should be sutured separately. Regarding the explantation procedure for the omental graft, special attention should be taken at the moment when the arteries close to the stomach (gastroepiploic arteries) are incised. It is important to pay attention to any hemorrhages that occur and stop them before suturing the animal, as any persistent bleeding leads to animal death within several days.
Troubleshooting may be necessary regarding the medium for carrying islets; the choice of hydrogel is up to the experimenter, as long as the hydrogel is injectable. The use of different materials can result in variable graft function (with or without supplementation, for example). The method described here has proven its efficiency for inert co-transplanting material with an islet ratio of 7660 IEQ/kg.
The present method may be limited by the omental size and hydrogel properties. To use a diabetic rodent model, insulin therapy is mandatory prior to islet implantation to provide sufficient grafting area. Diabetic rodents lose a lot of weight and fat mass due to STZ-induced diabetes. Considering the small weight of the animals enrolled in this study, grafting in an even smaller area is not possible.
Conservation of proper glycemia management (before transplant using pellets and thereafter using long-acting insulin) is mandatory. Here, we chose to conserve the insulin pellet until the day of transplantation for several reasons. First, the maintenance of a proper glycemic control considerably reduces oxidative stress induced by diabetes in the recipient organs, which can be deleterious for islet graft11 and allows continuation of intensive insulin therapy and recipient preparation observed in the clinic12. Second, we wanted to limit the maximum number of anesthesia procedures for the rats. Concerning the implementation of insulin therapy during the metabolic follow-up using long-acting insulin, the scheme used avoided massive complications due to glucotoxicity (which can destroy grafts) and to conserve a "real" glycemia value.
To monitor graft efficiency, glycemia is not a relevant parameter in the first few days if rats are receiving insulin therapy using pellets. This is why checking the C-peptide level several times prior to transplant is mandatory. These times include at the beginning of the study, to select animals after STZ injection, and then just preceding transplantation, to ensure that rats remain diabetic and that regeneration is minimal.
Hydrogel properties are also important. Liquid hydrogel will leak out of omental tissue and highly viscous hydrogel will not be injectable. Hydrogel viscosity and injectability has to be tested before use, but it appears essential to also test the material directly by evaluation of islet or another cells survival in vitro. Here, as islets are very sensitive to hypoxia and the use of a highly viscous carrier can affect oxygen diffusion.
In terms of the significance of the method described here with respect to existing methods, this intra-tissue transplantation technique has benefits in term of reproducibility. It is also atraumatic for the tissue and, because of its extra-vascular location, avoids bleeding and thrombosis risks. Also, when considering islet transplantation, instant blood-mediated inflammatory reaction is avoided14. Furthermore, hOMING allows transplanting islets into a non-vital organ, unlike transplantation into the liver, which poses risks in terms of hepatic function15.
To produce optimal performance, a hydrogel has to be adapted to the cells it will carry. Use of growth factors or specific proteins can have a positive effect on transplanted cells16,17.
To conclude, this technique can be used for in vivo validation of a hydrogel on different cell types, especially when an active metabolic environment is needed, as for islets. In addition, this surgical technique is applicable to multiple applications and could be, in the future, rapidly transferable to humans, as it can be easily performed using laparoscopy.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was funded by Région Alsace, BioArtMAtrix-Pôle Alsace Biovalley-CQDM; 53/14/C1. The authors are grateful to the team of Pr. Bruant-Rodier from the Hôpitaux Universitaires de Strasbourg for helping develop this innovative technique.
