Studying RNA Interactors of Protein Kinase RNA-Activated during the Mammalian Cell Cycle
Summary
We present experimental approaches for studying RNA-interactors of double-stranded RNA binding protein kinase RNA-activated (PKR) during the mammalian cell cycle using HeLa cells. This method utilizes formaldehyde to crosslink RNA-PKR complexes and immunoprecipitation to enrich PKR-bound RNAs. These RNAs can be further analyzed through high-throughput sequencing or qRT-PCR.
Abstract
Protein kinase RNA-activated (PKR) is a member of the innate immune response proteins and recognizes the double-stranded secondary structure of viral RNAs. When bound to viral double-stranded RNAs (dsRNAs), PKR undergoes dimerization and subsequent autophosphorylation. Phosphorylated PKR (pPKR) becomes active and induces phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) to suppress global translation. Increasing evidence suggests that PKR can be activated under physiological conditions such as during the cell cycle or under various stress conditions without infection. However, our understanding of the RNA activators of PKR is limited due to the lack of a standardized experimental method to capture and analyze PKR-interacting dsRNAs. Here, we present an experimental protocol to specifically enrich and analyze PKR bound RNAs during the cell cycle using HeLa cells. We utilize the efficient crosslinking activity of formaldehyde to fix PKR-RNA complexes and isolate them via immunoprecipitation. PKR co-immunoprecipitated RNAs can then be further processed to generate a high-throughput sequencing library. One major class of PKR-interacting cellular dsRNAs is mitochondrial RNAs (mtRNAs), which can exist as intermolecular dsRNAs through complementary interaction between the heavy-strand and the light-strand RNAs. To study the strandedness of these duplex mtRNAs, we also present a protocol for strand-specific qRT-PCR. Our protocol is optimized for the analysis of PKR-bound RNAs, but it can be easily modified to study cellular dsRNAs or RNA-interactors of other dsRNA binding proteins.
Introduction
Protein kinase RNA-activated (PKR), also known as eukaryotic initiation factor 2-alpha kinase 2 (EIF2AK2), is a well-characterized protein kinase that transmits information provided by RNAs. It belongs to the eukaryotic translation initiation 2 subunit alpha (eIF2α) kinase family and phosphorylates eIF2α at serine 51 in response to infection to suppress global translation1. In this context, PKR is activated by viral double-stranded RNAs (dsRNAs), which provide a platform for PKR dimerization and autophosphorylation2. In addition to eIF2α, PKR can also phosphorylate p53, insulin receptor substrate 1, inhibitor κB, and c-Jun N-terminal kinase (JNK) to regulate activity of numerous signal transduction pathways3,4,5,6.
PKR was originally identified as a kinase that phosphorylated eIF2α during poliovirus infection by recognizing poliovirus’ dsRNAs7,8. PKR is increasingly found to play multifaceted roles beyond immune response, and its aberrant activation or malfunction is implied in numerous human diseases. Activated/Phosphorylated PKR (pPKR) is frequently observed during apoptosis and is a common characteristic of patients with degenerative diseases, particularly neurodegenerative diseases such as Huntington’s, Parkinson’s, and Alzheimer’s disease9,10,11,12,13. In addition, PKR is activated under various stress conditions such as metabolic stress and heat shock14,15,16,17. On the other hand, inhibition of PKR results in increased cell proliferation and even malignant transformation18,19. PKR function is also important in normal brain function and during the cell cycle as the level of pPKR is elevated during the M phase20,21,22. In this context, pPKR suppresses global translation and provides cues to key mitotic signaling systems that are required for proper cell division20. Moreover, prolonged activation of PKR resulted in G2/M phase cell cycle arrest in Chinese hamster ovary cells23. Consequently, PKR phosphorylation is regulated by the negative feedback loop to ensure rapid deactivation during M/G1 transition21.
Despite the wide range of PKR function, our understanding of PKR activation is limited due to the lack of a standardized high-throughput experimental approach to capture and identify dsRNAs that can activate PKR. Previous studies have shown that PKR can interact with dsRNAs formed by two inverted Alu repeats (IRAlus)20,24, but the possibility of the existence of additional cellular dsRNAs that can activate PKR during the cell cycle or under stress conditions in human cells was unexplored. The conventional approach in identifying RNA-interactors of an RNA binding protein (RBP) uses UV light to crosslink RNA-RBP complexes25,26,27. A recent study applied this UV crosslinking approach in a mouse system and identified that small nucleolar RNAs can regulate PKR activation during metabolic stress16. By utilizing high crosslinking efficiency of formaldehyde, we presented an alternative method to identify PKR-interacting RNAs during the cell cycle in HeLa cells28. A similar approach has been applied to study other dsRBPs such as Staufen and Drosha29,30,31. We found that PKR can interact with various types of noncoding RNAs such as short interspersed nuclear element (SINE), long interspersed nuclear element (LINE), endogenous retrovirus element (ERV), and even alpha-satellite RNAs. In addition, we showed that PKR can interact with mitochondrial RNAs (mtRNAs), which form intermolecular dsRNAs through complementary interaction between the heavy-strand and the light strand RNAs28. A recent publication further supported our data that some mtRNAs exist in a duplex form and can activate dsRNA sensors such as melanoma differentiation-associated protein 5 to induce interferons32. More importantly, the expression and subcellular localization of mtRNAs are modulated during the cell cycle and by various stressors, which may be important in their ability to regulate PKR activation28.
In this article, we present a detailed protocol for a recently developed formaldehyde crosslinking and immunoprecipitation (fCLIP) method to capture and analyze PKR-interacting RNAs during the cell cycle. We demonstrate the method to prepare cell cycle arrest samples using thymidine and nocodazole. We then present the fCLIP process to isolate PKR-bound RNAs and a method to prepare high-throughput sequencing library to identify these RNAs. Furthermore, we delineate detailed procedures to analyze PKR-bound RNAs using qRT-PCR. Specifically, we present a strand-specific reverse transcription procedure to analyze the strandedness of mtRNAs. The described protocol is optimized for HeLa cells and PKR, but key steps such as the preparation of cell cycle sample, fCLIP, and strand-specific qRT-PCR analysis can be easily modified to study cellular dsRNAs or to identify RNA interactors of other dsRBPs.
Protocol
1. Solution and cell preparation
- Solution preparation
- For the cell culture medium, prepare medium for HeLa cell culture by adding 50 mL of fetal bovine serum (FBS) to 500 mL of Dulbecco’s Modified Eagle’s Medium (DMEM).
NOTE: Antibiotics can be added to the cell culture medium, but we do not use antibiotics. - For the 0.1% paraformaldehyde, dissolve 4% (w/v) paraformaldehyde in 1x Phosphate-Buffered Saline (PBS) with heating on a hot plate and dilute to make 30 mL of 0.1% (v/v) paraformaldehyde by adding 1x PBS.
CAUTION: Perform all steps in a fume hood and be careful not to boil the paraformaldehyde solution. Protect the 0.1% solution from light and store at 4 °C. Use the solution within one month.
NOTE: The pH of the final 0.1% (v/v) paraformaldehyde solution should be around 7. - Prepare fCLIP lysis buffer: 20 mM Tris-HCl (pH 7.5), 15 mM NaCl, 10 mM EDTA, 0.5% (v/v) nonidet-p40 (NP-40), 0.1% (v/v) Triton X-100, 0.1% (v/v) sodium dodecyl sulfate (SDS), and 0.1% (w/v) sodium deoxycholate. Add triple distilled water (TDW) to 40 mL. Store at 4 °C.
