Induction of Drug-Induced, Autoimmune Hepatitis in BALB/c Mice for the Study of Its Pathogenic Mechanisms
Summary
We describe an in vivo immunization, translational hepatitis model in BALB/c mice that can be utilized to study the pathogenesis of drug-induced autoimmune hepatitis including sex differences seen in this disease. We will describe how this model demonstrates reproducible analyses using in vivo and in vitro experimental techniques.
Abstract
Drug-induced autoimmune hepatitis (DIH) is the most common hepatic drug-induced hypersensitization process observed in approximately 9 to 12% of patients with autoimmune hepatitis. The overwhelming majority of patients with DIH are women. The underlying mechanisms of these sex differences in prevalence are unclear because of the paucity of animal models that mimic human disease. Even so, underlying mechanisms are widely believed to be associated with human leukocyte antigen haplotypes and sex hormones. In contrast, using a DIH mouse model, we have uncovered that IL-4 initiated CD4+ T cells directed against an epitope of cytochrome P450 2E1 induces influx of neutrophils, macrophages and mast cells into the livers of female BALB/c mice. Using this model, we have also shown that IL-33-induced FoxP3+regulatory T cells confer protection against DIH in female and male mice. This DIH model is induced by immunizing mice with an epitope of CYP2E1 that has been covalently altered with a drug metabolite that has been associated with DIH. This epitope is recognized by patients with DIH. Our method induces robust and reproducible hepatitis and autoantibodies that can be utilized to study the pathogenesis of DIH. While in vivo studies can cause undue pain and distress in mice when done improperly, the advantage of an in vivo model is the ability to evaluate the pathogenesis of disease in a large number of mice. Additionally, biological effects of the altered liver proteins can be studied using invasive procedures. The addition of in vitro studies to the experimental design allows rapid repetition and mechanistic analysis at a cellular level. Thus, we will demonstrate our model protocol and how it can be utilized to study in vivo and in vitro mechanisms of DIH.
Introduction
The purpose of this method is to describe a mouse model of drug-induced autoimmune hepatitis that develops in vivo and demonstrate how it can be utilized to investigate the molecular, immunologic and genetic basis of this disease. The long-term objective of our studies is to uncover mechanisms responsible for the development of chronic liver inflammation and injury by studying DIH in susceptible patients. Liver disease and cirrhosis constitute the sixth most common cause of death in adults between the ages of 25 and 64. Idiosyncratic DILI, sometimes referred to as drug-induced autoimmune hepatitis (DIH) is the third most common cause of acute liver failure in the United States. DIH is the most common hepatic drug-induced hypersensitization process observed in approximately 9 to 12% of patients with autoimmune hepatitis1. The overwhelming majority of patients with DIH are women2,3,4. A type of DIH develops in susceptible individuals following administration of halogenated volatile anesthetics such as isoflurane, sevoflurane, desflurane or halothane. These anesthetics covalently binds to liver proteins with reactive products of their metabolism, thus creating novel autoantigens capable of eliciting allergic or autoimmune responses5.
The study of pathogenic mechanisms involved in the development of anesthetic and any form of DIH has been previously hampered by the lack of an animal model that closely mimics the induction of human disease. We have developed an experimental murine model of DIH with features resembling immune-mediated DILI in patients. Hepatitis is induced by immunization with one of two autoantigens that have been covalently modified by the trifluoroacetyl chloride (TFA) metabolite that is formed following oxidative metabolism of the anesthetic by the enzyme cytochrome P450 2E1 (CYP2E1)5. One autoantigen is the hepatic cytosolic S100 liver fraction, which is a mixture of several proteins6, and the second autoantigen is an epitope of CYP2E1 that is recognized by sera from patients with anesthetic immune-mediated DILI7. By using BALB/c mice, which are relatively resistant to experimental autoimmune hepatitis, we distinguish our model from the S100-induced immunization model of autoimmune hepatitis in C57Bl/6J mice8.
Because of its diverse clinical presentations, DIH is difficult to study in patients. Translational experimental models offer the ability to evaluate the pathogenesis of disease in vivo and in vitro. At present, there are no other alternative methods for inducing DIH that fully examine in vivo or in vitro adaptive or innate immune responses without the use of animals. Moreover, since trifluoroacetylation of S-100 or the CYP2E1 epitope does not appear to produce an irritating immunogen, and we are inducing DIH by immunization with TFA-altered proteins, these animals will not receive ether, any halogenated anesthetic, barbiturate or alcohol prior to immunization or other procedures, considering that these agents may alter the parameters we are studying. Even so, we have decreased our mouse usage by utilizing computer simulation to confirm the binding preferences of our discovered CYP2E1 epitope9 and have mirrored human DIH implicating female sex by demonstrating that female BALB/c mice develop a more severe DIH10.
In spite of diverse presentations of DIH in patients and challenges in the study of clinical disease, post-translational modification of native proteins by reactive drug metabolites is an accepted key mechanism in the pathogenesis DIH that follows halogenated anesthetics11. Investigators also accept that CYP2E1 is a major autoantigen in this process12,13. The role of interleukin (IL)-4–upregulated CD4+T cells that recognize a post-translationally modified CYP2E1 and other liver proteins is an accepted initiator of anesthetic DIH by attracting neutrophils, eosinophils and mast cells into the liver14, and this mechanism has been confirmed in many forms of DIH15,16. Induced FoxP3-expressing CD4+CD25+T cells (Tregs) reduce the severity of DIH, and relative deficiencies of these cells in the spleen worsen DIH 10,7. Thus, the majority of advances in understanding DIH have been made possible by utilizing in vivo mouse models to evaluate the genetic, metabolic and immunologic mechanisms of DIH both in vivo and in vitro.