Materials
| Alginate (PRONOVA UP LMV) | Novamatrix | 4200206 | Hydrogel carrier |
| Atraumatic needle (Blunt) | B.Braun | 9180109 | |
| CMRL without FBS | Gibco | 11500576 | |
| C-peptide ELISA kit | Mercodia | 10-1172-01 | |
| Eosin | Leica Microsystems | 3801592E | |
| Ethilon 4/0isoflo | Ethicon | F2414 | Surgical suture |
| Hematoxylin | Leica Microsystems | 3801562E | |
| Insulin pellets | Linshin | INS-B14 | |
| Isofluorane | Centravet | ISO007 | |
| Lantus (Insulin-Glargin) | Sanofi Adventis | Lantus SoloStar | Long acting insulin |
| Metacam | Boehringer Ingelheim | MET019 | Anti-inflammatory drug |
| NaCl (for saline 0.9%) | Sigma | 10112640 | |
| Needle 26G | TERUMO | 050101B | |
| Oxygen | Linde | 2010152 | For isoflurane use |
| Pentobarbital sodique | Vetoquinol | Dolethal | For euthanasia |
| Steranios 2% | Anios | 11764046 | |
| Streptozotocin | Santa-Cruz | SC-200719A | |
| Syringe – Injekt-F | B.Braun | 9166017V | |
| Trocar & stylet (linshin) | Linshin | G12-SS | For pellet insertion |
References
- Bakhshandeh, B., et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnology & genetic engineering reviews. 33, 144-172 (2017).
- Lutolf, M. P., Gilbert, P. M., Blau, H. M. Designing materials to direct stem-cell fate. Nature. 462, 433-441 (2009).
- Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., Peppas, N. A. Hydrogels in regenerative medicine. Adv Mater. , 3307-3329 (2009).
- Rice, J. J., et al. Engineering the regenerative microenvironment with biomaterials. Advanced healthcare materials. 2, 57-71 (2013).
- Bai, X., et al. Bioactive hydrogels for bone regeneration. Bioactive Materials. 3, 401-417 (2018).
- Allbright, K. O., et al. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve. , (2018).
- Van Der Windt, D. J., Echeverri, G. J., Ijzermans, J. N. M., Cooper, D. K. C. The Choice of Anatomical Site for Islet Transplantation. Cell transplantation. 17, 1005-1014 (2008).
- Zweifach, B. W., Lipowsky, H. H. Quantitative studies of microcirculatory structure and function. III. Microvascular hemodynamics of cat mesentery and rabbit omentum. Circ Res. 41, 380-390 (1977).
- Bruant-Rodier, C., Dissaux, C., Baratte, A., Francois Fiquet, C., Bodin, F. The breast of the adolescent girl. Ann Chir Plast Esthet. 61, 629-639 (2016).
- Schaschkow, A., et al. Extra-Hepatic Islet Transplantation: Validation of the h-Omental Matrix Islet filliNG (hOMING) Technique on a Rodent Model Using an Alginate Carrier. Cell transplantation. 27, 1289-1293 (2018).
- Schaschkow, A., et al. Impact of the Type of Continuous Insulin Administration on Metabolism in a Diabetic Rat Model. Journal of diabetes research. 2016, 8310516 (2016).
- Schaschkow, A., et al. Impact of an autologous oxygenating matrix culture system on rat islet transplantation outcome. Biomaterials. 52, 180-188 (2015).
- Kissler, H. J., et al. Validation of methodologies for quantifying isolated human islets: an Islet Cell Resources study. Clinical transplantation. 24, 236-242 (2010).
- Delaune, V., Berney, T., Lacotte, S., Toso, C. Intraportal islet transplantation: the impact of the liver microenvironment. Transplant international : official journal of the European Society for Organ Transplantation. 30, 227-238 (2017).
- Leitao, C. B., et al. Liver fat accumulation after islet transplantation and graft survival. Cell transplantation. 23, 1221-1227 (2014).
- Narang, A. S., Mahato, R. I. Biological and biomaterial approaches for improved islet transplantation. Pharmacological reviews. 58, 194-243 (2006).
- Alvarado-Velez, M., Pai, S. B., Bellamkonda, R. V. Hydrogels as carriers for stem cell transplantation. IEEE Trans Biomed Eng. 61, 1474-1481 (2014).