- Prepare fCLIP wash buffer: 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 0.1% (v/v) NP-40, 0.1% (v/v) Triton X-100, 0.1% (v/v) SDS, and 0.1% (w/v) sodium deoxycholate. Add TDW to 40 mL. Store at 4 °C.
- Prepare 4x PK buffer: 400 mM Tris-HCl (pH 7.5), 200 mM NaCl, 40 mM EDTA, and 4% (v/v) SDS. Add TDW to 40 mL. Store at room temperature.
- Prepare fCLIP elution buffer: 20 mL of 4x PK buffer, 21 g of urea, and 8.5 mL of TDW. Prepare fresh.
- Prepare RNA elution buffer: 0.3 M NaOAc and 2% (w/v) SDS. Store at room temperature.
- Prepare 2x RNA loading dye: 0.025% (w/v) bromophenol blue, 0.025% (w/v) xylene cyanol, 5 mM EDTA, 0.05% (w/v) SDS, and 95% (v/v) formamide. Store at -20 °C.
- Prepare 1x TBE buffer: 0.089 M Tris-Borate and 0.002 M EDTA. Prepare 500 mL of TBE buffer.
- For the cell culture medium, prepare medium for HeLa cell culture by adding 50 mL of fetal bovine serum (FBS) to 500 mL of Dulbecco’s Modified Eagle’s Medium (DMEM).
- Preparation of S or M phase-arrested cells
- Seed ~750,000 or ~1,000,000 HeLa cells for S or M phase-arrested samples, respectively. Grow cells at 37 °C and 5% CO2 for 24 h.
- Treat the cells with 2 mM thymidine and incubate for 18 h at 37 °C.
- Wash the cells two times with PBS. Add fresh media and incubate for 9 h at 37 °C.
- For S phase-arrested cells, treat cells with 2 mM thymidine. For M phase-arrested cells, treat cells with 100 ng/mL nocodazole. Incubate for 15 h at 37 °C and harvest cells.
NOTE: The homogeneity of the cell cycle samples can be checked using FACS.
2. Formaldehyde cross-linking and immunoprecipitation
- Cell harvest
- For an S phase sample, collect cells with a cell scraper and transfer into a 15 mL conical tube. For an M phase sample, tap the side of the cell culture dish to detach M phase-arrested cells and transfer them into a 15 mL conical tube.
NOTE: To increase the homogeneity of the M phase-arrested cells, do not use a cell scraper. - Centrifuge the cells at 380 x g at room temperature for 3 min. Remove the supernatant and re-suspend with 1 mL of cold PBS and transfer into a 1.5 mL microcentrifuge tube.
- Centrifuge the cells at 10,000 x g at 4 °C for 30 s. Remove the supernatant completely.
- For an S phase sample, collect cells with a cell scraper and transfer into a 15 mL conical tube. For an M phase sample, tap the side of the cell culture dish to detach M phase-arrested cells and transfer them into a 15 mL conical tube.
- Formaldehyde crosslinking
- Add 750 μL of 0.1% paraformaldehyde for 10 min at room temperature to fix the cells.
- Add 250 μL of 1 M glycine and incubate an additional 10 min at room temperature to quench the reaction. Centrifuge the cells at 10,000 x g at 4 °C for 30 s. Remove the supernatant completely.
- Re-suspend with 1 mL of PBS. Centrifuge the cells at 10,000 x g at 4 °C for 30 s and remove the supernatant.
- Re-suspend with 400 μL of fCLIP lysis buffer supplemented with 0.2% (v/v) protease inhibitor and 2% (v/v) RNase inhibitor. Incubate on ice for 10 min and sonicate using an ultrasonicator.
- Add 11 μL of 5 M NaCl to adjust NaCl concentration to ~150 mM. Vortex briefly and centrifuge at 21,130 x g at 4 °C for 15 min. Transfer the supernatant to a pre-chilled 1.5 mL microcentrifuge tube.
NOTE: Retain 40 μL of supernatant in a new centrifuge tube as the input sample. Store the input sample at 4 °C.
- Immunoprecipitation
- Add 20 μL of Protein A beads into a 1.5 mL microcentrifuge tube.
- Wash the beads with 400 μL of fCLIP lysis buffer. Centrifuge the beads at 1,000 x g at 4 °C for 1 min. Remove the supernatant and add 400 μL of fCLIP lysis buffer carefully. Gently re-suspend the beads by inverting 3~4 times.
- Repeat the wash step three times. After the final wash, remove the supernatant completely.
- Re-suspend the beads in 300 μL of fCLIP lysis buffer. Add 8.3 μL of 5 M NaCl to adjust NaCl concentration to ~150 mM. Add 10 μL of PKR antibody and incubate for 3 h on a rotator at 4 °C.
- Centrifuge the beads at 1,000 x g at 4 °C for 1 min. Remove the supernatant and add 400 μL of fCLIP wash buffer. Gently re-suspend the beads by inverting 3~4 times.
- Repeat the wash step two times. After the final wash, remove the supernatant completely.
NOTE: Never use a vortex mixer. Invert the tube gently with hands. - Add 300 μL of lysate and incubate for 3 h at 4 °C on a rotator.
NOTE: Longer incubation time may result in increased background binding. - Centrifuge the beads at 1,000 x g at 4 °C for 1 min. Remove the supernatant and add 400 μL of fCLIP wash buffer. Gently re-suspend the beads by inverting 3~4 times.
- Repeat the wash step three times. After the final wash, remove the supernatant completely.
- Add 300 μL of fCLIP elution buffer. Incubate in a thermomixer for 3 h at 25 °C to elute PKR from the beads.
NOTE: Prepare the fCLIP elution buffer fresh. - Centrifuge at 1,000 x g at room temperature and transfer the supernatant to a clean siliconized tube.
NOTE: A microcentrifuge tube is also ok, but siliconized tube is preferred to prevent evaporation during the de-crosslinking step (step 2.3.12). - Add 300 μL of proteinase K (20 mg/mL) and incubate overnight at 65 °C.
NOTE: Use a thermomixer with heated cover to avoid evaporation.
- RNA extraction
- Add 580 μL of acid-phenol chloroform and vortex for 30 s. Incubate at 37 °C for 1 h.
- Vortex for 30 s and centrifuge at 12,000 x g at room temperature for 10 min.
- Transfer the top layer into a clean 1.5 mL microcentrifuge tube. Add 1/10 volume of 3 M NaOAc, 0.5 μL of coprecipitant (e.g., Glycoblue), and an equal volume of isopropanol. Incubate overnight at -20 °C.
- Precipitate pellets by centrifuging at maximum speed at 4 °C for 1 h.
NOTE: If RNA/DNA pellets were not observed, add an additional 0.5 μL of coprecipitant and centrifuge for an additional 1 h. Continue this step until RNA/DNA pellets are observed. - Remove the supernatant and add 1 mL of 75% ethyl alcohol. Centrifuge at 12,000 x g at 4 °C for 5 min. Repeat the wash step one more time. Remove the supernatant completely and dry the pellet at room temperature.
- Add 42 μL of TDW, 5 μL of DNase I buffer, 1 μL of RNase Inhibitor, and 2 μL of DNase I. Incubate at 37 °C for 1 h.