Because we and other investigators have uncovered roles for IL-4, neutrophils, and eosinophils in the initiation of DIH using different mouse models, we believe that this observation supports our contention that regardless of the DIH model utilized, hepatitis and injury are induced by IL-4. The strength of our protocol lies in the utilization of in vivo methodology, both male and female mice, and repetition of histology, CD4+ T cell proliferation assays and cytokines. The strength of our use of in vitro studies is that they reduce the numbers of mice needed while providing the methodology to isolate cellular interactions that drive DIH. We recommend the use of male and female mice because this reduces the possibility of unconscious bias in interpretation of results and strengthens the translation potential of our studies since the incidence, prevalence, and severity of DIH is higher in women17. We recommend that mice are obtained from a single vendor; however, if this is not possible, obtain litter mate controls or wild-type mice from the same vendor as the genetically altered mice.
Protocol
All procedures were approved by the animal care and use committee.
1. Trifluoroacetylation of hepatic S-100 cytosolic proteins or a CYP2E1 epitope
NOTE: First, prepare the trifluoroacetylated S100 (TFA-S100) and trifluoroacetylated CYP2E1 epitope (TFA-JHDN5). Because syngeneic S100 proteins are needed for immunizations, and BALB/c mice are required to produce the immunogen. The preparation yields a large amount of immunogen; so, anticipate performing this portion around four times a year. An identical method will be used to make the TFA-JHDN5. The CYP2E1 epitope (JHDN5), GII/ FNN/ GPT/ WKD/ IRR/ FSL/ TTL, can be sequenced or purchased.
- Isolation of the S100 fraction of the liver.
- Following sedation of 5 – 10 BALB/c mice with 40-60 mg/kg ketamine mixed with 4 – 6 mg/kg xylazine, confirm proper depth of anesthesia by observing a reduction in muscle tone and no response to painful stimuli, such as a toe pinch (withdrawal reflex), in addition to the loss of righting reflexes and the loss of palpebral reflexes.
- Using microsurgical scissors, expose the intra-abdominal contents using a midline incision and make a small cut in the inferior vena cava to remove the blood.
- Place a 24-gauge angiocatheter into the portal vein and perfuse the liver 10 mL/min with 40 mL of phosphate buffered saline (PBS) pH 7.4 in a water bath at 4 °C. Remove and weigh the pooled livers and cut it into small pieces (10 – 15 mm).
NOTE: Euthanasia is induced by intraperitoneal injection of additional ketamine/xylazine (80 mg/kg: 8 mg/kg), followed by cervical dislocation and opening the chest cavity to collapse the lungs. This method provides the least pain and discomfort to the animals. This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. - Add 4 times the weight of sucrose (250 mM) -TRIS (10 mM)- EDTA (1 mM) homogenization buffer (pH 7.4) supplemented with Complete Protease inhibitor Cocktail tablets (see Table of Materials) as per manufacturers recommendations. Homogenize in a 15 mL polypropylene tube, using a general laboratory tissue homogenizer on medium speed on ice until smooth. Homogenize on ice to prevent the tissue from becoming warm during homogenization.
- Centrifuge the liver homogenates at 1500 x g for 10 min and then pour off the supernatant. Centrifuge the supernatant for 1 h at 100,000 x g. Snap freeze the supernatant and store at -80 °C. The supernatant is cytosolic S-100.
- Trifluoroacetylation of S100 and JHDN5
NOTE: Trifluoroacetylation of the ε-amino groups of lysine residues of S-100 will be performed according to the procedure of Satoh18. All portions of this experiment with the exception of the latter days of dialysis are performed in the fume hood.- Determine the total protein concentration of the cytosolic S-100 using the bicinchoninic acid assay (BCA assay)7. Dilute 20 mg of BALB/c mouse S100 or JHDN5 to 10 mL with dH2O in a 50 mL Erlenmeyer flask. Adjust the pH to 10 with 1N KOH.
- Add 4.7 mmole of S-ethyltrifluorothioacetate (S-ETFA), to the solution. Maintain the pH between 9.9 – 10.0 with 1N KOH by administering KOH in droplet fashion for approximately 1 h. Record total volume of KOH for each reaction.
- Transfer the solutions into separate dialysis cassettes (Do not overfill). Dialyze the cassettes for 72 h against 4 L of dH2O with three changes per day. After dialysis, record the final volume of TFA-S100 or TFA-JHDN5 and then aliquot into labeled tubes. Snap freeze and store at –80 °C.
- An estimated concentration is determined by dividing the initial amount of S100 or JHDN5 (in mg) by the final volume following dialysis (mL). To determine the percent modification of the native protein19, dilute 1.0 mg of each native and TFA-altered protein (if the final concentration is greater than 1.0 mg) to 1.0 mL with dH2O in separate bullet tubes and prepare a blank using 1.0 mL dH2O. If the concentration of the TFA-altered protein is less than 1.0 mg, do not dilute
- To separate wells of a 96 well plate, add 50 µL of the blank, native and altered proteins. Add 50 µL of 4% NaHCO3 followed by 50 µL of 0.1% 2,4,6-trinitrobenzene sulfonic acid to each well.