- Add 150 μL of TDW and 200 μL of acid-phenol chloroform. Vortex for 30 s. Centrifuge at 12,000 x g at room temperature for 10 min.
- Transfer the top layer into a clean 1.5 mL microcentrifuge tube. Add 20 μL of 3 M NaOAc, 0.5 μL of coprecipitant, and 1 mL of 100% ethyl alcohol. Incubate overnight at -80 °C.
- Precipitate pellets and wash with 75% ethyl alcohol as described in steps 2.4.4 to 2.4.5.
- Re-suspend in the appropriate amount of TDW.
3. Sequencing Library Preparation
- rRNA removal
- Re-suspend the RNA pellets from step 2.4.10 with 28 μL of TDW.
NOTE: The maximum amount of total RNA should be less than 5 μg. - Follow the procedures provided by the rRNA removal kit’s reference guide to remove rRNA.
- Clean up rRNA depleted RNAs by adding 160 μL of magnetic beads.
- Mix by pipetting ~15 times and incubate at room temperature for 15 min.
- Attach the tube on a magnetic bar and remove the supernatant.
- Add 300 μL of 80% ethyl alcohol while the beads are still attached on the magnetic bar and incubate for 30 s.
- Replace ethyl alcohol with a fresh 300 μL solution. Air dry the beads on the magnetic bar for 12 min at room temperature.
- Re-suspend the beads in 12 μL of TDW and mix by pipetting 15 times.
- Attach the beads on the magnetic bar and move the supernatant to a clean PCR tube.
- Deplete the fragmented ribosomal RNAs (rRNAs) following the procedures provided by rRNA Depletion Kit.
- Clean up the RNAs by adding 110 μL of magnetic beads and repeating steps 3.1.3.1 to 3.1.3.6.
- Re-suspend the RNA pellets from step 2.4.10 with 28 μL of TDW.
- RNA labeling and adaptor ligation
- On rRNA-depleted RNAs, add 2 μL of 10x CIAP buffer, 1 μL of RNase inhibitor, and 2 μL of antarctic alkaline phosphatase. Incubate at 37 °C for 1 h and then at 65 °C for 5 min to inactivate the phosphatase.
- Add 3 μL of 10x PNK buffer, 1.5 μL of RNase inhibitor, 1.5 μL of T4 PNK enzyme, 0.8 μL of r-ATP, and 1.7 μL of TDW. Incubate at 37 °C for 50 min.
- Add 1.5 μL of 10 mM ATP and incubate at 37 °C for additional 40 min.
- Stop the reaction by adding 170 μL of the RNA elution buffer.
- Add 200 μL of acid-phenol chloroform and vortex for 30 s. Centrifuge at 12,000 x g at room temperature for 10 min.
- Transfer the top layer into a clean 1.5 mL microcentrifuge tube. Add 20 μL of 3 M NaOAc, 0.5 μL of coprecipitant, and 1 mL of 100% ethyl alcohol. Incubate overnight at -80 °C.
- Precipitate RNA pellets and wash with 75% ethyl alcohol as described in steps 2.4.4 to 2.4.5. Re-suspend in 4.5 μL of TDW and add 9 μL of 2x RNA loading dye.
- Heat the sample at 95 °C for 5 min and load on a 10% Urea-PAGE gel.
- Run the gel at 370 V for 40 min.
NOTE: Pre-run the gel at 370 V for 90 min before loading the sample. - Cut the gel at the ~100 – 500 nucleotide region and break the gel.
- Add 700 μL of 0.3 M NaCl and incubate overnight at 4 °C on a rotator.
- Transfer the solution to a column and centrifuge at maximum speed at room temperature for 5 min.
- Transfer the eluate into a clean 1.5 mL microcentrifuge tube. Add 1/10 volume of 3 M NaOAc, 0.5 μL of coprecipitant, and an equal volume of isopropanol. Incubate overnight at -20 °C.
- 3' adaptor ligation
- Precipitate RNA pellets and wash with 75% ethyl alcohol as described in steps 2.4.4 to 2.4.5.
- Re-suspend the RNA pellets in 6.5 μL of TDW and add 1 μL of 10 μM 3' adaptor.
- Transfer the solution to a PCR tube and incubate at 70 °C for 2 min.
- Add 1 μL of 10x ligation buffer, 0.5 μL of RNase inhibitor, and 1 μL of T4 RNA ligase 2. Incubate at 28 °C for 1 h, 25 °C for 6 h, and 22 °C for 6 h.
- Add 12 μL of 2x RNA loading dye and heat at 95 °C for 5 min.
- Gel purify the 3' adaptor ligated RNAs as described in 3.2.9 to 3.2.13.
- 5' adaptor ligation
- Precipitate RNA pellets and wash with 75% ethyl alcohol as described in steps 2.4.4 to 2.4.5.
- Re-suspend the RNA pellets in 4.2 μL of TDW and add 1 μL of 5 μM 5' adaptor.
- Transfer the solution to a PCR tube and incubate at 70 °C for 2 min.
- Add 0.8 μL of 10x ligase buffer, 0.4 μL of RNase inhibitor, 0.8 μL of 10 mM ATP, 0.8 μL of T4 RNA ligase 1. Incubate at 28 °C for 1 h, 25 °C for 6 h, and 22 °C for 6 h.
- Reverse transcription and PCR amplification
- On 5’ adaptor ligated RNAs, add 1 μL of 4 μM reverse transcription primer. Incubate at 70 °C for 2 min and immediately cool to 4 °C.
- Add 4 μL of 5x FS buffer, 4 μL of 2.5 mM dNTP, 1 μL of 0.1 M DTT, 1 μL of RNase inhibitor, and 1 μL of reverse transcriptase. Incubate at 50 °C for 1 h and 70 °C for 15 min.
- Prepare PCR amplification
- Mix 1 μL of RT product, 1 μL of 25 μM RPI primer, 1 μL of 25 μM RP1 primer, 10 μL of 5x HF buffer, 4 μL of 2.5 mM dNTP, 32.5 μL of TDW, and 0.5 μL of high-fidelity polymerase.
- Run PCR program for 11~13 cycles.
NOTE: The program for the PCR is: 98 °C for 30 s for a hot start, 98 °C for 10 s, 60 °C for 30 s and 72 °C for 45 s for amplification, and 72 °C for 5 min. The amplification step is repeated for 11-13 cycles.
- Clean up the PCR products using 40 μL of the magnetic beads and following steps 3.1.3.1 to 3.1.3.6 but using 10 μL of TDW to elute the DNA.
- Add 2 μL of 10x DNA loading dye. Load the sample on a 6% acrylamide gel and run at 200 V for 30 min.
- Stain with Sybr gold (0.01% (v/v) in 1x TBE buffer) for 5 min at room temperature.
- De-stain in 1x TBE buffer for 5 min at room temperature.
- Cut the gel at the ~200 – 700 nucleotide region and break the gel.
- Add 700 μL of 0.3 M NaCl and incubate overnight at room temperature to elute the DNA.
- Load everything onto a column and centrifuge at maximum speed at room temperature for 5 min.
- Transfer the eluate into a clean 1.5 mL microcentrifuge tube. Add 1/10 volume of 3 M NaOAc, 0.5 μL of coprecipitant, and an equal volume of isopropanol. Incubate overnight at -20 °C.
- Precipitate DNA pellets and wash with 75% ethyl alcohol as described in steps 2.4.4 to 2.4.5. Re-suspend in 20 μL of TDW.
- Analyze the sample using a sequencer.
4. Analysis of PKR-interacting RNAs using qRT-PCR
- Method 1: Random hexamer reverse transcription
- Re-suspend RNA pellets from step 2.4.10 with 8.5 μL of TDW and transfer the solution to a PCR tube.
- Add 0.5 μL of 100 μM random hexamers and 4 μL of 2.5 mM dNTP mix.
- Heat the solution at 65 °C for 5 min and immediately incubate on ice for at least 1 min.
- Make the reaction mix: 4 μL of 5x SSIV buffer, 1 μL of 100 mM DTT, 1 μL of RNase Inhibitor, and 1 μL of reverse transcriptase (200 U/μL). Add the reaction mix to the RNA solution.
- Incubate the mixture at 23 °C for 10 min, 50 °C for 10 min, and 80 °C for 10 min.
- Analyze the cDNA using a real-time PCR system.
NOTE: The program for the real-time PCR is: 95 °C for 3 min for hot start, 95 °C for 5 s and 60 °C for 10 s for amplification, and 95 °C for 30 s, 65 °C for 30 s, and 95 °C for 30 s for melt. The amplification step is repeated for 40 cycles.
- Method 2: Strand-specific reverse transcription
- Prepare a master mix of reverse primers: Mix equal amounts of gene specific reverse transcription primers containing CMV promoter sequence followed by ~20 nucleotides of gene specific sequences.
NOTE: The combined concentration of all gene specific RT primers is 4 μM. - Re-suspend RNA pellets from step 2.4.10 with 8.5 μL of TDW and transfer the solution to a PCR tube.
- Add 0.5 μL of 4 μM master mix of reverse transcription primers and 4 μL of 2.5 mM dNTP mix.
- Heat the solution at 65 °C for 5 min and immediately incubate on ice for at least 1 min.
- Prepare the reaction solution by mixing 4 μL of 5x SSIV buffer, 1 μL of 100 mM DTT, 1 μL of RNase Inhibitor, and 1 μL of reverse transcriptase (200 U/μL). Add the reaction solution to the RNA-primer mix.
- Incubate the solution at 50 °C for 10 min and 80 °C for 10 min.
- Analyze the cDNA using the real-time PCR system.
NOTE: The program for the real-time PCR is same as the one described in Method 1.
- Prepare a master mix of reverse primers: Mix equal amounts of gene specific reverse transcription primers containing CMV promoter sequence followed by ~20 nucleotides of gene specific sequences.
Representative Results
A schematic for the process to arrest HeLa cells at the S or M phase of the cell cycle is shown in Figure 1. For an M phase-arrested sample, we can clearly visualize round shaped cells under the microscope (Figure 2A). To examine the efficiency of the cell cycle arrest, the nuclear content of the cell can be analyzed using FACS (Figure 2B). Figure 3 shows representative data for immunoprecipitation efficiency test, where the D7F7 antibody shows a superior ability to immunoprecipitate PKR. This difference in the immunoprecipitation efficiency may have been reflected in the discrepancy in the class distribution of the high-throughput sequencing libraries prepared using two different PKR antibodies (Figure 4). The specificity of the D7F7 antibody is further confirmed using whole blot western analysis of the PKR immunoprecipitate (Figure 5A) and the total cell lysate (Figure 5B). Figure 6 shows the radioisotope signal before and after removal of rRNAs during high-throughput sequencing library preparation. Figure 7 shows the enrichment of mtRNAs in RNAs co-immunoprecipitated with PKR, but not in RNAs co-immunoprecipitated with rabbit IgG or DiGeorge syndrome chromosomal region 8 (DGCR8). Figure 8 shows the representative strand specific qRT-PCR analysis of PKR-bound mtRNAs in S or M-phase arrested samples.
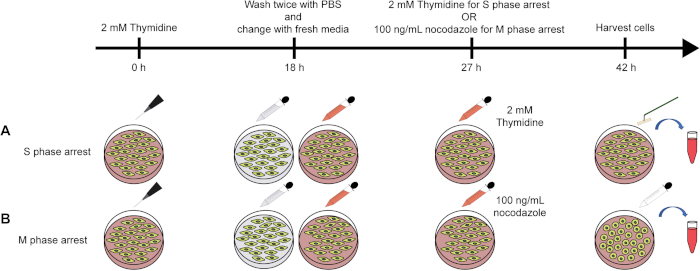
Figure 1: Schematic for the preparation cell cycle arrest samples. (A, B) Schematics of the preparation of S (A) or M (B) phase-arrested HeLa cells. Please click here to view a larger version of this figure.
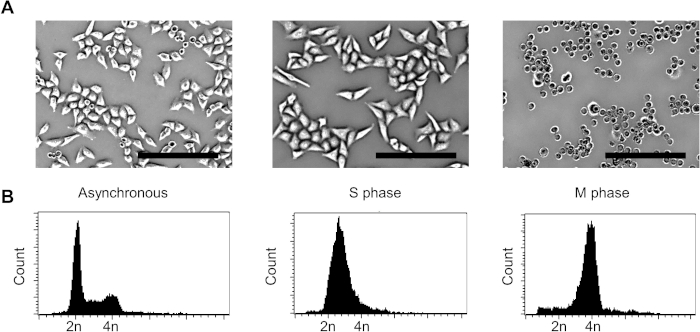
Figure 2: Analysis of cell cycle arrested samples. (A) Phase contrast images of S or M phase-arrested samples. Bars indicate 250 μm. (B) FACS analysis showing the nuclear content of the samples. Please click here to view a larger version of this figure.
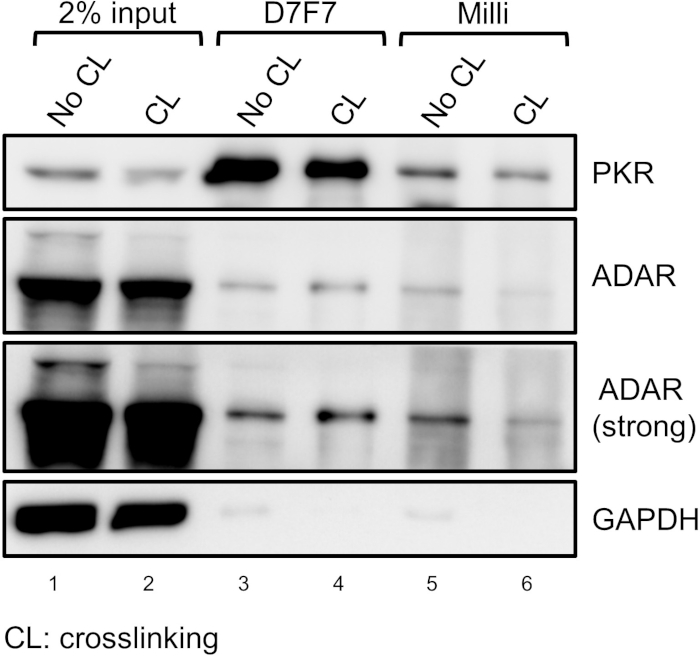
Figure 3: Immunoprecipitation efficiency test for PKR antibodies. For successful enrichment of PKR-bound RNAs, an antibody with a definitive immunoprecipitation efficiency such as the D7F7 antibody should be used. Please click here to view a larger version of this figure.