- Incubate the plate at 40 °C for 2 h. Following the incubation, add 50 µL of 10 % SDS to each well followed by 25 µL of 1N HCl.
- Read at OD of 334 nm and then record absorbance of each compound from 200 – 600 nm in order to confirm the characteristic drop in absorbance at 334 nm. Calculate the percent modification of lysine residues by TFA, using the following formula:

2. Immunization of mice to induce hepatitis
NOTE: DIH is modeled in BALB/c mice by immunizations with liver cytosolic proteins that have been covalently altered by trifluoracetyl chloride (TFA), a model drug-metabolite, TFA-S1006 or an epitope of CYP2E1 covalently altered by TFA9, TFA-JHDN5 that induces hepatitis, autoreactive T cells, and CYP2E1 autoantibodies. Mice exhibit a splenic activation phase 2 weeks after the initial immunization and a hepatic phase by 3 weeks that is characterized by granulocytic inflammation. Female BALB/c mice are more susceptible than males to hepatitis in this model.
- On day 0, immunize 6–8 week-old BALB/c mice subcutaneously at the base of the neck with 200 μg of TFA-S100 or 100 µg of TFA-JHDN5 emulsified in equal volumes of complete Freund’s adjuvant (CFA). On day 0, immunize the mice with 50 ng of pertussis toxin, intramuscularly in one of the hind legs. On day 7, immunize the mice subcutaneously at the base of the tail with either 200 μg of TFA-S100 or 100 µg of TFA-JHDN5 emulsified in equal volumes of CFA to ensure that each mouse receives two injections of the same immunogen.
NOTE: The mice are monitored hourly for the first 6 hours following the immunization and then at least twice daily for one week. If the mice demonstrate signs of pain or distress, such as hunched posture or ruffled fur, analgesics should be administered in accordance with the local ACUC. If significant pain or distress is noted, the mice should be evaluated by a veterinarian to determine if euthanasia is necessary. - Determination of CD4+ T cell immune responses to whole self-proteins, epitopes of self-proteins or the TFA hapten using flow cytometry
- Following sedation of mice with 40-60 mg/kg ketamine mixed with 4-6 mg/kg xylazine via intraperitoneal injection, confirm the proper depth of anesthesia as described in step 1.1.1 and then identify the spleen after exposing the intra-abdominal cavity using microsurgical scissors. Cut the spleen at the pedicle and place in a Petri dish with PBS/2% fetal calf serum (FCS).
NOTE: Euthanasia will be induced in the mice by intraperitoneal injection of additional ketamine/xylazine (80 mg/kg: 8 mg/kg), followed by cervical dislocation and opening the chest cavity to collapse the lungs. This method provides the least pain and discomfort to the animals. This method is consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. - Release the cells using frosted glass slides and transfer to a 50 mL conical polypropylene tube. Wash with PBS/2% FCS by bringing the volume up to 50 mL and centrifuging at 335 x g using a benchtop refrigerated centrifuge. Pour off the supernatant and repeat this step.
- Remove red cells using 1 mL of ACK Lysing buffer for 1 min and bring volume up to 50 mL with PBS/2% FCS. Centrifuge at 335 x g and pour off the supernatant.
- Count the cells. Label the cells with CFSE for 30 min on ice in the dark, as per manufacturer’s instructions. Suspend single cell suspensions into 6 well plates of 3×106 cells/mL per well in PBS/2% FCS.
- Stimulate labeled cells with either CYP2E1, JHDN5, or TFA-OVA (10 µg/mL) for 72 h at 37 °C in 5% CO2, 95% air (humidified). After incubation, stain the cells with CD4-APC (1:100) for 30 min on ice and analyze by flow cytometry within 3 days.
- Utilize the following gating strategy to identify CD4+CFSE+ cells: CD4+CFSE+ cells will be identified from the gated alive cells and displayed as histograms of proliferating cells.
- Following sedation of mice with 40-60 mg/kg ketamine mixed with 4-6 mg/kg xylazine via intraperitoneal injection, confirm the proper depth of anesthesia as described in step 1.1.1 and then identify the spleen after exposing the intra-abdominal cavity using microsurgical scissors. Cut the spleen at the pedicle and place in a Petri dish with PBS/2% fetal calf serum (FCS).
- Isolation of infiltrating immune cells from immunized mice.
- To isolate infiltrating immune cells in the livers on day 14 or day 21, anesthetize the mice by intraperitoneal injection with 40-60 mg/kg ketamine mixed with 4-6 mg/kg xylazine and confirm the proper depth of anesthesia as described in step 1.1.1. After laparotomy using a midline incision made with micro surgical scissors as described in 1.1.2, cannulate the portal vein with a 25 gauge needle and then cut the inferior vena cava below the renal veins.
- Perfuse each liver at a flow rate of 10 mL/min with 40 mL of PBS in a water bath 37 °C. After perfusion, using micro surgical scissors, cut the liver at the hepatic pedicle, remove the gall bladder and then cut the liver at the hilum.
- Disrupt the liver on a mesh stainless steel sieve using a 20 mL sterile syringe pestle and cold PBS. Filter the resulting cell suspension into 50 mL pre-sterilized centrifuge tubes using a 300 mesh screen. Bring each suspension to 50 mL using cold PBS and then wash the suspension by centrifugation for 10 min at 370 x g.