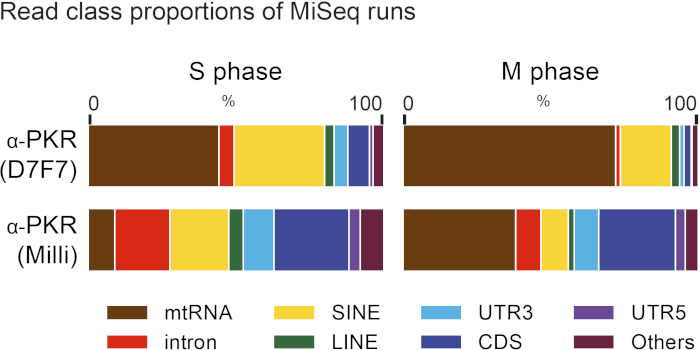
Figure 4: RNA class distribution of sequencing libraries prepared using different PKR antibodies. Using different PKR antibodies for fCLIP resulted in a different class distribution of mapped sequencing reads. The discrepancy is likely due to the differences in the immunoprecipitation efficiency. This figure has been modified from Kim et al.28. Please click here to view a larger version of this figure.
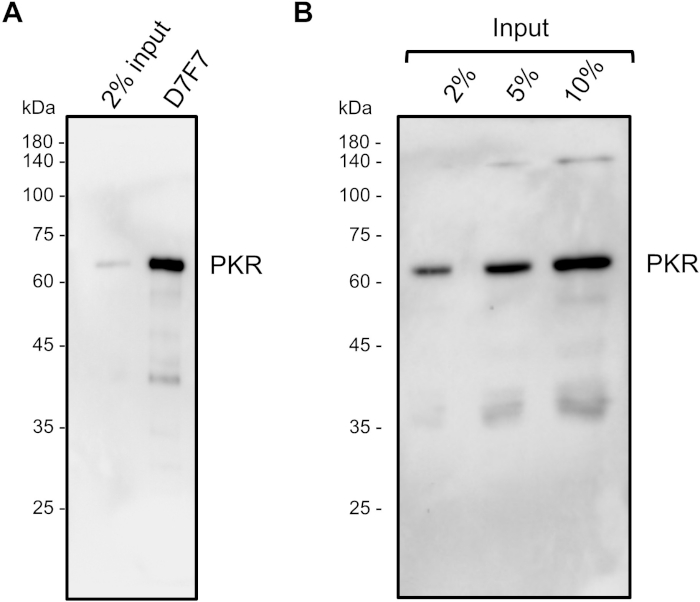
Figure 5: Specificity of the D7F7 PKR antibody. (A, B) The whole blot western analysis of PKR immunoprecipitated (A) or total HeLa lysate (B) showed only one strong band corresponding to the size of PKR, indicating that the D7F7 antibody is highly specific in recognizing PKR. Please click here to view a larger version of this figure.
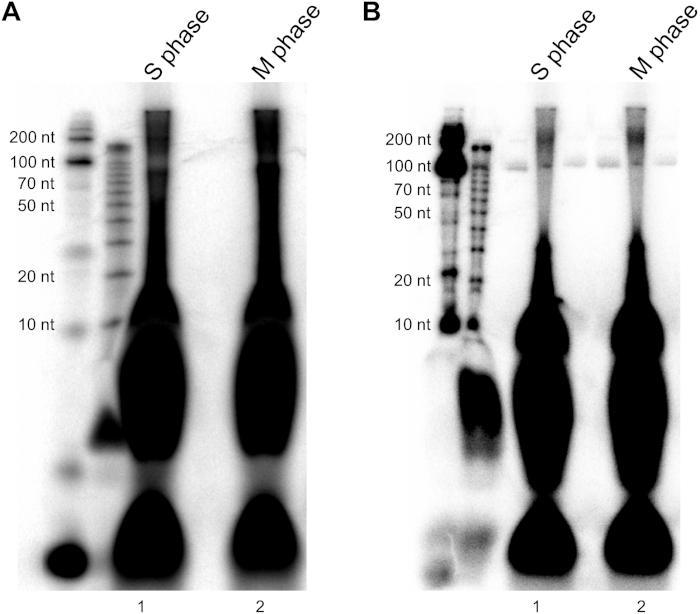
Figure 6: Radioisotope signal for PKR co-immunoprecipitated RNAs. (A) PKR co-immunoprecipitated RNAs could be detected by labeling their 5' ends with r-ATP. (B) Decrease in radioistope signal was observed upon successful rRNA removal. Please click here to view a larger version of this figure.
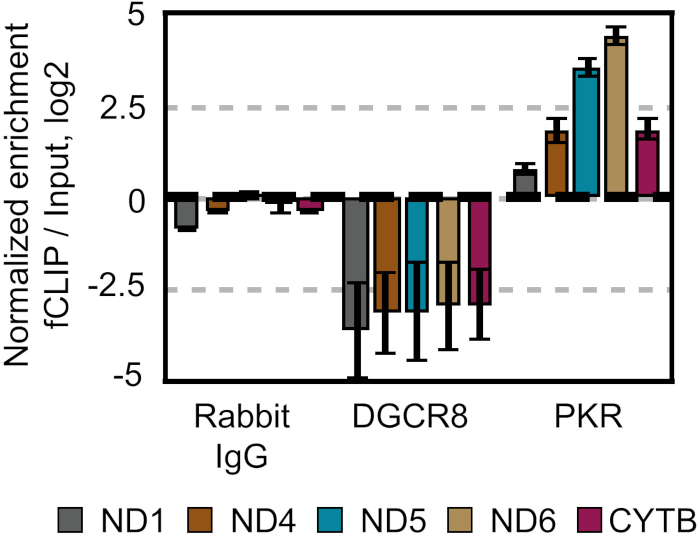
Figure 7: Validation of PKR-mtRNA interactions. Log2 fold enrichment of mtRNAs in RNAs co-immunoprecipitated with indicated antibodies. Only the PKR co-immunoprecipitated RNA sample showed strong enrichment of mtRNAs. Rabbit IgG and DGCR8 antibodies were used as negative controls. This figure has been modified from Kim et al.28. Please click here to view a larger version of this figure.
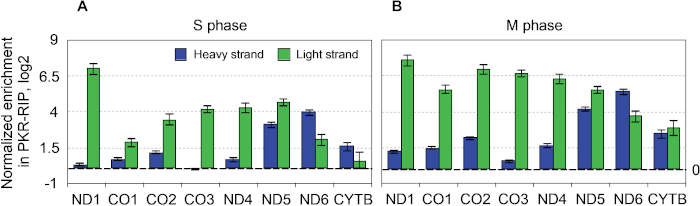
Figure 8: Strand-specific qRT-PCR analysis of PKR-bound mtRNAs. (A, B) Strand-specific reverse transcription was used to analyze the strandedness of mtRNAs that were co-immunoprecipitated with PKR for S (A) or M (B) phase-arrested cells. This figure has been modified from Kim et al.28. Please click here to view a larger version of this figure.