- Discard the supernatant and then pool each pellet by treatment into new 50 mL tubes (One tube per mouse is recommended; however, if samples are pooled, 2-4 pellets/tube is recommended). Suspend the pooled pellets in 45 mL Percoll 35% (in PBS), and 100 IU/mL heparin.
- Spin each tube at 500 x g for 10 min at 20 °C. Discard the supernatant and suspend the pellet in 5 mL of PBS and then add 1 mL of ACK lysing buffer to each pellet for 10 min on ice.
- Bring each tube to 50 mL with PBS and wash by centrifugation for 10 min at 370 x g. Discard the supernatant and then wash the cells with PBS/2% FCS by centrifugation for 10 min at 370 x g. Count the cells.
- Analysis of cell type using flow cytometry
NOTE: Here is an example of how induced Foxp3+Tregs can be detected.- Incubate 1×106 cells with FcR blocking reagent and stain with 1:100 dilutions of CD4-FITC, CD25-PE, and CD45-PerCP for 30 min on ice. Next, stain the cells intracellularly with FoxP3-APC.
- Fix the cells with 250 µL of fixation buffer (see Table of Materials), and store at 4 °C until analysis by flow cytometry within 3 days.
- The following gating strategy is recommended in order to detect induced Foxp3+Tregs in single cell suspensions from liver, spleen or lymph nodes using flow cytometry: Identify live cells using Live/Dead Fixable Aqua Dead Cell stain kit. Next, gate on liver cells that are CD45+ (PerCP, clone RA3-6B2), and gate CD4+ cells (FITC, cloneGK1.5) from the CD45+ gate. From the CD4+ cells, identify the percentages of CD25+(PE, clone 7D4) and FoxP3+ (APC, clone 3G3) cells.
- Histological analysis of liver tissues for hepatitis
- On day 21, fix the liver sections (5 µm thick) in 10% neutral buffered formalin and stain with Hematoxylin &Eosin.
- Determine histology scores first at low power (40X) in an average of 2 views and confirm at 64X. Score the tissue sections as follows: Grade 0=no inflammation or necrosis; Grade 1= minor lobular inflammation with no necrosis; Grade 2= lobular inflammation involving <50% of the section; Grade 3=lobular inflammation involving ≥ 50% of the section; and Grade 4=inflammation with necrosis.
- Determination of tissue cytokine levels in spleens and livers.
- On day 14 or day 21, homogenize the liver or spleen samples (1 g) from each mouse in 1 mL of RPMI/2% FCS until smooth using a general laboratory homogenizer on medium setting. Keep the sample cold on ice.
- Centrifuge the homogenate for 15 min at 1455 x g at 4 °C using a refrigerated desktop centrifuge. Snap freeze the supernatant and store at -80 °C until ready for use. Cytokine and chemokine levels can be measured with commercial ELISA kits, as per kit instructions. Standardize the levels of cytokines by converting the levels (in mL or µL) to pg/g of tissue.
- Detection of serum antibodies to CYP2E1, the CYP2E1 epitope JHDN5 and the TFA drug metabolite.
- On days 14 or 21, sedate the mice with 40-60 mg/kg ketamine mixed with 4-6 mg/kg xylazine. Confirm proper depth of anesthesia by observing a reduction in muscle tone and no response to painful stimuli, such as a toe pinch (withdrawal reflex), in addition to the loss of righting reflexes and the loss of palpebral reflexes. Utilize a 25 G needle attached to a tuberculin syringe and approach the heart by slowly advancing the needle into the thoracic cavity under the ribs and immediately lateral to the xiphoid process. Collect blood using intracardiac puncture.
- Once blood is collected, allow it to clot at room temperature. Centrifuge the blood samples at 295 x g for 20 min at room temperature. Carefully remove the sera, aliquot the sera and snap freeze at -20 °C.
- Apply 100 µL of CYP2E1, JHDN5, or TFA-ovalbumin (OVA) test antigens (5 µg/mL in PBS) to 96 well plates for at least 18 h at 4 °C overnight. The next day, wash the plates with wash buffer (PBS/2%FCS), 2 cycles (4 washes each).
- Apply 100 µL of mouse sera (1:100) in PBS/2% FCS in triplicate on the plates and incubate at room temperature for 2 h. After 2 h, wash the plates with wash buffer as described in step 2.6.2.
- Add 100 µL of Alkaline phosphatase (AKP)-goat anti-mouse IgG, AKP-rat anti-mouse IgG1, or AKP-rat anti-mouse IgG2a secondary antibodies (1:1000) for 2 h followed by a wash step of 1 cycle with wash buffer and 1 cycle with PBS. Detect the antibodies using an AKP substrate kit and measure at OD 405 nm every 15 min with a spectrophotometer. TFA usually develops completely in 15 min while CYP2E1 and the CYP2E1 epitope can develop from 30 to 60 min7.
- Studies of the development of JHDN5 IgG-induced oxidative stress in vitro.
- Using 12 well plates, incubate 106 terminally differentiated hepatic cells per well on fibronectin-covered cover slips in 1000 µL of Williams E media supplemented with glutamine and general supplement (see Table of Materials) at 37 °C, 5% CO2, 95% humidity for 7 days as recommended to maximize CYP2E1 activity.