Discussion
The process to prepare S or M phase-arrested samples is illustrated in Figure 1. To arrest cells at the S phase, we used a thymidine double block method where we treated cells with thymidine two times with a 9 h release in between to ensure high arrest efficiency (Figure 1A). For M phase arrest, we treated cells once with thymidine followed by a 9 h release and then applied nocodazole to block cells at prometaphase (Figure 1B). One key step in preparing the cell cycle sample is the release step after the first thymidine block. To completely remove thymidine, it is critical to wash the cells at least two times with fresh PBS. Improper washing and residual thymidine can result in increased heterogeneity and decreased cell viability. The success of M phase arrest can be confirmed visually based on the increase in the number of round-shaped cells (Figure 2A). However, the M phase sample still contains many interphase cells based on their morphology. To collect only the M phase arrested cells, we applied physical force to detach M phase cells from the surface. The nuclear content of the harvested cells was further examined using FACS, which showed a broad peak between 2n and 4n for the S phase and a sharp peak at 4n for the M phase-arrested sample (Figure 2B). The presented protocol is optimized for HeLa cells, which have a doubling time of approximately 24 h. The idea of double thymidine and thymidine-nocodazole block can be applied to other cell lines, but the exact drug treatment and release durations need to be optimized based on the doubling time of the target cells.
To identify PKR-interacting RNAs, we crosslinked PKR-RNA complexes with formaldehyde and enriched them through immunoprecipitation. A key factor that determines the accuracy of the subsequent analysis is the efficiency of immunoprecipitation. We have tested numerous antibodies that target different epitopes of the PKR protein in order to determine the antibody for the high-throughput sequencing library preparation. As shown in Figure 3, we found that the D7F7 antibody that recognizes the linker region between the dsRNA binding domains and the catalytic domain showed superior ability in capturing PKR. The other antibody shown in Figure 3 (Milli) recognizes the N-terminal region, but shows a poor ability in immunoprecipitating PKR. Consequently, the high-throughput sequencing library prepared using the Milli antibody contained many background sequencing reads that are dispersed throughout the genome, particularly in introns, without distinct accumulations at specific regions28 (Figure 4). We believe the discrepancies in the two sequencing libraries are mostly due to the differences in the antibodies’ abilities in capturing PKR during the immunoprecipitation step. We further examined the specificity of the D7F7 PKR antibody through whole blot western analyses of immunoprecipitate (Figure 5A) and total HeLa lysates (Figure 5B). In both blots, we only observed one strong band that corresponds to PKR. This indicates that the D7F7 antibody is highly specific and that the sequencing data obtained using the D7F7 antibody likely reflect true RNA interactors of PKR.
A critical step during the high-throughput sequencing library preparation is the removal of rRNAs. Since we crosslinked RNA-RBP complexes with formaldehyde, we used an ultrasonicator for complete lysis of the cells. This process resulted in fragmentation of rRNAs, which significantly reduced the efficiency of rRNA removal using the rRNA Removal Kit. To resolve this problem, we first used the rRNA Removal Kit followed by the rRNA Depletion Kit (see Table of Materials), which almost completely removed rRNAs and less than 1% of the total sequencing reads were mapped to rRNAs. We used these two kits sequentially because the rRNA Depletion Kit has a maximum capacity of only 1 μg while the rRNA Removal Kit has a maximum capacity of 5 μg. We have experienced that using more than the recommended amount of the total RNA results in significant amount of sequencing reads mapped to rRNAs. The successful removal of rRNA can be confirmed after labeling the RNAs with r-ATP through the PNK reaction. While the rRNA depleted RNAs showed a distinct band around 150 nt, the sample before rRNA removal shows strong signal throughout the region corresponding to the 50 ~ 300 nt (Figure 6).
One limitation of formaldehyde crosslinking is the decrease immunoprecipitation efficiency. Other applications of formaldehyde crosslinking such as immunocytochemistry typically use 4% paraformaldehyde solution for fixation. However, such a strong fixation condition cannot be applied for fCLIP experiment because it significantly decreases the immunoprecipitation efficiency, which results in a higher background. Moreover, formaldehyde fixation crosslinks protein-protein complexes in addition to protein-RNA complexes. Therefore, one needs to pay caution in interpreting the fCLIP data.
As reported previously, mtRNAs form intermolecular dsRNAs that are recognized by PKR28. We first validated our sequencing data by examining PKR-mtRNA interactions through qRT-PCR. We used rabbit IgG and DGCR8 antibodies as negative controls, which did not show any enrichment of mtRNAs (Figure 7). Of note, DGCR8 was used as a nuclear dsRNA binding protein that is physically separated from mtRNAs. At the same time, PKR co-immunoprecipitated RNAs showed strong enrichment of mtRNAs (Figure 7).
To further analyze PKR-mtRNA interactions, we performed strand-specific reverse transcription to distinguish the heavy-strand mtRNAs from the light-strand mtRNAs (Figure 8). We designed reverse transcription primers that have a CMV promoter sequence followed by a gene-specific sequence and then used a CMV promoter sequence as the left primer and gene-specific right primers for the qPCR analysis. We have also tested SP6 promoter and pGEX sequencing primer sequences instead of the CMV promoter sequence. We found that while CMV promoter and pGEX sequencing primer sequences showed good result, using the SP6 promoter sequence did not. The difference is due to the low GC content of the SP6 promoter sequence (~33%) compared to those of the CMV promoter (~67%) and the pGEX sequencing primer (~65%) sequences. The proposed scheme for strand-specific reverse transcription can easily be applied to other intermolecular dsRNAs, but when designing the reverse transcription primers, the GC content needs to be taken into consideration.
Overall, we demonstrated the preparation of cell cycle arrested samples and the enrichment of PKR-interacting RNAs through formaldehyde crosslinking and immunoprecipitation. Using a highly efficient D7F7 antibody, we have successfully isolated PKR-bound RNAs and identified these RNAs by generating and analyzing a high-throughput sequencing library. Furthermore, to analyze the strandedness of mtRNAs bound to PKR, we presented a strand-specific reverse transcription approach. We expect that the presented protocol can be easily optimized to study RNA interactors of other dsRBPs and strandedness of intermolecular dsRNAs that are formed via complementary interaction between sense and antisense transcripts.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korean government Ministry of Science and ICT (NRF-2016R1C1B2009886).
Materials
| 0.5 M EDTA, pH 8.0 | Thermo Fisher Scientific | AM9260G | |
| 1 M Tris, pH 7.0 | Thermo Fisher Scientific | AM9855G | |
| 1 M Tris, pH 8.0 | Thermo Fisher Scientific | AM9855G | |
| 1.7 mL microcentrifuge tube | Axygen | MCT-175-C | |
| 10% Nonidet-p40 (NP-40) | Biosolution | BN015 | |
| 10% Urea-acrylamide gel solution | 7 M (w/v) Urea and 0.5X TBE, stored protected from light at 4 °C | ||
| 10X DNA loading buffer | TaKaRa | 9157 | |
| 15 mL conical tube | SPL | 50015 | |
| 3' adaptor | 5'-rApp NN NNT GGA ATT CTC GGG TGC CAA GG/3ddC/-3' | ||
| 3 M Sodium Acetate pH 5.5 | Thermo Fisher Scientific | AM9740 | |
| 5' adaptor | 5'-GUU CAG AGU UCU ACA GUC CGA CGA UCN NNN-3' | ||
| 5 M NaCl | Thermo Fisher Scientific | AM9760G | |
| 50 mL conical tube | SPL | 50050 | |
| Acid-phenol chloroform, pH 4.5 | Thermo Fisher Scientific | AM9722 | |
| Agencourt AMPure XP | Beckman Coulter | A63881 | Magnetic beads DNA/RNA clean up |
| Antarctic alkaline phosphatase | New England Biolabs | M0289S | |
| Anti-DGCR8 | Made in house | ||
| Anti-PKR (D7F7) | Cell signaling technology | 12297S | |
| Anti-PKR (Milli) | Millipore EMD | 07-151 | |
| ATP (100 mM) | GE Healthcare | GE27-2056-01 | |
| Bromophenol blue sodium salt | Sigma-aldrich | B5525 | |
| Calf intestinal alkaline phosphatase | TaKaRa | 2250A | |
| Cell scraper 25 cm 2-position | Sarstedt | 83.183 | |
| CMV promoter sequence | 5'-CGCAAATGGGCGGTAGGCGTG-3' | ||
| Dulbecco's modified eagle medium | Welgene | LM001-05 | |
| dNTP mixture (2.5 mM) | TaKaRa | 4030 | |
| Ethanol, Absolute, ACS Grade | Alfa-Aesar | A9951 | |
| Fetal bovine serum | Merck | M-TMS-013-BKR | |
| Formamide | Merck | 104008 | |
| Glycine | Bio-basic | GB0235 | |
| GlycoBlue coprecipitant (15 mg/mL) | Thermo Fisher Scientific | AM9516 | |
| Isopropanol | Merck | 8.18766.1000 | |
| NEBNext rRNA Depletion Kit | New England Biolabs | E6318 | rRNA Depletion Kit |
| Nocodazole | Sigma-Aldrich | M1404 | |
| Normal rabbit IgG | Cell signaling technology | 2729S | |
| Paraformaldehyde | Sigma-Aldrich | 6148 | |
| PCR forward primer (RP1) | 5'-AAT GAT ACG GCG ACC ACC GCG ATC TAC ACG TTC AGA GTT CTA CAG TCC GA-3' | ||
| PCR index reverse primer (RPI) | 5'-CAA GCA GAA GAC GGC ATA CGA GAT NNN NNN GTG ACT GGA GTT CCT TGG CAC CCG AGA ATT CCA-3' | ||
| PCR tubes with flat cap, 0.2 mL | Axygen | PCR-02-C | |
| Phosphate bufered saline (PBS) Tablet | TaKaRa | T9181 | |
| Phusion high-fidelity DNA polymerase | New England Biolabs | M0530 | High-fidelity polymerase |
| PlateFuge microcentrifuge with swing-out rotor | Benchmark | c2000 | |
| Polynucleotide kinase (PNK) | TaKaRa | 2021A | |
| Protease inhibitor cocktail set III | Merck | 535140-1MLCN | |
| Proteinase K, recombinant, PCR Grade | Sigma-Aldrich | 3115879001 | |
| qPCR primer sequence: CO1 Heavy | Forward/Reverse: 5′-GCCATAACCCAATACCAAACG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CO1 Light | Forward/Reverse: 5′-TTGAGGTTGCGGTCTGTTAG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CO2 Heavy | Forward/Reverse: 5′-CTAGTCCTGTATGCCCTTTTCC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CO2 Light | Forward/Reverse: 5′-GTAAAGGATGCGTAGGGATGG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CO3 Heavy | Forward/Reverse: 5′-CCTTTTACCACTCCAGCCTAG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CO3 Light | Forward/Reverse: 5′-CTCCTGATGCGAGTAATACGG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CYTB Heavy | Forward/Reverse: 5′-CAATTATACCCTAGCCAACCCC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: CYTB Light | Forward/Reverse: 5′-GGATAGTAATAGGGCAAGGACG -3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: GAPDH | Forward/Reverse: 5′-CAACGACCACTTTGTCAAGC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND1 Heavy | Forward/Reverse: 5′-TCAAACTCAAACTACGCCCTG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND1 Light | Forward/Reverse: 5′-GTTGTGATAAGGGTGGAGAGG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND4 Heavy | Forward/Reverse: 5′-CTCACACTCATTCTCAACCCC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND4 Light | Forward/Reverse: 5′-TGTTTGTCGTAGGCAGATGG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND5 Heavy | Forward/Reverse: 5′-CTAGGCCTTCTTACGAGCC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND5 Light | Forward/Reverse: 5′-TAGGGAGAGCTGGGTTGTTT-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND6 Heavy | Forward/Reverse: 5′-TCATACTCTTTCACCCACAGC-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| qPCR primer sequence: ND6 Light | Forward/Reverse: 5′-TGCTGTGGGTGAAAGAGTATG-3′/5′-CGCAAATGGGCGGTAGGCGTG-3′ | ||
| Random hexamer | Thermo Fisher Scientific | SO142 | |
| Recombinant Dnase I (Rnase-free) (5 U/μL) | TaKaRa | 2270A | |
| Recombinant Rnase inhibitor (40 U/μL) | TaKaRa | 2313A | |
| Ribo-Zero rRNA Removal Kit | Illumina | MRZH116 | rRNA Removal Kit |
| Rotator | FINEPCR, ROTATOR AG | D1.5-32 | |
| RT primer sequence: CO1 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGTTGAGGTTGCGGTCTGTTAG-3′ | ||
| RT primer sequence: CO1 Light | 5′-CGCAAATGGGCGGTAGGCGTGGCCATAACCCAATACCAAACG-3′ | ||
| RT primer sequence: CO2 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGGTAAAGGATGCGTAGGGATGG-3′ | ||
| RT primer sequence: CO2 Light | 5′-CGCAAATGGGCGGTAGGCGTGCTAGTCCTGTATGCCCTTTTCC-3′ | ||
| RT primer sequence: CO3 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGCTCCTGATGCGAGTAATACGG-3′ | ||
| RT primer sequence: CO3 Light | 5′-CGCAAATGGGCGGTAGGCGTGCCTTTTACCACTCCAGCCTAG-3′ | ||
| RT primer sequence: CYTB Heavy | 5′-CGCAAATGGGCGGTAGGCGTGGGATAGTAATAGGGCAAGGACG-3′ | ||
| RT primer sequence: CYTB Light | 5′-CGCAAATGGGCGGTAGGCGTGCAATTATACCCTAGCCAACCCC-3′ | ||
| RT primer sequence: GAPDH | 5′-CGCAAATGGGCGGTAGGCGTGTGAGCGATGTGGCTCGGCT-3′ | ||
| RT primer sequence: ND1 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGGTTGTGATAAGGGTGGAGAGG-3′ | ||
| RT primer sequence: ND1 Light | 5′-CGCAAATGGGCGGTAGGCGTGTCAAACTCAAACTACGCCCTG-3′ | ||
| RT primer sequence: ND4 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGTGTTTGTCGTAGGCAGATGG-3′ | ||
| RT primer sequence: ND4 Light | 5′-CGCAAATGGGCGGTAGGCGTGCCTCACACTCATTCTCAACCC-3′ | ||
| RT primer sequence: ND5 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGTTTGGGTTGAGGTGATGATG-3′ | ||
| RT primer sequence: ND5 Light | 5′-CGCAAATGGGCGGTAGGCGTGCATTGTCGCATCCACCTTTA-3′ | ||
| RT primer sequence: ND6 Heavy | 5′-CGCAAATGGGCGGTAGGCGTGGGTTGAGGTCTTGGTGAGTG-3′ | ||
| RT primer sequence: ND6 Light | 5′-CGCAAATGGGCGGTAGGCGTGCCCATAATCATACAAAGCCCC-3′ | ||
| Siliconized polypropylene 1.5 mL G-tube | Bio Plas | 4167SLS50 | |
| Sodium dedecyl sulfate | Biosesang | S1010 | |
| Sodium deoxycholate | Sigma-Aldrich | D6750 | |
| SUPERase In Rnase inhibitor | Thermo Fisher Scientific | AM2694 | |
| SuperScript III reverse transcriptase | Thermo Fisher Scientific | 18080093 | Reverse transcriptase for library preparation |
| SuperScript IV reverse transcriptase | Thermo Fisher Scientific | 18090010 | Reverse transcriptase for qRT-PCR |
| SYBR gold nucleic acid gl stain | Thermo Fisher Scientific | S11494 | |
| T4 polynucleotide kinase | New England Biolabs | M0201S | |
| T4 RNA ligase 1 (ssRNA Ligase) | New England Biolabs | M0204 | |
| T4 RNA ligase 2, truncated KQ | New England Biolabs | M0373 | |
| Thermomixer | Eppendorf ThermoMixer C with ThermoTop | ||
| Thymidine | Sigma-Aldrich | T9250 | |
| Tris-borate-EDTA buffer (TBE) | TaKara | T9122 | |
| Triton X-100 | Promega | H5142 | |
| Ultralink Protein A sepharose beads | Thermo Fisher Scientific | 22810 | Protein A beads |
| Ultrasonicator | Bioruptor | ||
| Urea | Bio-basic | UB0148 | |
| Vortex mixer | DAIHAN Scientific | VM-10 | |
| Xylene cyanol | Sigma-Aldrich | X4126 | |
| γ-32P-ATP (10 μCi/μL, 3.3 μM) | PerkinElmer | BLU502A100UC |
References
- Meurs, E. F., et al. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. Journal of Virology. 66 (10), 5805-5814 (1992).