- Add JHDN5 IgG (1:40) or mouse IgG (1:1000) to separate wells and incubate for 2 h at 37 °C, 5% CO2, 95% humidity. Hybridoma sera was very dilute. Add deep red fluorescent antibody detector (see Table of Materials) for an additional 30 min to all wells.
- Wash the wells 3x with 1 mL of PBS in the dark. Fix the cells in 3.7% formaldehyde for 10 min. Examine by confocal microscopy within 24 h.
- Co-localization studies of JHDN5 IgG with intracellular organelles such as mitochondria in vitro.
- To demonstrate co-localization of JHDN5 IgG with mitochondria, sparsely culture (~30% confluence) terminally differentiated hepatic cells on fibronectin-covered cover slips for 7 days in dye–free Williams’s media E supplemented as described in step 2.7.
- After determining the correct absorption wavelengths, add green fluorescent, 488 nm -conjugated mouse IgG or JHDN-5 (1:100) and Red fluorescent, 594-conjugated Mito-tracker Red (1:100) for 2 h (37 °C), 5% CO2, 95% humidity.
- Mount labeled fibronectin-covered cover slips with Anti-fade Reagent with DAPI, and examine by confocal microscopy.
3. General protocol notes
- Utilize non-pharmaceutical grade tools when compounds are not available in a clinical use formulation. However, obtain each of these tools from reliable commercial suppliers identified in this method. Always use chemicals that conform to specifications defined by the Committee on Analytical Reagents of the American Chemical Society of at least the reagent grade level. For our methods, utilize analytical grade level reagents whenever possible.
- Follow strict aseptic technique for the formulation of the TFA-altered proteins in order to prevent contamination that could adversely affect animal welfare or the interpretation of data.
- Store and use non-pharmaceutical grade formulations at durations for which the formulation will remain potent, as per available technical information. Store CFA at room temperature, CYP2E1 and its epitopes at -20 or -80 °C. Store TFA-altered proteins at -80 °C and allowed to come to 4 °C prior to emulsification with CFA. Store TFA altered proteins at -80 °C and store in aliquots in order to prevent repeat freeze-thaw cycles.
Representative Results
The immunization schedule utilized to induce DIH shown in Figure 1 represents the two immunizations required at the base of the neck (day 0) and the base of the tail (day 7). Figure 2 shows representative proliferation data obtained on day 14 using CFSE in response to CYP2E1, JHDN5, the CYP2E1 epitope and the trifluoroacetyl (TFA) metabolite of the anesthetics. Figure 3 shows the gating strategy and representative flow cytometry analysis of induced CD4+CD25+FoxP3+ Tregs obtained on day 14. Figure 4 shows representative hematoxylin and eosin stained slides demonstrating the evolution of hepatitis on day 21 6. Figure 5 shows representative hematoxylin and eosin stained slides demonstrating more severe hepatitis in female BALB/c mice when compared to males on day 21 in addition to the comparative cellular content in these livers10. Figure 6 shows representative confocal microscopy slides demonstrating the absence of co-localization of mouse IgG with mitochondria. Figure 7 shows representative confocal microscopy demonstrating co-localization of JHDN5 IgG with mitochondria.
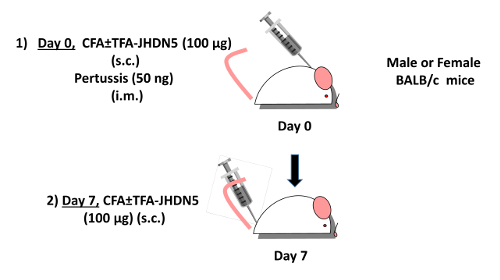
Figure 1: Immunization of mice to induce hepatitis. DIH can be induced in female BALB/c mice (as an example) by immunization with TFA-JHDN5 (100 µg) emulsified in complete Freund’s adjuvant (CFA) subcutaneously (s.c.) at the base of the neck and 50 ng of pertussis toxin intramuscularly (i.m.) in the hind leg on day 0 (Step 1). On day 7, BALB/c mice can then be immunized with TFA-JHDN5 (100µg) emulsified in CFA (s.c.) at the bae of the tail. Please click here to view a larger version of this figure.
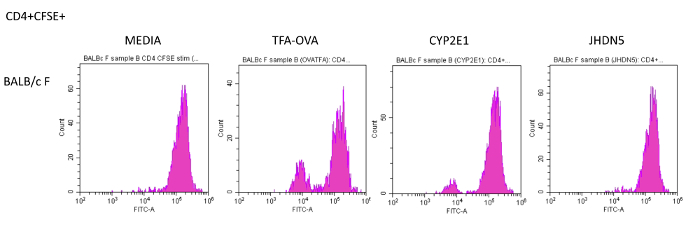
Figure 2: Determination of CD4+ T cell immune responses to whole self-proteins, epitopes of self-proteins or the TFA hapten using flow cytometry. Single cell suspensions of splenocytes from 6 – 8 week-old BALB/c mice isolated day 14 after the initial immunization, labeled with CFSE, stimulated with TFA-OVA, CYP2E1 or JHDN5 (10 µg/mL) for 72 h at 37 °C in 5% CO2, 95% air (humidified), stained with CD4-APC and analyzed by flow cytometry. Wells without antigen (media) were used as controls. BALB/c mice developed proliferation in response to OVA-TFA and CYP2E1 and not JHDN5, when compared to media. Please click here to view a larger version of this figure.