- Patel, R. C., Stanton, P., McMillan, N. M., Williams, B. R., Sen, G. C. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proceedings of the National Academy of Sciences. 92 (18), 8283-8287 (1995).
- Bennett, R. L., Pan, Y., Christian, J., Hui, T., May, W. S. The RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G(1) arrest. Cell Cycle. 11 (1), 407-417 (2012).
- Yang, X., Nath, A., Opperman, M. J., Chan, C. The double-stranded RNA-dependent protein kinase differentially regulates insulin receptor substrates 1 and 2 in HepG2 cells. Molecular and Cellular Biology. 21 (19), 3449-3458 (2010).
- Zamanian-Daryoush, M., Mogensen, T. H., DiDonato, J. A., Williams, B. R. G. NF-kappa B Activation by Double-Stranded-RNA-Activated Protein Kinase (PKR) Is Mediated through NF-kappa B-Inducing Kinase and Ikappa B Kinase. Molecular and Cellular Biology. 20 (4), 1278-1290 (2000).
- Takada, Y., Ichikawa, H., Pataer, A., Swisher, S., Aggarwal, B. B. Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase. JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 26 (8), 1201-1212 (2007).
- Dabo, S., Meurs, E. F. dsRNA-dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses. 4 (11), 2598-2635 (2012).
- Black, T. L., Safer, B., Hovanessian, A., Katze, M. G. The Cellular 68,000-Mr Protein-Kinase Is Highly Autophosphorylated and Activated yet Significantly Degraded during Poliovirus Infection – Implications for Translational Regulation. Journal of Virology. 63 (5), 2244-2251 (1989).
- Bando, Y., et al. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson’s disease and Huntington’s disease. Neurochemistry International. 46 (1), 11-18 (2005).
- Onuki, R., et al. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer’s disease. The EMBO Journal. 23 (4), 959-968 (2004).
- Peel, A. Activation of the cell stress kinase PKR in Alzheimer’s disease and human amyloid precursor protein transgenic mice. Neurobiology of Disease. 14 (1), 52-62 (2003).
- Peel, A. L. Double-stranded RNA-dependent protein kinase, PKR, binds preferentially to Huntington’s disease (HD) transcripts and is activated in HD tissue. Human Molecular Genetics. 10 (15), 1531-1538 (2001).
- Suen, K. C., Yu, M. S., So, K. F., Chang, R. C., Hugon, J. Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. Journal of biological chemistry. 278 (50), 49819-49827 (2003).
- Nakamura, T., et al. A critical role for PKR complexes with TRBP in Immunometabolic regulation and eIF2alpha phosphorylation in obesity. Cell Reports. 11 (2), 295-307 (2015).
- Saito, S. Enhancement of the interferon-induced double-stranded RNA-dependent protein kinase activity by Sindbis virus infection and heat-shock stress. Microbiology and Immunology. 34 (10), 859-870 (1990).
- Youssef, O. A., et al. Potential role for snoRNAs in PKR activation during metabolic stress. Proceedings of the National Academy of Sciences. 112 (16), 5023-5028 (2015).
- Murtha-Riel, P., Davies, M. V., Choi, S. Y., Hershey, J. W., Kaufman, R. J. Expression of a Phosphorylation-resistant Eukaryotic Initiation Factor 2 a-Subunit Mitigates Heat Shock Inhibition of Protein Synthesis. The Journal of Biological Chemistry. 268, 12946-12951 (1993).
- Benkirane, M., et al. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. The EMBO Journal. 16 (3), 611-624 (1997).
- Koromilas, A., Roy, S., Barber, G., Katze, M., Sonenberg, N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 257 (5077), 1685-1689 (1992).
- Kim, Y., et al. PKR is activated by cellular dsRNAs during mitosis and acts as a mitotic regulator. Genes & Development. 28 (12), 1310-1322 (2014).
- Kim, Y., et al. Deletion of human tarbp2 reveals cellular microRNA targets and cell-cycle function of TRBP. Cell Reports. 9 (3), 1061-1074 (2014).
- Zhu, P. J., et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 147 (6), 1384-1396 (2011).
- Dagon, Y., et al. Double-stranded RNA-dependent protein kinase, PKR, down-regulates CDC2/cyclin B1 and induces apoptosis in non-transformed but not in v-mos transformed cells. Oncogene. 20 (56), 8045-8056 (2001).
- Elbarbary, R. A., Li, W., Tian, B., Maquat, L. E. STAU1 binding 3′ UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes & Development. 27 (13), 1495-1510 (2013).
- Cho, J., et al. LIN28A is a suppressor of ER-associated translation in embryonic stem cells. Cell. 151 (4), 765-777 (2012).
- Licatalosi, D. D., et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 456 (7221), 464-469 (2008).
- Van Nostrand, E. L., et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nature Methods. 13 (6), 508-514 (2016).
- Kim, Y., et al. PKR Senses Nuclear and Mitochondrial Signals by Interacting with Endogenous Double-Stranded RNAs. Molecular Cell. 71 (6), 1051-1063 (2018).
- Kim, B., Jeong, K., Kim, V. N. Genome-wide Mapping of DROSHA Cleavage Sites on Primary MicroRNAs and Noncanonical Substrates. Molecular Cell. 66 (2), 258-269 (2017).
- Ricci, E. P., et al. Staufen1 senses overall transcript secondary structure to regulate translation. Nature Structural & Molecular Biology. 21 (1), 26-35 (2014).
- Kim, B., Kim, V. N. fCLIP-seq for transcriptomic footprinting of dsRNA-binding proteins: Lessons from DROSHA. Methods. , (2018).
- Dhir, A., et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature. 560 (7717), 238-242 (2018).