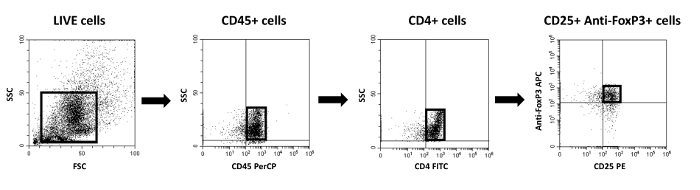
Figure 3: Gating strategy for the identification of CD4+CD25+FoxP3+ induced Tregs using the method described in 2.3.7. The first gate identifies live cells. To detect immune cells, CD45+ cells were initially gated. Next, CD4+ T cells were identified and gated, followed by identification of CD25+FoxP3+ cells within the CD4+ T cell population. Please click here to view a larger version of this figure.
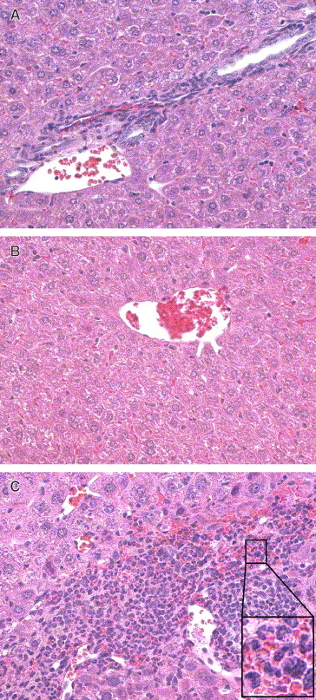
Figure 4: Histological analysis of liver tissues for hepatitis. CFA-immunized mice (top panel) were used as vehicle controls. S100-immunized mice (middle panel) were evaluated following immunizations on the same schedule. On day 21, mice were euthanized, and the liver fixed in formalin. Sections 5 μm thick were made and stained with hematoxylin and eosin (H&E). Minimal hepatic inflammation (blue cells) is demonstrated following CFA (top) and S100 (middle) immunizations and large amounts of inflammation following immunizations with TFA-S100. (H&E, magnification 64X). This figure is used with permission6. Please click here to view a larger version of this figure.
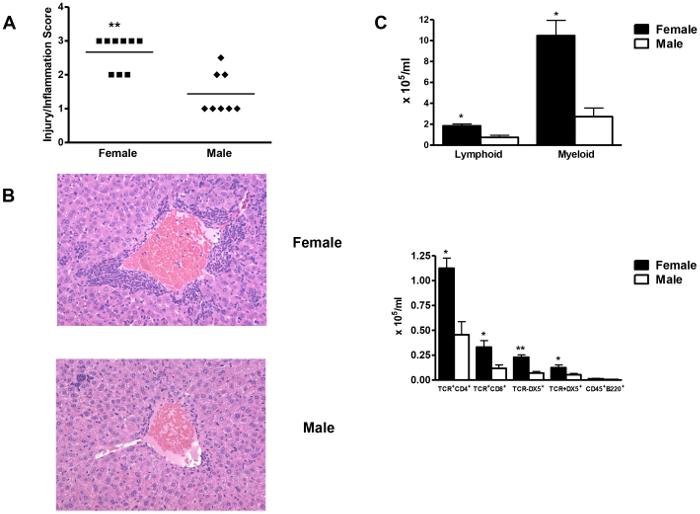
Figure 5: Female BALB/c mice develop more DIH when compared to male BALB/c mice. (A) Female mice (n = 8) had significantly more severe hepatitis 3 weeks after TFA-S100/CFA immunizations than did males (n = 7/group). (B) Representative liver sections from female and male mice (H&E, magnification 64X). (C) Numbers of hepatic CD4+, CD8+, NK+, and NKT+ cells were significantly higher in females than in males. Mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. This figure is used with permission10. Please click here to view a larger version of this figure.
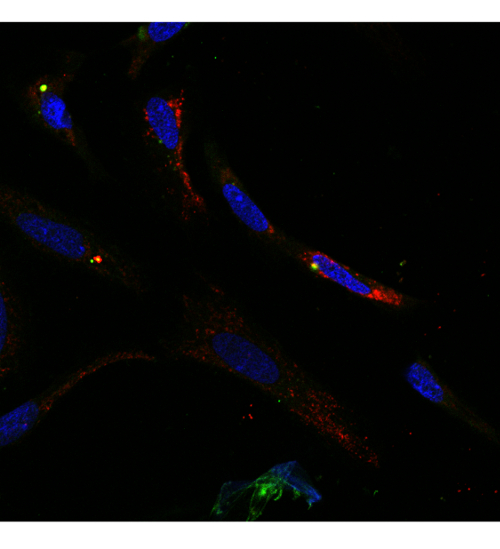
Figure 6: Mouse IgG does not co-localize with mitochondria. Confocal image of terminally differentiated hepatic cells stained with green fluorescent (488nm) -labeled mouse IgG (1:100) (green) in addition to red fluorescent (594)–-labeled Mitotracker Red (1:100). Green fluorescent (488nm)–labeled Mouse IgG does not co-localize with Mitotracker Red (63X magnification). Please click here to view a larger version of this figure.
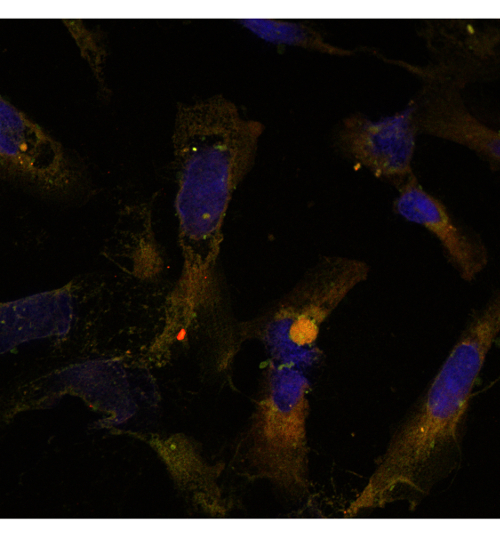
Figure 7: JHDN5 IgG co-localizes with mitochondria. Confocal image of terminally differentiated hepatic progenitor cells stained with green fluorescent (488)–- labeled JHDN-5 IgG (1:100) in addition to red fluorescent (594)–-labeled Mitotracker Red (1:100). Green fluorescent (488nm)–labeled JHDN-5 IgG co-localizes with Mito-tracker Red, demonstrated by the yellow hue on the representative image (63X Magnification). Please click here to view a larger version of this figure.
Discussion
The strength of this protocol rests in its reproducibility; so, it is critical to adhere to the suggested steps. Formulation of the immunogen can be a barrier for some; however, we have reproduced our model using the epitope described in our document, which removes the need to isolate the S100 fraction of the liver. It is likely that additional epitopes or proteins can be altered and induce hepatitis following immunizations; however, we describe those proteins that we have used with reliable results. Several proteins have been demonstrated to be trifluoroacetylated upon halogenated anesthetic exposure. Most likely due to epitope spreading, some of these proteins are also target of autoantibodies in their native, non-trifluoroacetylated state. As an example, the E2 subunit of the pyruvate dehydrogenase complex (PDH-E2) carries epitopes (the lipoic acid prosthetic group) with a structural similarity to the TFA-moiety in TFA-adducts. Antibodies generated in patients with halothane hepatitis have been demonstrated to be cross-reactive to TFA-proteins and PDH-E2 20,21.
Our DIH model requires two immunizations that are emulsified in CFA along the back of the mice. We know that footpad injections with CFA have been associated with pain and distress in the mouse. Hence, the development of the experimental DIH model included several experimental trials. When we evaluated the model using one immunization using CFA, with the second immunization using incomplete Freund’s adjuvant (IFA) or IFA in both injections with our immunogen, hepatic inflammation did not develop. In sharp contrast, when we immunized the mice with the immunogen using two immunizations with CFA, significant hepatic inflammation was present. The original description of another model of autoimmune hepatitis included studies similar to ours and required two immunizations with CFA in order to demonstrate significant hepatic inflammation8. Even so, we continuously re-evaluate adjuvants using literature searches to attempt to optimize inflammation without CFA. As an example, there is a well-known adjuvant Titer Max that would augment B cell responses; however, although antibodies are a component of DIH, we and others have demonstrated a critical role for T cell responses in our model14.
The development of reliable models facilitates the investigations of the pathogenesis of DIH. We demonstrate that liver histology and immune cells can be reliably evaluated at various stages in this model. We demonstrate identification of antigen-specific T cells, serum antibodies and tissue cytokines that can be utilized to study the development of DIH. We demonstrate the methods for isolating cells from the inflamed liver and suggest the use of heparin. However, we have performed this technique without heparin and the results were indistinguishable. Additionally, current methods of tissue disruption may also release cells from the liver.
Recently, we have utilized modern tools such next gen sequencing and quantitative PCR and have experienced reproducible results. We have published results regarding DIH using IL-4, IL-4 receptor, IL-6, IL-6 receptor IL-33 and ST2 deficient mice that were all derived on a BALB/c background so that we can utilize the BALB/c mouse as our control mouse. Future applications of this model of DIH will include development of knock in mice in addition to the utilization of CRISPR technology in order uncover previously unrecognized mechanisms responsible for the pathogenesis of DIH.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Dr. Njoku would like to acknowledge Dr. Noel R. Rose, MD PhD, for his guidance and insightful discussions that resulted in the formulation of this model.
Materials
| 0.1% 2,4,6-trinitrobenzene sulfonic acid (TNBS) | ThermoFisher | 28997 | |
| AKP Substrate Kit | BioRad | 172-1063 | |
| BALB/c mice | Jackson | ||
| CellTrace™ CFSE Cell Proliferation Kit | ThermoFisher | C34554 | |
| CFA H37Ra | Becton Dickinson (Difco Bacto) | 231131 | |
| FcR Blocking reagent | Milteyi | 130-092-575 | |
| General supplement | ThermoFisher | HPRG770 | |
| HepaRG™ cells cryopreserved | ThermoFisher | HPR GC10 | |
| Live/Dead Fixable Aqua Dead Cell stain kit | ThermoFisher | L34965 | |
| NaHC03 | Millipore Sigma | S5761 | |
| Percoll® | Millipore Sigma | P1644-1L | |
| Pertussis Toxin | List Biologicals | 180 | |
| Phosphate Buffered Saline pH 7.4 | Various | ||
| Pierce™ Protease Inhibitor Mini Tablets, EDTA Free | ThermoFisher | 88666 | |
| Potassium Hydroxide | JT Baker | 3140-01 | |
| S-ethyltrifluorothioacetate (S-ETFA) | Millipore Sigma | 177474 | |
| Slide-a-lyzer dialysis cassettes (10 K, 12 ml) | ThermoFisher | 66810 | |
| UltraPure™ SDS Solution, 10% | ThermoFisher | 24730020 | |
| Williams Media E, no phenol red | ThermoFisher | A1217601 |
References
- Castiella, A., Zapata, E., Lucena, M. I., Andrade, R. J. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J. Hepatology. 6 (4), 160-168 (2014).
- Bjornsson, E. S., Bergmann, O. M., Bjornsson, H. K., Kvaran, R. B., Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 144 (7), 1419-1425 (2013).
- Castiella, A., Lucena, M. I., Zapata, E. M., Otazua, P., Andrade, R. J. Drug-induced autoimmune-like hepatitis: a diagnostic challenge. Digestive Diseases and Sciences. 56 (8), 2501-2502 (2011).
- Czaja, A. J. Drug-induced autoimmune-like hepatitis. Digestive Diseases and Sciences. 56 (4), 958-976 (2011).
- Pohl, L. R., Thomassen, D., Pumford, N. R., Butler, L. E., Satoh, H., Ferrans, V. J., Perrone, A., et al. Hapten carrier conjugates associated with halothane hepatitis. Advances in Experimental Medicine and Biology. 283, 111-120 (1991).
- Njoku, D. B., Talor, M. V., Fairweather, D., Frisancho-Kiss, S., Odumade, O. A., Rose, N. R. A novel model of drug hapten-induced hepatitis with increased mast cells in the BALB/c mouse. Experimental and Molecular Pathology. 78 (2), 87-100 (2005).
- Cottagiri, M., Nyandjo, M., Stephens, M., Mantilla, J., Saito, H., Mackay, I. R., et al. In drug-induced, immune-mediated hepatitis, interleukin-33 reduces hepatitis and improves survival independently and as a consequence of FoxP3+ T-cell activity. Cellular and Molecular Immunology. , (2018).
- Lohse, A. W., Manns, M., Dienes, H. P., Meyer zum Buschenfelde, K. H., Cohen, I. R. Experimental autoimmune hepatitis: disease induction, time course and T-cell reactivity. Hepatology. 11 (1), 24-30 (1991).
- McCarthy, E. K., Vakos, A., Cottagiri, M., Mantilla, J. J., Santhanam, L., Thomas, D. L., et al. Identification of a Shared Cytochrome p4502E1 Epitope Found in Anesthetic Drug-Induced and Viral Hepatitis. mSphere. 3 (5), (2018).
- Cho, J., Kim, L., Li, Z., Rose, N. R., Talor, M. V., Njoku, D. B. Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for Tregs, estrogen, and IL-6. PLoS. One. 8 (4), 61186 (2013).
- Satoh, H., Gillette, J. R., Takemura, T., Ferrans, V. J., Jelenich, S. E., Kenna, J. G., et al. Investigation of the immunological basis of halothane-induced hepatotoxicity. Advances in Experimental Medicine and Biology. 197, 657-673 (1986).
- Eliasson, E., Kenna, J. G. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Molecular Pharmacology. 50 (3), 573-582 (1996).
- Bourdi, M., Chen, W., Peter, R. M., Martin, J. L., Buters, J. T., Nelson, S. D., et al. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chemical Research in Toxicology. 9 (7), 1159-1166 (1996).
- Njoku, D. B., Li, Z., Washington, N. D., Mellerson, J. L., Talor, M. V., Sharma, R., et al. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury. European Journal of Immunology. 39 (6), 1652-1663 (2009).
- Aithal, G. P., Ramsay, L., Daly, A. K., Sonchit, N., Leathart, J. B., Alexander, G., et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 39 (5), 1430-1440 (2004).
- Higuchi, S., Kobayashi, M., Yoshikawa, Y., Tsuneyama, K., Fukami, T., Nakajima, M., et al. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicology Letters. 200 (3), 139-145 (2011).
- Rubtsova, K., Marrack, P., Rubtsov, A. V. Sexual dimorphism in autoimmunity. Journal of Clinical Investigation. 125 (6), 2187-2193 (2015).
- Satoh, H., Fukuda, Y., Anderson, D. K., Ferrans, V. J., Gillette, J. R., Pohl, L. R. Immunological studies on the mechanism of halothane-induced hepatotoxicity: immunohistochemical evidence of trifluoroacetylated hepatocytes. Journal of Pharmacology and Experimental Therapeutics. 233 (3), 857-862 (1985).
- Habeeb, A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Analytical Biochemistry. 14 (3), 328-336 (1966).
- Christen, U., Burgin, M., Gut, J. Halothane metabolism: immunochemical evidence for molecular mimicry of trifluoroacetylated liver protein adducts by constitutive polypeptides. Molecular Pharmacology. 40 (3), 390-400 (1991).
- Christen, U., Quinn, J., Yeaman, S. J., Kenna, J. G., Clarke, J. B., Gandolfi, A. J., et al. Identification of the dihydrolipoamide acetyltransferase subunit of the human pyruvate dehydrogenase complex as an autoantigen in halothane hepatitis. Molecular mimicry of trifluoroacetyl-lysine by lipoic acid. European Journal of Biochemistry. 223 (3), 1035-1047 (1994).

